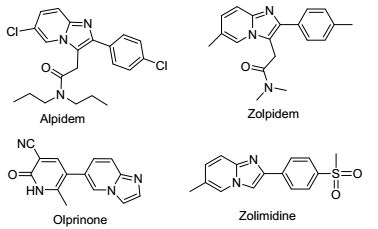
 Figure 1.
Some examples of imidazo-fused drugs
Figure 1.
Some examples of imidazo-fused drugs

CeCl3·7H2O催化Groebke-Blackburn-Bienayme反应简便合成咪唑稠杂环化合物
-
关键词:
- 咪唑稠杂环
- / CeCl3·7H2O
- / LaCl3·7H2O
English
Convenient Synthesis of Imidazo-Fused Heterocycles via CeCl3·7H2O Catalyzed Groebke-Blackburn-Bienayme Reaction
-
Isocyanide-based multicomponent reactions are in accord with green chemistry standards as a powerful tool for the construction of novel and diverse molecular structures due to "one-pot" advantages over conventional multistep syntheses. [1]The Groebke-Blackburn-Bienayme (GBB) reaction is a four-centre, three-component reaction, which involves starting materials of aldehyde, aminoazine and isocyanide in the presence of a suitable catalyst to afford a highly substituted and imidazo-fused heterocycles. [2] In recent decades, imidazo-fused heterocycles exhibit a broad spectrum of biological activities. Many commercially available drugs with these scaffolds have been developed, such as the anxiolytic drug alpidem, [3] the sedative drug zol-pidem, [4] the anti-ulcer drug zolimidine, [5]the heart failure drug olprinone [6] (Figure 1) and many other compounds are being investigated in the stage of biological testing or preclinical evaluation. [7]
The GBB reaction catalysts are generally acid in various kinds, occasionally base, such as Br nsted acids like CH3COOH, [8] HClO4, [9] cellulose sulfuric acid, [10] p-toluene sulfonic acid, [11]CH3SO3H; [12] Lewis acids like Sc(OTf)3, [13] SnCl2, [14] LaCl3, [15] LaCl3•7H2O, [16] MgCl2, [17] CeCl3• 7H2O, [18] ZrCl4, [19] ZnCl2, [20] RuCl3 [21] and InCl3, [22]solid acid catalyst like LaMnO3, [23] ionic liquid like 1-butyl-3-methylimidazolium bromide, [24] base like piperidine. [25] However, most of these catalysts suffer from several drawbacks such as long reaction time, harsh reaction conditions, poor yield, vastvast toxic metal-containing waste, and expensive reagents. In addition, different catalysts for a variety of substrates have different catalytic effect and each catalyst is not generally highly efficient. In order to overcome these disadvantages, development of novel methods to construct a variety of imidazo-fused heterocycles is still necessary and attractive. As for substrates, this reaction has a broad reactivity domain. Aminoazines include 2-amino-pyridine, 2-aminopyrimidine, 2-aminopyrazine, 2-amino-thiazole, 2-aminopyrazole, and a few more. Similarly, aromatic and aliphatic aldehydes participate in GBB reaction. [26] Commercial isocyanides can be widely used.
CeCl3 and CeCl3•nH2O, as ecofriendly, efficient and economical catalysts, [27] have been continuously studied to enhance the reactivity and selectivity of organic reactions. [28] With our continuous interest in exploring green catalyst, we knew that LaCl3 [15] and LaCl3•7H2O [16] catalysts had exhibited high activities at relative low reaction temperature of 60 ℃ in ethanol in GBB reaction. However, CeCl3•7H2O was reported as catalyst of GBB reaction in DMSO at 70 ℃ with a relatively low yield at 42% (Eq. 1). [18] Therefore, these findings gave us full interest to enhance the activities of CeCl3•7H2O catalyst by optimization of the reaction conditions applied for the GBB reaction. According to further attempts with different equivalent catalyst, temperature and solvent, we have successfully developed an efficient method for synthesis of imidazo-fused heterocycles using simple starting materials in the presence of 10 mol% CeCl3•7H2O in ethanol at 60 ℃. Using this method the reaction yield could be enhanced to 97%. In addition, we made a comparative study of CeCl3•7H2O with LaCl3•7H2O, which was reported to be a good catalyst in GBB reaction, and a series of new compounds were obtained.
1 Results and discussion
The GBB reaction is a very powerful tool for the efficient synthesis of libraries of diverse complex small molecules. To explore a kind of green and available method, we tried to search a high-performance catalyst to improve the synthetic reactions of imidazo-fused heterocycles into one step. A preliminary examination of the reaction of 5-chloropyridin-2-amine, benzaldehyde and t-butyl isocyanide in ethanol was selected as the model reaction for optimum reaction conditions. The time taken to achieve complete conversions, temperature and the isolated yields were recorded in Table 1.

Entry Solvent CeCl3•7H2O/ mol% T/℃ Time/min Yieldb/% 1 Ethanol —c r.t. 120 N.R.d 2 Ethanol 5 r.t. 150 82 3 Ethanol 5 35 100 86 4 Ethanol 5 60 60 89 5 Ethanol 10 r.t. 110 96 6 Ethanol 10 60 60 97 7 Methanol 10 60 90 65 8 Trifluoroethanol 10 60 90 78 9 H2O 10 60 160 74 10 H2O 10 80 120 83 11 —e 10 60 60 56 12 Ethanol 10 70 60 96 13 Ethanol 15 60 60 95 14 DMSO 10 70 120 83 15 Toluene 10 60 120 77 16 Dichloromethane 10 35 120 81 17 Acetonitrile 10 60 120 84 a Reaction conditions: 5-chloropyridin-2-amine (1.0 mmol), benzaldehyde (1.0 mmol), t-butyl isocyanide (1.0 mmol); b Isolated yield; c No catalyst used; dN.R. means no reaction; eSolvent-free condition. Table 1. Optimization of GBB reaction conditionsaIn our investigation, initially we found that no reaction occurred in the absence of catalysts at room temperature. However, when CeCl3•7H2O (5 mol%) was added at room temperature, the corresponding N-(tert-butyl)-6-chloro-2-phenylimidazo[1, 2-a]-pyridine-3-amine was obtained in 82% yield (Table 1, Entry 2). Upon varying the temperature of the reaction from room temperature to 70 ℃, the best yield was obtained at 60 ℃. Further increase of the temperature neither increased the yield nor shortened the reaction time. The solvents including methanol, water, DMSO, toluene, trifluoroethanol, dichloromethane and acetonitrile did not give excellent yields. Hence, we chose 10 mol% CeCl3•7H2O under ethanol and 60 ℃ conditions as the GBB reaction optimal condition for our further study.
To explore the scope of substrates in the CeCl3•7H2O-catalyzed GBB reaction substrates, we examined the generality of the reaction by using different aldehydes, aminoazines and isocyanides to achieve a wide range of imidazo-fused heterocycles and a series of novel compounds in satisfactory yields with short reaction time (Table 2). A variety of aldehydes were used, some with electron-with-drawing and eletron-donating groups being attached. It was found that electron-withdrawing groups like Cl and CN on the phenyl ring gave the products in good yields 93%~94% (Table 2, 4b~4c). At the same time, electron-donating groups like methoxyl on the phenyl ring afforded the unexpected yield in 93% (Table 2, 4d). When we further tried to replace the phenyl ring with the pyridyl ring on the aldehyde, yield of some products decreased slightly, and the reaction time need to be extended to 6 h (Table 2, 4m~4o). Further, scope of the substrates was extended to bicyclic aldehyde, which provided products in high yield 91% (Table 2, 4f). Different scaffolds of imidazo-fused heterocycles were successfully obtained by extension of various aminoa-zines. What's more, to extend scope of the reaction, t-butyl isocyanide, n-butyl isocynide and cyclohexylisocyanide were also used as synthetic substrates.

Entry Aminoazine 1 Aldehyde 2 R3 Compd. Time/h Yieldb/% 1 R1=5-Cl R2=Ph t-Butyl 4a 1 97 2 R1=5-Cl R2=4-ClC6H4 t-Butyl 4b 1 93 3 R1=5-Cl R2=4-CNC6H4 t-Butyl 4c 1 94 4 R1=5-Cl R2=4-MeOC6H4 t-Butyl 4d 1 93 5 R1=5-Cl R2=4-Et2NC6H4 t-Butyl 4e 2.5 94 6 R1=5-Cl R2=4-C8H7O t-Butyl 4f 3 91 7 R1=5-Cl R2=Ph n-Butyl 4g 3 88 8 R1=5-Cl R2=4-ClC6H4 Cyclohexyl 4h 3 91 9 2-NH2-pyrazine R2=Ph Cyclohexyl 4i 3.5 93 10 2-NH2-thiazole R2=Ph t-Butyl 4j 3 84 11 2-NH2-pyrazine R2=Ph t-Butyl 4k 3 90 12 2-NH2-pyrimidine R2=3, 5-Me2C6H3 t-Butyl 4l 3.5 85 13 R1=H R2=3-BrC5H3N Cyclohexyl 4m 6 85 14 3-NH2-1, 2, 4-triazole R2=3-BrC5H3N t-Butyl 4n 6 87 15 2-NH2-5-Cl-pyrazine R2=3-BrC5H3N t-Butyl 4o 6 80 a Reaction conditions: aldehyde (1.0 mmol), aminoazine (1.0 mmol), isocyanide (1.0 mmol), CeCl3•7H2O (10 mol %), ethanol, 60 ℃; bIsolated yield. Table 2. Synthesis of imidazo-fused heterocycles via GBB reactionaDue to obtain the best conditions of the CeCl3•7H2O-catalyzed GBB reaction, the catalytic activity of CeCl3• 7H2O for the synthesis of compounds 4n and 4o at a relatively low yield was compared with LaCl3• 7H2O in ethanol at 60 ℃. As shown in Table 3, CeCl3• 7H2O-catalyzed GBB product 4o resulting in 83% yield is substantially the same as CeCl3•7H2O in a 80% yield. However, LaCl3• 7H2O-catalyzed GBB product 4n resulting in 76% yield is lower than CeCl3•7H2O yield 87%. Hence, it is sure that CeCl3•7H2O as a very valuable and available catalyst is comparable to LaCl3•7H2O in GBB reaction.
 Table 3.
Comparison of efficiency of CeCl3•7H2O and LaCl3• 7H2O in the synthesis of products 4o and 4n
Table 3.
Comparison of efficiency of CeCl3•7H2O and LaCl3• 7H2O in the synthesis of products 4o and 4n
Entry Compd. Catalysta Yieldb/% 1 4o CeCl3•7H2O 80 2 4o LaCl3•7H2O 83 3 4n CeCl3•7H2O 87 4 4n LaCl3•7H2O 76 a10 mol%; bIsolated yield. Table 3. Comparison of efficiency of CeCl3•7H2O and LaCl3• 7H2O in the synthesis of products 4o and 4nThe structures of obtained GBB products 4a~4o were fully characterized by HRMS, 1H NMR and 13C NMR spectra. The single crystal structures of the two GBB products 4m and 4o were also determined by X-ray diffraction analysis in order to confirm the products (Figure 2). Crystallographic data of 4m (CCDC 1498341) and 4o (CCDC 1498345) have been deposited at the Cambridge Crystallographic Database Centre.
2 Conclusions
In conclusion, we had successfully developed an efficient and simple protocol for the synthesis of imidazo-fused heterocycles via one-pot three component GBB reaction catalyzed by CeCl3•7H2O. The salient features of our protocol include less reaction time, high substrate adaptability, easy purification, fewer column chromatography, cheap and green catalyst and easy separation for catalyst. Hence, this method can be easily applied to synthesis of N-fused and S-fused heterocycles at industrial scale. In addition, a serious of new imidazo-fused heterocycles which can ultimately lead to diverse medicinally relevant compounds were synthesized. This green methodology might provide a better manners alternative to the existing literature reports.
3 Experimental section
3.1 Instruments and reagents
All reagents and solvents were obtained from commercial suppliers and used as received without further purification. Melting points were determined using a WRS-1B digital melting point apparatus from Shanghai Measuring Instruments Equipment Co., Ltd. 1H NMR and 13C NMR spectra were measured on a VARIAN Mercury 600 MHz spectrometer in deuterated solvents with TMS as an internal standard. 1H NMR and 13C NMR spectra were obtained in CDCl3 or DMSO-d6 solutions as indicated. High-resolution mass spectra (HRMS) were recorded on Thermo Scientific LTQ Orbitrap Discovery. Thin-layer chromatography (TLC) was performed using silica gel GF254. All reactions progress was monitored by TLC and column chromatography was performed with silica gel (200~300 mesh).
3.2 Experimental method
A mixture of aldehyde (1.0 mmol), aminoazine (1.0 mmol), isocyanide (1.0 mmol) and 10 mol% CeCl3•7H2O in ethanol (2.0 mL) was stirred at 60 ℃ in an oil bath. After completion of the reaction as indicated by TLC, the resulting mixture was cooled to room temperature. Then ethanol was removed at reduced pressure and the residue was distributed in dichloromethane (20 mL) and water (10 mL). The organic layer was separated and the aqueous layer was extracted with dichloromethane. The combined organic phases were dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford a crude product which was purified by column chromatography on silica gel using petroleum ether and ethyl acetate (V:V=5:1) as eluent (4g~4o). In some cases, TLC showed that the product was nearly pure, so that the crude residue could be used directly purification by recrystallization with petroleum and slight ethyl acetate to obtain pure compounds (4a~4f).
N-(tert-Butyl)-6-chloro-2-phenylimidazo[1, 2-a]pyri-din-3-amine (4a): White solid, yield 97%. m.p. 207~209 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.50 (s, 1H), 8.14 (d, J=7.5 Hz, 2H), 7.51 (d, J=9.4 Hz, 1H), 7.38 (t, J=7.6 Hz, 2H), 7.27 (t, J=7.3 Hz, 1H), 7.20 (dd, J=9.4, 1.5 Hz, 1H), 4.67 (s, 1H), 0.98 (s, 9H); 13C NMR (150 MHz, DMSO-d6) δ: 139.9, 139.5, 135.4, 128.4, 128.1, 127.6, 125.1, 122.2, 118.9, 118.1, 56.4, 30.4. HRMS calcd for C17H19ClN3 [M+H]+ 300.1262, found 300.1263.
N-(tert-Butyl)-6-chloro-2-(4-chlorophenyl)imidazo[1, 2-a]pyridin-3-amine (4b): White solid, yield 93%. m.p. 196~198 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.55~8.52 (m, 1H), 8.22~8.20 (m, 1H), 8.20~8.19 (m, 1H), 7.56~7.51 (m, 1H), 7.49~7.44 (m, 2H), 7.24 (dd, J=9.4, 2.1 Hz, 1H), 4.74 (s, 1H), 1.01 (s, 9H); 13C NMR (150 MHz, DMSO-d6) δ: 140.0, 138.2, 134.3, 132.2, 129.7, 128.4, 125.4, 122.3, 119.0, 118.1, 56.6, 30.4. HRMS calcd for C17H18Cl2N3 [M+H]+ 334.0872, found 334.0871.
4-(3-(tert-Butylamino)-6-chloroimidazo[1, 2-a]pyridin-2-yl)benzonitrile (4c): Light yellow solid, yield 94%. m.p. 226~228 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.57 (d, J=0.5 Hz, 1H), 8.40~8.37 (m, 1H), 7.89~7.84 (m, 1H), 7.57 (d, J=9.4 Hz, 1H), 7.28 (d, J=9.5 Hz, 1H), 4.85 (s, 1H), 1.02 (d, J=1.3 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 140.6, 139.3, 138.5, 132.1, 128.4, 126.3, 124.83, 121.3, 120.5, 119.0, 118.0, 110.9, 56.8, 30.4. HRMS calcd for C18H18ClN4 [M+H]+ 325.1215, found 325.1217.
N-(tert-Butyl)-6-chloro-2-(4-methoxyphenyl)imida-zo[1, 2-a]pyridin-3-amine (4d): White solid, yield 93%. m.p. 175~177 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.50 (s, 1H), 8.11 (d, J=9.6 Hz, 1H), 7.50 (dd, J=9.4, 1.9 Hz, 1H), 7.21~7.17 (m, 1H), 6.97 (d, J=9.7 Hz, 1H), 4.64 (d, J=1.8 Hz, 1H), 3.79 (d, J=1.4 Hz, 1H), 1.00 (d, J=1.6 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 159.2, 140.7, 140.2, 129.3, 127.4, 125.1, 123.4, 121.3, 119.6, 117.5, 113.7, 56.4, 55.2, 30.3. HRMS calcd for C18H21ClN3O [M+H]+ 330.1368, found 330.1371.
N-(tert-Butyl)-6-chloro-2-(4-(diethylamino)phenyl)im-idazo[1, 2-a]pyridin-3-amine (4e): Yellow solid, yield 94%. m.p. 163~165 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.45~8.43 (m, 1H), 8.01~7.97 (m, 1H), 7.45 (dd, J=9.4, 0.4 Hz, 1H), 7.15 (dd, J=9.4, 2.1 Hz, 1H), 6.67 (d, J=9.0 Hz, 1H), 4.54 (s, 1H), 3.36 (t, J=7.0 Hz, 1H), 1.11 (t, J=7.0 Hz, 2H), 1.03 (s, 5H); 13C NMR (150 MHz, CDCl3) δ: 147.3, 141.3, 140.1, 124.6, 122.7, 121.6, 121.1, 119.2, 117.2, 111.5, 56.4, 44.3, 30.4, 12.6. HRMS calcd for C21H28ClN4 [M+H]+ 371.1997, found 371.1997.
N-(tert-Butyl)-6-chloro-2-(2, 3-dihydrobenzofuran-5-yl)-imidazo[1, 2-a]pyridin-3-amine (4f): Yellow solid, yield 91%. m.p. 185~187 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.48 (s, 1H), 8.03 (s, 1H), 7.93 (d, J=8.1 Hz, 1H), 7.48 (d, J=9.4 Hz, 1H), 7.19 (d, J=9.3 Hz, 1H), 6.78 (d, J=8.3 Hz, 1H), 4.61 (s, 1H), 4.56 (t, J=8.6 Hz, 2H), 3.22 (t, J=8.6 Hz, 2H), 1.01 (s, 9H); 13C NMR (150 MHz, CDCl3) δ: 159.8, 141.0, 140.1, 128.0, 127.2, 125.1, 124.9, 123.2, 121.2, 119.6, 117.4, 108.9, 71.4, 56.4, 30.4, 29.6. HRMS calcd for C19H21ClN3O [M+H]+ 342.1368, found 342.1368.
N-Butyl-6-chloro-2-phenylimidazo[1, 2-a]pyridin-3-ami-ne (4g): White solid, yield 88%. m.p. 128~130 ℃; 1H NMR (600 MHz, CDCl3) δ: 8.08 (d, J=1.8 Hz, 1H), 7.95 (d, J=7.3 Hz, 2H), 7.51~7.44 (m, 3H), 7.34 (t, J=7.4 Hz, 1H), 7.09 (dd, J=9.4, 2.0 Hz, 1H), 3.14 (t, J=6.3 Hz, 1H), 3.05 (dd, J=13.8, 6.8 Hz, 2H), 1.63~1.57 (m, 2H), 1.47~1.40 (m, 2H), 0.94 (t, J=7.3 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ: 139.4, 136.3, 133.4, 128.7, 127.7, 126.9, 126.8, 125.5, 120.3, 117.6, 48.0, 32.8, 20.2, 13.9. HRMS calcd for C17H19ClN3 [M+H]+ 300.1262, found 300.1262.
6-Chloro-2-(4-chlorophenyl)-N-cyclohexylimidazo[1, 2-a]pyridin-3-amine (4h): White solid, yield 91%. m.p. 219~221 ℃; 1H NMR (600 MHz, CDCl3) δ: 8.11 (d, J=1.6 Hz, 1H), 7.98 (d, J=8.5 Hz, 2H), 7.47 (d, J=9.5 Hz, 1H), 7.42 (d, J=8.5 Hz, 2H), 7.10 (dd, J=9.4, 1.9 Hz, 1H), 3.04 (d, J=4.5 Hz, 1H), 2.97~2.91 (m, 1H), 1.79 (d, J=11.9 Hz, 2H), 1.74~1.67 (m, 2H), 1.28~1.13 (m, 5H); 13C NMR (150 MHz, CDCl3) δ: 139.6, 136.5, 133.5, 132.1, 128.7, 128.2, 125.8, 125.3, 120.6, 120.4, 117.6, 56.8, 34.1, 25.6, 24.7. HRMS calcd for C19H20Cl2N3 [M+H]+ 360.1029, found 360.1032.
N-Cyclohexyl-2-phenylimidazo[1, 2-a]pyrazin-3-amine (4i): White solid, yield 93%. m.p. 156~158 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.92 (d, J=1.4 Hz, 1H), 8.36 (dd, J=4.6, 1.4 Hz, 1H), 8.22 (dd, J=8.3, 1.2 Hz, 2H), 7.85 (d, J=4.6 Hz, 1H), 7.53~7.41 (m, 2H), 7.38~7.30 (m, 1H), 5.05 (d, J=7.0 Hz, 1H), 2.90~2.83 (m, 1H), 1.71 (d, J=11.9 Hz, 2H), 1.68~1.59 (m, 2H), 1.49 (s, 1H), 1.34~1.22 (m, 2H), 1.15~1.02 (m, 3H); 13C NMR (150 MHz, CDCl3) δ: 143.4, 138.9, 136.7, 133.6, 129.0, 128.7, 128.1, 127.3, 115.5, 56.9, 34.3, 25.6, 24.8. HRMS calcd for C18H21N4 [M+H]+ 293.1761, found 293.1764.
N-(tert-Butyl)-6-phenylimidazo[2, 1-b]thiazol-5-amine (4j): White solid, yield 84%. m.p. 150~152 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.09 (dd, J=8.2, 1.0 Hz, 2H), 7.72 (d, J=4.5 Hz, 1H), 7.34 (t, J=7.7 Hz, 2H), 7.19 (t, J=7.4 Hz, 1H), 7.16 (d, J=4.5 Hz, 1H), 4.49 (s, 1H), 1.02 (s, 9H); 13C NMR (150 MHz, CDCl3) δ: 145.5, 140.1, 135.3, 128.2, 127.2, 126.7, 125.5, 117.8, 111.4, 55.8, 30.2. HRMS calcd for C15H18N3S [M+H]+ 272.1216, found 272.1217.
N-(tert-Butyl)-2-phenylimidazo[1, 2-a]pyrazin-3-amine (4k): Gray solid, yield 90%. m.p. 148~150 ℃; 1H NMR (600 MHz, CDCl3) δ: 9.00 (s, 1H), 8.14 (dd, J=4.6, 1.2 Hz, 1H), 7.93~7.89 (m, 2H), 7.86 (d, J=4.6 Hz, 1H), 7.46 (t, J=7.7 Hz, 2H), 7.37 (t, J=7.4 Hz, 1H), 1.05 (s, 9H); 13C NMR (150 MHz, CDCl3) δ: 143.3, 142.2, 137.23, 134.2, 128.8, 128.4, 128.1, 125.0, 116.3, 56.9, 30.3. HRMS calcd for C16H19N4 [M+H]+ 267.1604, found 267.1607.
N-(tert-Butyl)-2-(3, 5-dimethylphenyl)imidazo[1, 2-a]pyr-imidin-3-amine (4l): Yellow solid, yield 85%. m.p. 189~191 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.79 (dd, J=6.8, 2.0 Hz, 1H), 8.47~8.44 (m, 1H), 7.85 (s, 2H), 7.01 (dd, J=6.8, 4.0 Hz, 1H), 6.94 (s, 1H), 4.69 (s, 1H), 2.33 (s, 6H), 1.01 (s, 9H); 13C NMR (150 MHz, CDCl3) δ: 149.2, 145.0, 141.1, 137.6, 134.2, 130.9, 129.5, 126.1, 121.8, 107.7, 56.6, 30.4, 21.4. HRMS calcd for C18H23N4 [M+H]+ 295.1917, found 295.1918.
2-(3-Bromopyridin-4-yl)-N-cyclohexylimidazo[1, 2-a]-pyridin-3-amine (4m): Light yellow solid, yield 85%. m.p. 123~125 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.84 (s, 1H), 8.60 (d, J=4.9 Hz, 1H), 8.31 (dt, J=7.0, 1.0 Hz, 1H), 7.65 (d, J=4.7 Hz, 1H), 7.51 (dt, J=9.1, 0.9 Hz, 1H), 7.22 (ddd, J=9.0, 6.6, 1.2 Hz, 1H), 6.95 (td, J=6.8, 1.1 Hz, 1H), 4.59 (d, J=6.0 Hz, 1H), 2.70~2.63 (m, 1H), 1.63~1.56 (m, 2H), 1.53 (dd, J=11.5, 4.2 Hz, 2H), 1.44~1.36 (m, 1H), 1.09~0.92 (m, 5H); 13C NMR (150 MHz, DMSO-d6) δ: 152.1, 148.1, 144.1, 142.0, 134.1, 126.9, 126.6, 124.5, 123.0, 120.5, 117.8, 112.0, 56.6, 33.8, 25.5, 24.5. HRMS calcd for C18H20BrN4 [M+H]+ 371.0866, found 371.0871.
6-(3-Bromopyridin-4-yl)-N-(tert-butyl)-1H-imidazo[2, 1-c] [1, 2, 4]triazol-5-amine) (4n): White solid, yield 87%. m.p. 197~200 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 11.99 (s, 1H), 8.88 (s, 1H), 8.64 (d, J=4.9 Hz, 1H), 7.87 (s, 1H), 7.67 (d, J=4.9 Hz, 1H), 4.30 (s, 1H), 0.97 (s, 9H); 13C NMR (150 MHz, CDCl3) δ: 153.1, 152.8, 148.2, 139.3, 126.4, 123.0, 120.3, 56.0, 29.9. HRMS calcd for C13H16BrN6 [M+H]+ 335.0614, found 335.0617.
2-(3-Bromopyridin-4-yl)-N-(tert-butyl)-6-chloroimidazo-[1, 2-a]pyrazin-3-amine (4o): White solid, yield 80%. m.p. 193~195 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.95 (d, J=1.3 Hz, 1H), 8.89 (s, 1H), 8.70 (d, J=1.3 Hz, 1H), 8.64 (d, J=4.9 Hz, 1H), 7.72~7.70 (m, 1H), 4.64 (s, 1H), 0.90 (s, 9H); 13C NMR (150 MHz, CDCl3) δ: 152.3, 148.4, 143.3, 142.5, 140.9, 136.6, 134.9, 127.2, 126.8, 120.9, 114.5, 56.8, 30.0. HRMS calcd for C15H16BrClN5 [M+H]+ 380.0272, found 380. 0272.
Supporting Information 1H NMR and 13C NMR spec-tra for all products associated with this article. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn/.
-
-
[1]
(a) Ma, Z. ; Xiang, Z. ; Luo, T. ; Lu, K. ; Xu, Z. ; Chen, J. ; Yang, Z. J. Comb. Chem. 2006, 8, 696. (b) Riva, R. ; Banfi, L. ; Basso, A. ; Cerulli, V. ; Guanti, G. ; Pani, M. J. Org. Chem. 2010, 15, 5134. (c) Erb, W. ; Neuville, L. ; Zhu, J. J. Org. Chem. 2009, 74, 3109. (d) Zhang, Z. R. ; Zheng, X. L. ; Guo, C. B. Chin. J. Org. Chem. 2016, 36, 1241 (in Chinese). (张钊瑞, 郑晓霖, 郭长彬, 有机化学, 2016, 36, 1241. )(e) Singh, S. ; Samanta, S. Chin. J. Chem. 2015, 33, 1244. (f) Wang, H. ; Xu, B. Chin. J. Org. Chem. 2015, 35, 588 (in Chinese). (王浩, 许斌, 有机化学, 2015, 35, 588. )(g) Sun, X. Y. ; Wang, W. M. ; Ma, J. ; Yu, S. Y. Acta Chim. Sinica 2017, 75, 115 (in Chinese). (孙晓阳, 王文敏, 马晶, 俞寿云, 化学学报, 2017, 75, 115. )(h) Ren, S. B. ; Zhang, H. F. ; Zhang, J. ; Zhang, W. ; Liu, Y. K. Chin. J. Org. Chem. 2016, 36, 1954 (in Chinese). (任少波, 张海峰, 张剑, 张巍, 刘运奎, 有机化学, 2016, 36, 1954. )(i) Xu, W. S. ; Zhao, S. J. ; Luo, X. P. ; Song, J. N. ; Liu, J. Q. ; Bi, X. H. ; Liao, P. Q. Chin. J. Org. Chem. 2015, 35, 2095 (in Chinese). (徐文帅, 赵寿经, 骆晓沛, 宋金娜, 刘建全, 毕锡和, 廖沛球, 有机化学, 2015, 35, 2095. )(j) Li, Z. H. ; Yan, N. N. ; Xie, J. W. ; Liu, P. ; Zhang, J. ; Dai, B. Chin. J. Chem. 2015, 33, 589.
-
[2]
Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183. doi: 10.1016/j.tet.2014.10.032
-
[3]
Berson, A.; Descatoire, V.; Sutton, A.; Fau, D.; Maulny, B.; Vadrot, N.; Feldmann, G.; Berthon, B.; Tordjmann, T.; Pessayre, D. J. Pharmacol. Exp. Ther. 2001, 299, 793.
-
[4]
Harrison, T. S.; Keating, G. M. CNS Drugs 2005, 19, 65. doi: 10.2165/00023210-200519010-00008
-
[5]
Kaminski, J. J.; Wallmark, B.; Briving, C.; Andersson, B. M. J. Med. Chem. 1991, 34, 533. doi: 10.1021/jm00106a008
-
[6]
Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Cardiovasc. Drug Rev. 2002, 20, 163.
-
[7]
(a) Palmer, A. M.; Chrismann, S.; Munch, G.; Brehm, C.; Zimmermann, P. J.; Buhr, W.; Senn-Bilfinger, J.; Feth, M. P.; Simon, W. A. Bioorg. Med. Chem. 2009, 17, 368.(b) Mori, H.; Tanaka, M.; Kayasuga, R.; Masuda, T.; Ochi, Y.; Yamada, H.; Kishikawa, K.; Ito, M.; Nakamura, T. Bone 2008, 43, 840.
-
[8]
Sharma, A.; Li, H. Y. Synlett 2011, 1407.
-
[9]
Huang, Y.; Hu, X. Q.; Shen, D. P.; Chen, Y. F.; Xu, P. F. Mol. Diversity 2007, 11, 73. doi: 10.1007/s11030-007-9059-3
-
[10]
Groebke, K.; Weber, L.; Mehlin, F. Synlett 1998, 661.
-
[11]
Che, C.; Xiang, J.; Wang, G. X.; Fathi, R.; Quan, J. M.; Yang, Z. J. Comb. Chem. 2007, 9, 982.
-
[12]
Mert-Balci, F.; Conrad, J.; Meindl, K.; Schulz, T.; Stalke, D.; Beifuss, U. Synthesis 2008, 3649.
-
[13]
McKeown, M. R.; Shaw, D. L.; Fu, H.; Liu, S.; Xu, X.; Marineau, J. J.; Huang, Y.; Zhang, X.; Buckley, D. L.; Kadam, A.; Zhang, Z.; Blacklow, S. C.; Qi, J.; Zhang, W.; Bradner, J. E. J. Med. Chem. 2014, 57, 9019. doi: 10.1021/jm501120z
-
[14]
Shaabani, A.; Soleimani, E.; Sarvary, A.; Rezayan, H.; Maleki, A. Chin. J. Chem. 2009, 27, 369. doi: 10.1002/cjoc.v27:2
-
[15]
Xi, G. L.; Liu, Z. Q. Tetrahedron 2015, 71, 9602. doi: 10.1016/j.tet.2015.10.080
-
[16]
Shinde, A. H.; Srilaxmi, M.; Satpathi, B.; Sharada, D. S. Tetrahedron Lett. 2014, 55, 5915. doi: 10.1016/j.tetlet.2014.08.126
-
[17]
Odell, L. R.; Nilsson, M.; Gising, J.; Lagerlund, O.; Muthas, D.; Nordqvist, A.; Karlen, A.; Larhed, M. Bioorg. Med. Chem. Lett. 2009, 19, 4790. doi: 10.1016/j.bmcl.2009.06.045
-
[18]
Guchhait, S. K.; Madaan, C. Tetrahedron Lett. 2011, 52, 56. doi: 10.1016/j.tetlet.2010.10.143
-
[19]
Guchhait, S. K.; Maadan, C. Synlett 2009, 628.
-
[20]
Rousseau, A. L.; Matlaba, P.; Parkinson, C. J. Tetrahedron Lett. 2007, 48, 4079. doi: 10.1016/j.tetlet.2007.04.008
-
[21]
Rostamnia, S.; Hassankhanib, A. RSC Adv. 2013, 3, 18626. doi: 10.1039/C2RA22527A
-
[22]
Akbarzadeh, R.; Shakibaei, G. I.; Bazgir, A. Monatsh. Chem. 2010, 141, 1077. doi: 10.1007/s00706-010-0372-7
-
[23]
Sanaeishoara, T.; Tavakkolia, H.; Mohave, F. Appl. Catal., A 2014, 470, 56. doi: 10.1016/j.apcata.2013.10.026
-
[24]
Shaabani, A.; Soleimani, E.; Maleki, A. Tetrahedron Lett. 2006, 47, 3031.
-
[25]
Lee, C. H.; Hsu, W. S.; Chen, C. H.; Sun, C. M. Eur. J. Org. Chem. 2013, 11, 2201.
-
[26]
Neochoritis, C. G.; Stotani, S.; Mishra, B.; Domling, A. Org. Lett. 2015, 17, 2002. doi: 10.1021/acs.orglett.5b00759
-
[27]
Kolo, K.; Sajadi, S. M. Lett. Org. Chem. 2013, 10, 688. doi: 10.2174/15701786113109990021
-
[28]
Bartoli, G.; Marcantoni, E.; Marcolini, M.; Sambri, L. Chem. Rev. 2010, 110, 6104. doi: 10.1021/cr100084g
-
[1]
-
Table 1. Optimization of GBB reaction conditionsa

Entry Solvent CeCl3•7H2O/ mol% T/℃ Time/min Yieldb/% 1 Ethanol —c r.t. 120 N.R.d 2 Ethanol 5 r.t. 150 82 3 Ethanol 5 35 100 86 4 Ethanol 5 60 60 89 5 Ethanol 10 r.t. 110 96 6 Ethanol 10 60 60 97 7 Methanol 10 60 90 65 8 Trifluoroethanol 10 60 90 78 9 H2O 10 60 160 74 10 H2O 10 80 120 83 11 —e 10 60 60 56 12 Ethanol 10 70 60 96 13 Ethanol 15 60 60 95 14 DMSO 10 70 120 83 15 Toluene 10 60 120 77 16 Dichloromethane 10 35 120 81 17 Acetonitrile 10 60 120 84 a Reaction conditions: 5-chloropyridin-2-amine (1.0 mmol), benzaldehyde (1.0 mmol), t-butyl isocyanide (1.0 mmol); b Isolated yield; c No catalyst used; dN.R. means no reaction; eSolvent-free condition. Table 2. Synthesis of imidazo-fused heterocycles via GBB reactiona

Entry Aminoazine 1 Aldehyde 2 R3 Compd. Time/h Yieldb/% 1 R1=5-Cl R2=Ph t-Butyl 4a 1 97 2 R1=5-Cl R2=4-ClC6H4 t-Butyl 4b 1 93 3 R1=5-Cl R2=4-CNC6H4 t-Butyl 4c 1 94 4 R1=5-Cl R2=4-MeOC6H4 t-Butyl 4d 1 93 5 R1=5-Cl R2=4-Et2NC6H4 t-Butyl 4e 2.5 94 6 R1=5-Cl R2=4-C8H7O t-Butyl 4f 3 91 7 R1=5-Cl R2=Ph n-Butyl 4g 3 88 8 R1=5-Cl R2=4-ClC6H4 Cyclohexyl 4h 3 91 9 2-NH2-pyrazine R2=Ph Cyclohexyl 4i 3.5 93 10 2-NH2-thiazole R2=Ph t-Butyl 4j 3 84 11 2-NH2-pyrazine R2=Ph t-Butyl 4k 3 90 12 2-NH2-pyrimidine R2=3, 5-Me2C6H3 t-Butyl 4l 3.5 85 13 R1=H R2=3-BrC5H3N Cyclohexyl 4m 6 85 14 3-NH2-1, 2, 4-triazole R2=3-BrC5H3N t-Butyl 4n 6 87 15 2-NH2-5-Cl-pyrazine R2=3-BrC5H3N t-Butyl 4o 6 80 a Reaction conditions: aldehyde (1.0 mmol), aminoazine (1.0 mmol), isocyanide (1.0 mmol), CeCl3•7H2O (10 mol %), ethanol, 60 ℃; bIsolated yield. Table 3. Comparison of efficiency of CeCl3•7H2O and LaCl3• 7H2O in the synthesis of products 4o and 4n
Entry Compd. Catalysta Yieldb/% 1 4o CeCl3•7H2O 80 2 4o LaCl3•7H2O 83 3 4n CeCl3•7H2O 87 4 4n LaCl3•7H2O 76 a10 mol%; bIsolated yield. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 8
- 文章访问数: 1248
- HTML全文浏览量: 114



 下载:
下载:

 下载:
下载:

