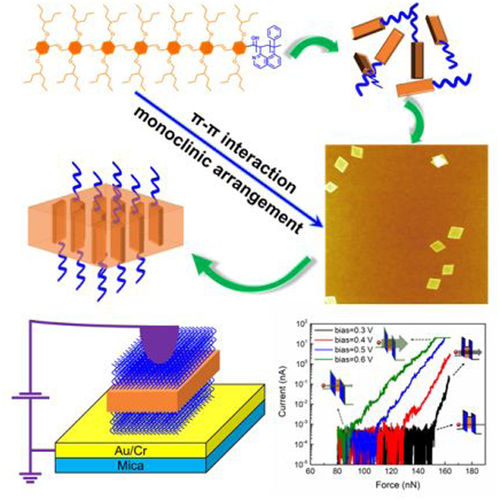
A new dinuclear cadmium(II) complex,[Cd(NPHSNPAB)(H2O)2(CH3CH2OH)]2 (1), was synthesized by the hydrothermal reaction of N-phenyl-2-[2-hydroxyl-3-sulfo-5-nitrophenylazo] butadiene-1,3 (NPHSNPAB) and Cd(NO3)2·4H2O, and characterized by elemental analysis, infrared, UV-visible, fluorescence, thermal behavior and single-crystal X-ray diffraction. For this complex:C36H42Cd2N8O22S2, Mr=1227.74, triclinic system, space group 1, a=7.555(9), b=12.006(14), c=13.943(16)Å, α=67.955(2), β=88.573(2), γ=77.550(2)°, V=1142.5(2)Å3, Z=2, Dc=1.784 g/cm3, λ=0.71073Å, F(000)=620, S=1.125, R=0.0460 and wR=0.1159 for 3949 observed reflections with I>2(I). X-ray diffraction analysis reveals that the central Cd(II) ion is bound by six oxygen atoms, forming a slightly distorted octahedral geometry. Density functional theory of complex 1 was studied. Noticeably, the application of 1 on metallic yarn got good effect.





 Login In
Login In