
Citation: Fu Yang, Sheng Li, Gao Lixin, Li Jia, Sun Liangpeng. Synthesis and PTP1B Inhibitory Activity of Novel Chalcone Derivatives Bearing 1H-Benzo[d]imidazol or 1H-Benzo[d][1,2,3]triazol Moieties[J]. Chinese Journal of Organic Chemistry, 2019, 39(4): 1029-1036. doi: 10.6023/cjoc201811030

含1H-苯并[d]咪唑或1H-苯并[d][1,2,3]三唑结构的新型查尔酮衍生物的合成及PTP1B抑制活性研究
-
关键词:
- PTP1B抑制剂
- / 1H-苯并[d]咪唑
- / 1H-苯并[d] [1, 2, 3]三唑
- / 查尔酮
English
Synthesis and PTP1B Inhibitory Activity of Novel Chalcone Derivatives Bearing 1H-Benzo[d]imidazol or 1H-Benzo[d][1,2,3]triazol Moieties
-
Key words:
- PTP1B inhibitor
- / 1H-benzo[d]imidazol
- / 1H-benzo[d] [1, 2, 3]triazol
- / chalcone
-
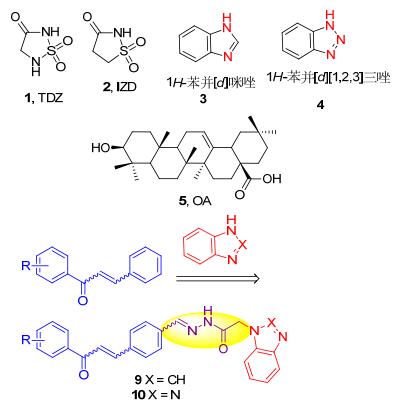
蛋白酪氨酸磷酸酯酶1B (PTP1B)在胰岛素信号转导通路中起着关键的负调控作用, 通过对胰岛素受体的去磷酸化作用使胰岛素受体失活, 降低胰岛素的敏感性, 产生胰岛素抵抗. PTP1B在体内过度表达, 还会造成瘦素受体不能对瘦素应答而引起肥胖症[1, 2].因此, PTP1B已经成为治疗Ⅱ型糖尿病和肥胖症等相关疾病的重要的药物靶点.近年来, 随着PTP1B酶三维结构的阐明, 发现了大量的PTP1B抑制剂[3].然而, 这些化合物多数具有较高的负电荷, 导致细胞通透性差, 药代动力学性质欠佳, 难于成药.研究者试图利用杂环结构来有效替代磷酸、羧酸等具有较高负电荷基团, 有可能解决因极性强而导致膜通透性差的难题.通过复合物X射线晶体结构分析发现, 1, 2, 5-噻二唑烷-3-酮-1, 1-二氧化物(1, TDZ)和异噻唑烷-3-酮-1, 1-二氧化物(2, IZD)都可以非常完美的嵌入PTP1B的活性位点, 并且与之结合[4~7].这些结果为寻找类药性好、非酸类PTP1B抑制剂的研究提供了重要思路.
基于PTP1B酶的催化结构域、第二活性位点以及其周边的结构域设计的多位点结合型PTP1B抑制剂不仅可以增强抑制剂与酶的亲和性, 而且还可以改善选择性[8~10].根据本实验室前期研究发现[11~14], 查尔酮类化合物由于具有较好的分子柔性及易与不同的受体结合的特点, 可以作为理想的分子模板; 若在其结构上引入能与酶活性部位相匹配的杂环, 则可以发现活性较好的新型PTP1B抑制剂.考虑到1H-苯并[d]咪唑(3)和1H-苯并[d][1,2,3]三唑(4)具有广泛生理活性, 如抗炎、抗菌、抗HIV-1、抗肿瘤、抗惊厥、肝保护及降糖作用[15, 16], 且结构中的含有2~3个氮原子, 氮原子作为电子供体易与氨基酸残基形成氢键作用, 可以增加配体与受体结合的机会.因此, 根据活性亚结构拼接和活性叠加原理, 将查尔酮和1H-苯并[d]咪唑或1H-苯并[d][1,2,3]三唑通过腙构筑于同一分子中, 设计了目标化合物(图 1).
图 1
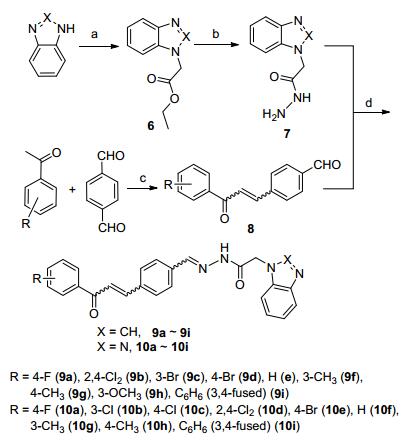
合成了18个未见报道的化合物, 其结构经1H NMR, 13C NMR, HRMS确认, CH=N和CH=CH结构单元的顺反异构未经确证.合成路线如Scheme 1所示.
图式 1
1. 结果与讨论
1.1 目标化合物的合成与表征
1H-苯并[d]咪唑和1H-苯并[d][1,2,3]三唑分别与氯乙酸乙酯反应合成了化合物6, 化合物6肼解得化合物7; 取代苯乙酮和对苯二甲醛为起始原料经过Claisen-Schmidt反应, 合成了4-(3-取代苯基-3-氧亚基-丙-1-烯基)苯甲醛(8); 再在冰醋酸的作用下, 化合物7与8缩合得到目标化合物9和10.通过1H NMR, 13C NMR和HRMS分析方法对所合成的化合物进行了表征. 1H NMR谱图中, HC=N上的质子信号分别在δ 5.6和6.1左右处呈现单峰, N—NH上的质子信号在δ 12.0左右处呈现单峰, 在δ 7.00~8.90处为芳环氢和查尔酮双键氢的质子信号; HRMS谱图中, 所有的化合物均出现了[M+H]+峰.
1.2 生物活性测试
目标化合物未分离得到单一构型的异构体, 以混合物的形式直接进行活性测试.在20 μg/mL的浓度下, 对PTP1B进行初筛, 再对抑制率高于50%的化合物进行复筛, 得到IC50.从表 1可知, 所有化合物对PTP1B均有抑制活性.在20 μg/mL的浓度下, 抑制率为58.76%~97.99%; IC50为(2.98±0.04)~(22.72±3.14) μmol•L-1, 其中化合物10i (2.98±0.04 μmol•L-1)活性最强, 近似于阳性对照物(2.69±0.15 μmol•L-1).分析化合物9和10的构效关系, 查尔酮A环上的取代基的类型与取代位置对活性影响的规律性不明显; 比较化合物9e, 9i, 10f和10i的IC50值发现, 含有萘环的9i和10i的活性优于含有苯环的9e和10f, 推测可能由于查尔酮末端芳环部分延伸至催化中心周边的疏水性区域, 萘环比苯环能与之形成更加稳定的疏水性作用所致[12].比较化合物9a与10a、9b与10d、9d与10e、9e与10f、9f与10g、9g与10h、9i与10i的IC50发现,化合物10比化合物9对PTP1B显示了更强的活性.该结论与分子对接结果相吻合, 通过比较化合物9a和10a与酶的分子对接结果可以发现, 化合物10a的三唑环上2位的氮原子与酶催化活性中心的氨基酸残基ALA217额外多形成了一根氢键.
表 1
Compd. X R PTP1B Inhibition ratea/% IC50b/(μmol•L-1) 9a CH 4-F 58.76±4.74 22.72±3.14 9b CH 2, 4-Cl2 83.88±2.22 8.51±0.88 9c CH 3-Br 81.34±7.38 19.46±3.17 9d CH 4-Br 76.86±4.16 8.31±0.04 9e CH H 69.28±2.77 10.19±3.14 9f CH 3-CH3 97.99±1.21 9.33±0.12 9g CH 4-CH3 93.46±2.50 6.30±0.24 9h CH 3-OCH3 77.80±3.23 17.23±0.07 9i CH C6H6 (3, 4-fused) 79.46±1.98 8.05±0.39 10a N 4-F 81.68±1.80 5.92±1.43 10b N 3-Cl 73.48±5.08 3.32±0.41 10c N 4-Cl 80.70±1.57 8.53±0.68 10d N 2, 4-Cl2 73.94±2.94 5.14±1.11 10e N 4-Br 92.61±3.97 4.58±0.76 10f N H 80.62±3.64 6.18±1.42 10g N 3-CH3 87.53±3.92 3.19±0.12 10h N 4-CH3 78.94±4.24 4.30±0.21 10i N C6H6 (3, 4-fused) 75.14±5.21 2.98±0.04 OAc 2.69±0.15 a Values tested at 20 μg/mL concentration. b The pNPP assay. IC50 values were determined by regression analyses and expressed as means±SD of three replications. c Positive control. 选取活性较好的化合物9g, 9i, 10h, 10i对其他相关PTP, 如TCPTP, 细胞周期分裂蛋白25B (cell division cycle 25 homolog B, CDC25B)和白细胞共同抗原相关蛋白(leukocyte antigen-related phosphatase, LAR), 及含SH2结构域蛋白酪氨酸磷酸酶1和2 (Src homology phosphatase, SHP-1和SHP-2)进行选择性评价, 结果如表 2所示, 除了10h以外, 9g, 9i, 10i在20 μg/mL的浓度下对CDC25B, LAR, SHP-1和SHP-2没有抑制活性, 显示了较好的选择性; 对于TCPTP, 9g, 9i, 10i显示了近2~4倍的选择性, 而化合物10h在20 μg/mL的浓度下对TCPTP没有活性, 显示了较好的选择性.
表 2
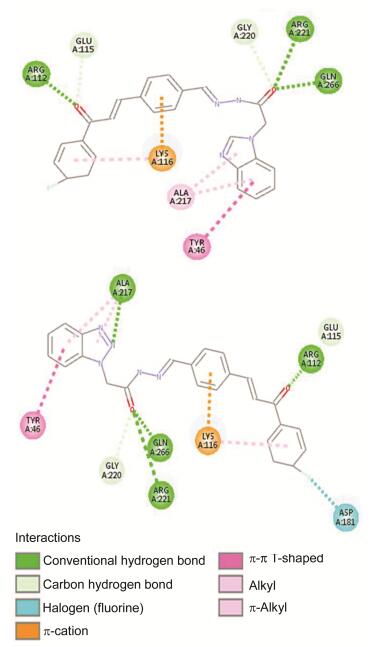
表 2 部分化合物对相关PTPs的抑制活性[IC50/(μmol•L-1)]aTable 2. Inhibitory activity [IC50/(μmol•L-1)] of selected compounds against related PTPsCompd. PTP1B TCPTP CDC25B LAR SHP-1 SHP-2 9g 6.30±0.24 13.27±0.52 NAb NA NA NA 9i 8.05±0.39 20.72±0.35 NA NA NA NA 10h 4.30±0.21 NA 42.93±6.99 NA NA NA 10i 2.98±0.04 10.43±0.04 NA NA NA NA a The pNPP assay. IC50 values were determined by regression analyses and expressed. b Not active at 20 μg/mL concentration. 为了阐述该类化合物与酶的作用机理, 利用Discovery Studio 2016 (DS)软件将复合物晶体结构(ID: 1NNY)及配体小分子(9a和10a)进行分子对接.如图 2所示, 化合物的苯并咪唑乙酰肼和苯并三唑乙酰肼部分均能占据PTP1B催化活性中心, 羰基与ARG221, GLN266形成氢键相互作用, 与GLY220形成C—H键相互作用; 苯并咪唑环和苯并三唑环分别与ALA217形成Alkyl相互作用, 苯环还与TYR46发生π-π相互作用.值得注意的是, 比较二者发现, 苯并三唑环上2位的N原子与ALA217额外多形成一条氢键相互作用.查尔酮部分延伸至催化活性中心周边区域, LYS116分别与查尔酮的A、B环形成π-Cation和π-Alkyl相互作用, 羰基分别与ARG112和GLU115形成氢键和C—H键相互作用.分子对接结果符合我们的设计预期, 目标化合物与PTP1B酶通过氢键及疏水性作用等相互结合.
图 2
2. 结论
在查尔酮的B环上通过腙结构引入了1H-苯并[d]咪唑和1H-苯并[d][1,2,3]三唑杂环, 设计合成了18个新型的查尔酮衍生物, 目标化合物经1H NMR, 13C NMR和HRMS确定其结构.活性结果表明, 该类化合物对PTP1B具有较好的抑制作用; 对于其他相关PTP显示了较好的选择性, 特别是化合物10h在20 μg/mL的浓度下对TCPTP没有活性.分子对接结果表明, 苯并咪唑乙酰肼和苯并三唑乙酰肼部分占据酶的催化活性中心, 查尔酮部分延伸至周边区域, 与酶形成了多位点结合.通过该研究, 发现了具有良好PTP1B抑制活性的含有1H-苯并[d]咪唑和1H-苯并[d][1,2,3]三唑杂环的查尔酮衍生物, 并初步确立此类化合物抑制PTP1B活性的构效关系, 为进一步结构优化奠定了基础.
3. 实验部分
3.1 仪器和试剂
熔点用毛细管法测定, 温度计未经校正; 1H NMR, 13C NMR用BRUKER AV-300型核磁共振仪测定(TMS为内标, DMSO-d6为溶剂); ESI-HRMS用Thermo scientific LTQ Orbitrap XL高分辨质谱仪测定.本实验所用试剂均为AR级或CP级, 阳性对照药齐墩果酸(oleanolic acid, 5)为HPLC≥98%的标准品.
3.2 化合物的合成
3.2.1 4-(3-取代苯基-3-氧亚基-丙-1-烯基)苯甲醛(8)的合成
3.2.2 2-(1H-苯并[d]咪唑-1-基)乙酸乙酯或2-(1H-苯并[d][1,2,3]三唑-1-基)乙酸乙酯(6)的合成
将11.8 mmol 1H-苯并[d]咪唑(或1H-苯并[d][1,2,3]三唑)、23.6 mmol氯乙酸乙酯、3.1 mL三乙胺和2 mL N, N-二甲基甲酰胺(DMF)分别加入到100 mL圆底烧瓶中. N2保护, 升温至80 ℃, 反应8 h, 薄层色谱(TLC)跟踪反应至完毕.减压蒸除DMF, 加少量水和CH2Cl2萃取3次.合并有机层, 无水硫酸镁干燥, 抽滤, 减压蒸除溶剂后得到粗品, 粗品经硅胶柱层析分离[V(二氯甲烷):V(甲醇)=100:1]得到化合物6.
3.2.3 2-(1H-苯并[d]咪唑-1-基)乙酰肼或2-(1H-苯并[d][1,2,3]三唑-1-基)乙酰肼(7)的合成
将2.16 mmol化合物6、2 mL水合肼和10 mL无水乙醇分别加入到100 mL圆底烧瓶中, N2保护, 升温至80 ℃, 反应12 h, TLC跟踪反应至结束, 减压蒸除溶剂后得粗品, 粗品经硅胶柱层析分离[V(二氯甲烷):V(甲醇)=100:1]得到化合物7.
3.2.4 化合物9a~9i和10a~10i的合成
分别取1 mmol的化合物7和8于100 mL圆底烧瓶, 加入适量的无水乙醇, 催化量的冰乙酸, N2保护, 加热至50 ℃, 反应4 h. TLC监测至反应完全, 趁热抽滤, 少量无水乙醇洗涤, 干燥, 重结晶得到目标化合物9a~9i和10a~10i.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(4-氟苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9a):收率93.9%, 浅黄绿色粉末. m.p. 270.5~272.1 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.92 (s, 1H, NH), 8.32~8.28 (m, 2H, ArH), 8.24 (s, 1H, NH, ImidazoH), 8.11 (s, 1H, CH=N), 8.01~7.23 (m, 12H, ArH, CH=CH), 5.62 (s, 2H); 13C NMR (125 MHz, DMSO-d6) δ: 188.13, 169.14, 166.60 (d, JCF=251.25 Hz), 145.61, 143.83, 143.81, 143.68, 136.57, 136.42, 135.22, 134.69, 134.37, 132.12 (d, JCF=10 Hz), 129.90, 127.90, 123.06, 122.71, 121.82, 119.72, 116.41 (d, JCF=21.25 Hz), 111.07, 45.93; ESI-HRMS calcd for C25H20FN4O2 [M+H]+ 427.1565, found 427.1569.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(2, 4-二氯苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9b):收率56.0%, 浅黄绿色粉末. m.p. 219.3~221.1 ℃; 1H NMR (DMSO- d6, 300 MHz) δ: 11.88 (s, 1H, NH), 8.21 (s, 1H, ImidazoH), 8.09 (s, 1H, CH=N), 7.87~7.21 (m, 13H, ArH, CH=CH), 5.59 (s, 2H, CH2); 13C NMR (75 MHz, DMSO- d6) δ: 192.81, 169.13, 146.39, 145.60, 143.68, 137.78, 136.87, 136.18, 135.94, 135.21, 131.74, 131.22, 130.19, 129.90, 128.05, 127.18, 122.72, 121.83, 119.72, 111.04, 49.08; ESI-HRMS calcd for C25H19Cl2N4O2 [M+H]+ 477.0880, found 477.0885.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(3-溴苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9c):收率43.4%, 浅黄色粉末. m.p. 268.4~271.1 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.90 (s, 1H, NH), 8.24 (s, 1H, ImidazoH), 8.11 (s, 1H, CH=N), 8.07~7.22 (m, 14H, ArH, CH=CH), 5.60 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 206.93, 169.13, 145.61, 144.46, 143.73, 139.80, 136.51, 135.31, 135.21, 134.35, 133.41, 131.38, 130.05, 128.69, 127.87, 127.66, 122.77, 121.81, 119.73, 111.06, 45.92; ESI-HRMS calcd for C25H20BrN4O2 [M+H]+ 487.0764, found 487.0754.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(4-溴苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9d):收率90.0%, 浅黄色粉末. m.p. 235.3~263.1 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.91 (s, 1H, NH), 8.23 (s, 1H, ImidazoH), 8.11 (s, 1H, CH=N), 8.14~7.22 (m, 14H, ArH, CH=CH), 5.61 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 188.75, 169.14, 145.61, 144.17, 143.79, 143.68, 136.51, 136.50, 132.37, 131.09, 129.95, 127.90, 122.94, 122.71, 111.07, 45.93; ESI-HRMS calcd for C25H20BrN4O2 [M+H]+ 487.0764, found 487.0759.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-苯基-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9e):收率64.2%, 浅黄绿色粉末. m.p. 266.5~268.0 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.90 (s, 1H, NH), 8.24 (s, 1H, ImidazoH), 8.11 (s, 1H, CH=N), 8.20~7.22 (m, 13H, ArH, CH=CH), 5.61 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 189.58, 169.18, 147.22, 145.63, 143.78, 137.94, 136.60, 136.37, 133.75, 129.88, 129.32, 129.07, 127.91, 123.21, 122.73, 121.84, 119.69, 111.10, 45.95; ESI-HRMS calcd for C25H21N4O2 [M+H]+ 409.1659, found 409.1649.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(3-甲基苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9f):收率69.0%, 浅黄色粉末. m.p. 247.5~249.5 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.91 (s, 1H, NH), 8.24 (s, 1H, ImidazoH), 8.11 (s, 1H, CH=N), 8.05~7.22 (m, 14H, ArH, CH=CH), 5.59 (s, 2H, CH2), 2.43 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6) δ: 189.58, 169.16, 145.63, 143.67, 138.73, 137.98, 136.63, 136.33, 135.23, 134.36, 129.86, 129.32, 127.96, 126.30, 123.28, 122.73, 121.84, 119.71, 111.11, 45.94, 21.39; ESI-HRMS calcd for C26H23N4O2 [M+H]+ 423.1816, found 423.1811.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(4-甲基苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9g):收率49.8%, 浅黄色粉末. m.p. 239.5~241.5 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.87 (s, 1H, NH), 8.23 (s, 1H, ImidazoH), 8.11 (s, 1H, CH=N), 8.23~7.22 (m, 14H, ArH, CH=CH), 5.61 (s, 2H, CH2), 2.41 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δ: 189.02, 169.13, 145.61, 144.19, 143.84, 143.68, 143.29, 129.87, 129.81, 129.21, 127.90, 123.32, 122.71, 121.81, 111.08, 45.93, 21.69; ESI-HRMS calcd for C26H23N4O2 [M+H]+ 423.1816, found 423.1807.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(3-甲氧基苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9h): 收率73.7%, 浅黄色粉末. m.p. 230.4~231.2 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.89 (s, 1H, NH), 8.23 (s, 1H, ImidazoH), 8.11 (s, 1H, CH=N), 8.02~7.22 (m, 14H, ArH, CH=CH), 5.61 (s, 2H, CH2), 3.86 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6) δ: 189.32, 169.14, 160.05, 145.61, 143.76, 143.66, 139.41, 136.48, 135.22, 134.81, 130.45, 129.91, 127.88, 123.28, 122.71, 121.70, 119.73, 113.52, 111.08, 55.86, 49.07; ESI-HRMS calcd for C26H23N4O2 [M+H]+ 439.1765, found 439.1770.
2-(1H-苯并[d]咪唑-1-基)-N'-(4-(3-(2'-萘基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(9i):收率89.6%, 浅黄绿色粉末. m.p. 257.9~259.0 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 11.89 (s, 1H, NH), 8.23 (s, 1H, ImidazoH), 8.11 (s, 1H, CH=N), 8.18~7.21 (m, 17H, ArH, CH=CH), 5.60 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 189.13, 169.13, 145.63, 143.70, 143.55, 136.71, 136.38, 135.56, 135.31, 132.80, 131.02, 130.10, 129.87, 128.97, 128.21, 127.92, 127.47, 124.61, 123.32, 122.73, 121.83, 119.75, 111.07, 45.96; ESI-HRMS calcd for C29H23N4O2 [M+H]+ 459.1816, found 459.1806.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(4-氟苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10a):收率56.2%, 浅黄色粉末. m.p. 210.4~211.5 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.00 (s, 1H, NH), 8.13 (s, 1H, CH=N), 8.32~7.39 (m, 14H, ArH, CH=CH), 6.11 (s, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 188.12, 168.06, 166.59 (d, JCF=250 Hz), 145.58, 144.23, 143.82, 136.63, 136.34, 134.68, 134.66, 134.56, 132.12 (d, JCF=10 Hz), 129.87, 127.99, 127.72, 124.28, 123.07, 119.47, 116.40 (d, JCF=21.25 Hz), 111.56, 49.33; ESI-HRMS calcd for C24H19FN5O2 [M+H]+ 428.1517, found 428.1505.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(3-氯苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10b):收率84.3%;浅黄色粉末. m.p. 245.1~246.4 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.02 (s, 1H, NH), 8.13 (s, 1H, CH=N), 8.32~7.42 (m, 14H, ArH, CH=CH), 6.11 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 188.31, 168.11, 145.56, 144.47, 144.18, 141.89, 139.75, 136.46, 134.34, 133.45, 131.29, 130.05, 128.70, 127.83, 124.30, 122.78, 119.47, 111.57, 49.07; ESI-HRMS calcd for C24H19ClN5O2 [M+H]+ 444.1222, found 444.1217.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(4-氯苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10c):收率81.4%, 浅黄色粉末. m.p. 272.7~275.3 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.00 (s, 1H, NH), 8.13 (s, 1H, CH=N), 8.22~7.42 (m, 14H, ArH, CH=CH), 6.11 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 188.50, 168.08, 145.57, 144.17, 143.40, 138.68, 136.51, 134.55, 130.98, 129.93, 129.41, 128.04, 127.72, 124.28, 122.95, 119.47, 111.56, 49.07; ESI-HRMS calcd for C24H19ClN5O2 [M+H]+ 444.1222, found 444.1226.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(2, 4-二氯苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10d):收率54.9%, 浅黄色粉末. m.p. 240.5~241.3 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.00 (s, 1H, NH), 8.11 (s, 1H, CH=N), 7.32~8.30 (m, 13H, ArH, CH=CH), 6.09 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 192.80, 168.07, 146.38, 145.60, 144.10, 137.78, 136.79, 136.09, 134.55, 131.75, 131.23, 130.19, 129.89, 128.09, 127.71, 127.20, 124.27, 119.46, 111.53, 49.33; ESI-HRMS calcd for C24H18Cl2N5O2 [M+H]+ 478.0832, found 478.0839.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(4-溴苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10e):收率81.5%, 浅黄色粉末. m.p. 282.2~283.5 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.02 (s, 1H, NH), 8.13 (s, 1H, CH=N), 8.11~7.40 (m, 14H, ArH, CH=CH), 6.12 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 188.69, 168.07, 145.57, 144.16, 136.93, 136.55, 136.40, 134.55, 132.34, 131.07, 129.92, 127.94, 127.72, 124.27, 122.89, 119.47, 111.56, 49.08; ESI-HRMS calcd for C24H19BrN5O2 [M+H]+ 488.0717, found 488.0722.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-苯基-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10f):收率92.9%, 浅黄绿色粉末. m.p. 235.5~237.0 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.01 (s, 1H, NH), 8.13 (s, 1H, CH=N), 8.20~7.43 (m, 15H, ArH, CH=CH), 6.12 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 189.56, 168.10, 145.56, 144.22, 143.69, 137.94, 136.65, 136.28, 134.56, 133.74, 129.86, 129.19, 127.87, 124.28, 123.22, 119.46, 111.57, 49.35; ESI-HRMS calcd for C24H20N5O2 [M+H]+ 410.1612, found 410.1608.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(3-甲基苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10g):收率96.8%, 浅黄色粉末. m.p. 223.3~224.3 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.01 (s, 1H, NH), 8.13 (s, 1H, CH=N), 8.09~7.42 (m, 14H, ArH, CH=CH), 6.10 (s, 2H, CH2), 2.43 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6) δ: 189.58, 168.08, 145.56, 144.23, 143.52, 138.73, 137.98, 136.69, 134.45, 129.84, 129.46, 129.18, 127.98, 127.73, 126.31, 124.29, 123.34, 119.46, 111.57, 49.06, 21.38; ESI-HRMS calcd for C25H22N5O2 [M+H]+424.1768, found 424.1758.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(4-甲基苯基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10h):收率74.3%, 浅黄色粉末. m.p. 251.3~252.6 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.00 (s, 1H, NH), 8.13 (s, 1H, CH=N), 8.11~7.38 (m, 14H, ArH, CH=CH), 6.11 (s, 2H, CH2), 2.41 (s, 3H, CH3); 13C NMR (75 MHz, DMSO-d6) δ: 188.96, 168.09, 145.56, 144.21, 143.32, 136.72, 136.19, 135.43, 134.56, 129.83, 129.21, 127.86, 124.29, 123.22, 119.46, 111.57, 49.33, 21.68; ESI-HRMS calcd for C25H22N5O2 [M+H]+ 424.1768, found 424.1756.
2-(1H-苯并[d][1,2,3]三唑-1-基)-N'-(4-(3-(2'-萘基)-3-氧亚基-丙-1-烯基)苯亚甲基)乙酰肼(10i):收率93.5%, 浅黄绿色粉末. m.p. 243.6~244.8 ℃; 1H NMR (DMSO-d6, 300 MHz) δ: 12.02 (s, 1H, NH), 8.15 (s, 1H, CH=N), 7.39~8.33 (m, 17H, ArH, CH=CH), 6.12 (s, 2H, CH2); 13C NMR (75 MHz, DMSO-d6) δ: 193.35, 168.09, 145.56, 144.23, 143.52, 136.76, 136.30, 135.56, 135.27, 134.55, 132.79, 131.06, 129.99, 128.12, 127.61, 124.57, 123.30, 119.46, 111.47, 49.06; ESI-HRMS calcd for C28H221N5O2 [M+H]+ 460.1768, found 460.1759.
3.3 PTP1B及其PTPs抑制活性的测试
药理筛选由中国国家新药筛选中心完成.具体测试方法参照文献[17], 以对硝基苯磷酸二钠(pNPP)为底物, 以已知的PTP1B抑制剂齐墩果酸为阳性对照药物[18, 19], PTP1B筛选体系为: 30 nmol•L-1 GST-PTP1B, 2 mmol• L-1 pNPP, 50 mmol•L-1 3-吗啉丙磺酸(Mops), pH=6.5, 2 mmol•L-1二硫苏糖醇(DTT), 1 mmol•L-1乙二胺四乙酸(EDTA).对初筛抑制率高于50%的化合物测试活性剂量依赖关系, 通过样品活性对样品浓度进行非线性拟和得到IC50, 计算所用软件为Graphpad Prism 5, 拟合所使用的模型为sigmoidal dose-response (varible slope), 拟合曲线底部和顶部分别设定为0和100.其他相关PTP酶抑制活性的测试原理及方法同PTP1B的测试.
3.4 分子对接
分子对接采用Accelrys公司的DS 2016软件, 参考文献[20, 21], 以PTP1B晶体结构(ID: 1NNY)作为对接受体, 1NNY直接通过DS从蛋白质结构数据库(PDB)下载[22]. pH值设定为7.00, 蛋白质晶体结构经Macro- molecules/Prepare Protein模块下的Clean Protein去除蛋白多余构象, 补充非完整的氨基酸残基和加氢处理.利用Sketching构建化合物9a和10a的3D空间构象, 经Small Molecules/Prepare or Filter Ligands模块下的Prepare Ligands处理后, 再经Small Molecules/ Minimize Ligands模块下的Full Minimization对分子结构进行优化.利用Receptor-Ligands Interactions模块下的Define and Edit Bing Site定义活性位点后, 执行Dock Ligands (LibDock)分子对接计算, Input Receptor: 1NNY, Input Site Sphere: 31.1939, 26.6815, 25.6829, 15.6, 其他参数采用缺省值, 不同构象的配体分子分别与受体形成比较合适的相互作用, 然后进行能量优化, 最后保留打分较高的分子对接构象.
辅助材料(Supporting Information) 化合物9a~9i和10a~10i的1H NMR, 13C NMR和HRMS图谱.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
(a) Hunter, T. Cell 2000, 100, 113.
(b) Zhang, Z. Y.; Lee, S. Y. Expert Opin. Invest. Drugs 2003, 12, 223.
(c) Tonks, N. K. Cell 2005, 121, 667.
(d) Zhang, Z. Y. Biochim. Biophys. Acta 2005, 1754, 100. -
[2]
(a) Bialy, L.; Waldmann, H. Angew. Chem., Int. Ed. 2005, 44, 3814.
(b) Yip, S. C.; Saha, S.; Chernoff, J. Trends Biochem. Sci. 2010, 35, 442. -
[3]
(a) Lee, S.; Wang, Q. Med. Res. Rev. 2007, 27, 553.
(b) Combs, A. P. J. Med. Chem. 2010, 53, 2333.
(c) Barr, A. J. Future Med. Chem. 2010, 2, 1563.
(d) Tamrakar, A. K.; Maurya, C. K.; Rai, A. K. Expert Opin. Ther. Pat. 2014, 24, 1. -
[4]
Combs, A. P.; Zhu, W.; Crawley, M. L.; Glass, B.; Polam, P.; Sparks, R. B.; Modi, D.; Takvorian, A.; McLaughlin, E.; Yue, E. W.; Wasserman, Z.; Bower, M.; Wei, M.; Rupar, M.; Ala, P. J.; Reid, B. M.; Ellis, D.; Gonneville, L.; Emm, T.; Taylor, N.; Yeleswaram, S.; Li, Y.; Wynn, R.; Burn, T. C.; Hollis, G.; Liu, P. C. C.; Metcalf, B. J. Med. Chem. 2006, 49, 3774. doi: 10.1021/jm0600904
-
[5]
Sparks, R. B.; Polam, P.; Zhu, W.; Crawley, M. L.; Takvorian, A.; McLaughlin, E.; Wei, M.; Ala, P. J.; Gonneville, L.; Taylor, N.; Li, Y.; Wynn, R.; Burn, T. C.; Liu, P. C. C.; Combs, A. P. Bioorg. Med. Chem. Lett. 2007, 17, 736. doi: 10.1016/j.bmcl.2006.10.079
-
[6]
Douty, B.; Wayland, B.; Ala, P. J.; Bower, M. J.; Pruitt, J.; Bostrom, L.; Wei, M.; Klabe, R.; Gonneville, L.; Wynn, R.; Burn, T. C.; Liu, P. C. C.; Combs, A. P.; Yue, E. W. Bioorg. Med. Chem. Lett. 2008, 18, 66. doi: 10.1016/j.bmcl.2007.11.012
-
[7]
Black, E.; Breed, J.; Breeze, A. L.; Embrey, K.; Garcia, R.; Gero, T. W.; Godfrey, L.; Kenny, P. W.; Morley, A. D.; Minshull, C. A. Bioorg. Med. Chem. Lett. 2005, 15, 2503. doi: 10.1016/j.bmcl.2005.03.068
-
[8]
Du, Y.; Ling, H.; Zhang, M.; Shen, J.; Li, Q. Bioorg. Med. Chem. 2015, 23, 4891. doi: 10.1016/j.bmc.2015.05.032
-
[9]
Liu, P.; Du, Y.; Song, L.; Shen, J.; Li, Q. Eur. J. Med. Chem. 2016, 118, 27. doi: 10.1016/j.ejmech.2016.04.014
-
[10]
Maccari, R.; Paoli, P.; Ottanà, R.; Jacomelli, M.; Ciurleo, R.; Manao, G.; Steindl, T.; Langer, T.; Vigorita, M. G.; Camici, G. Bioorg. Med. Chem. 2007, 15, 5137. doi: 10.1016/j.bmc.2007.05.027
-
[11]
孙良鹏, 姜哲, 高立信, 李英哲, 孙立云, 李佳, 朴虎日, 有机化学, 2012, 32, 2108. Sun, L. P.; Jiang, Z.; Gao, L. X.; Li, Y. Z.; Sun, L. Y.; Li, J.; Piao, H. R. Chin. J. Org. Chem. 2012, 32, 2108 (in Chinese).
-
[12]
孙良鹏, 姜哲, 高立信, 刘晓芳, 全迎春, 郑光浩, 李佳, 朴虎日, 有机化学, 2013, 33, 1496. Sun, L. P.; Jiang, Z.; Gao, L. X.; Liu, X. F.; Quan, Y. C.; Zheng, G. H.; Li, J.; Piao, H. R. Chin. J. Org. Chem. 2013, 33, 1496 (in Chinese).
-
[13]
Chen, Z. H.; Zheng, C. J.; Sun, L. P.; Piao, H. R. Eur. J. Med. Chem. 2010, 45, 5739. doi: 10.1016/j.ejmech.2010.09.031
-
[14]
Jin, X.; Zheng, C. J.; Song, M. X.; Wu, Y.; Sun, L. P.; Li, Y. J.; Yu, L. J.; Piao, H. R. Eur. J. Med. Chem. 2012, 56, 203. doi: 10.1016/j.ejmech.2012.08.026
-
[15]
(a) Yang, Y. H.; Cheng, M. S.; Wang, Q. H.; Nie, H.; Liao, N.; Wang, J.; Chen, H. Eur. J. Med. Chem. 2009, 44, 1808.
(b) Rajasekaran, A.; Murugesan, S.; AnandaRajagopal, K. Arch. Pharm. Res. 2006, 29, 535.
(c) Shingalapur, R. V.; Hosamani, K. M.; Keri, R. S.; Hugar, M. H. Eur. J. Med. Chem. 2010, 45, 1753.
(d) Achar, K. C.; Hosamani, K. M.; Seetharamareddy, H. R. Eur. J. Med. Chem. 2010, 45, 2048. -
[16]
(a) Kuş, C.; Ayhan-Kilcigil, G.; Ozbey, S.; Kaynak, F. B.; Kaya, M.; Coban, T.; Can-Eke, B. Bioorg. Med. Chem. 2008, 16, 4294.
(b) Mavrova; A. T.; Wesselinova, D.; Tsenov, Y. A.; Denkova, P. Eur. J. Med. Chem. 2009, 44, 63.
(c) Kumar, H.; Javed, S. A.; Khan, S. A.; Amir, M. Eur. J. Med. Chem. 2008, 43, 2688.
(d) Khan, I.; Ali, S.; Hameed, S.; Rama, N. H.; Hussain, M. T.; Wadood, A.; Uddin, R.; Ul-Haq, Z.; Khan, A.; Ali, S.; Choudhary, M. I. Eur. J. Med. Chem. 2010, 45, 5200. -
[17]
(a) Shi, L.; Yu, H. P.; Zhou, Y. Y.; Du, J. Q.; Shen, Q.; Li, J. Y.; Li, J. Acta Pharmacol. Sin. 2008, 29, 278.
(b) Zhang, W.; Hong, D.; Zhou, Y. Y.; Zhang, Y. N.; Shen, Q.; Li, J. Y.; Hu, L. H.; Li, J. Biochim. Biophys. Acta 2006, 1760, 1505. -
[18]
(a) Qiu, W. W.; Shen, Q.; Yang, F.; Wang, B.; Zou, H.; Li, J. Y.; Li, J.; Tang, J. Bioorg. Med. Chem. Lett. 2009, 19, 6618.
(b) Qian, S.; Li, H. J.; Chen, Y.; Zhang, W. Y.; Yang, S. Y.; Wu, Y.; J. Nat. Prod. 2010, 73, 1743. -
[19]
(a) Chung, Y. H.; Jang, S. C.; Kim, S. J.; Sung, N. D. J. Appl. Biol. Chem. 2007, 50, 52.
(b) Lin, Z. H.; Zhang, Y.; Zhang, Y. N.; Shen, H.; Hu, L. H.; Jiang, H. L.; Shen, X. Biochem. Pharmacol. 2008, 76, 1251.
(c) Zhang, Y. N.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J. Y.; Li J.; Hu, L. H. Bioorg. Med. Chem. 2008, 16, 8697. -
[20]
Szczepankiewicz, B. G.; Liu, G.; Hajduk, P. J.; Abad-Zapatero, C.; Pei, Z. H.; Xin, Z. L.; Lubben, T. H.; Trevillyan, J. M.; Stashko, M. A.; Ballaron, S. J.; Liang, H.; Huang, F.; Hutchins, C. W.; Stephen, W. F.; Jirousek, M. R. J. Am. Chem. Soc. 2003, 125, 4087. doi: 10.1021/ja0296733
-
[21]
Chen, J.; Gao, L. X.; Gong, J. X.; Jiang, C. S.; Yao, L. G.; Li, J. Y.; Li, J.; Xiao. W.; Guo, Y. W. Bioorg. Med. Chem. Lett. 2015, 25, 2211. doi: 10.1016/j.bmcl.2015.03.060
- [22]
-
[1]
-
表 1 化合物9和10对PTP1B的抑制活性a
Table 1. Inhibitory activities of compounds 9 and 10 against PTP1B
Compd. X R PTP1B Inhibition ratea/% IC50b/(μmol•L-1) 9a CH 4-F 58.76±4.74 22.72±3.14 9b CH 2, 4-Cl2 83.88±2.22 8.51±0.88 9c CH 3-Br 81.34±7.38 19.46±3.17 9d CH 4-Br 76.86±4.16 8.31±0.04 9e CH H 69.28±2.77 10.19±3.14 9f CH 3-CH3 97.99±1.21 9.33±0.12 9g CH 4-CH3 93.46±2.50 6.30±0.24 9h CH 3-OCH3 77.80±3.23 17.23±0.07 9i CH C6H6 (3, 4-fused) 79.46±1.98 8.05±0.39 10a N 4-F 81.68±1.80 5.92±1.43 10b N 3-Cl 73.48±5.08 3.32±0.41 10c N 4-Cl 80.70±1.57 8.53±0.68 10d N 2, 4-Cl2 73.94±2.94 5.14±1.11 10e N 4-Br 92.61±3.97 4.58±0.76 10f N H 80.62±3.64 6.18±1.42 10g N 3-CH3 87.53±3.92 3.19±0.12 10h N 4-CH3 78.94±4.24 4.30±0.21 10i N C6H6 (3, 4-fused) 75.14±5.21 2.98±0.04 OAc 2.69±0.15 a Values tested at 20 μg/mL concentration. b The pNPP assay. IC50 values were determined by regression analyses and expressed as means±SD of three replications. c Positive control. 表 2 部分化合物对相关PTPs的抑制活性[IC50/(μmol•L-1)]a
Table 2. Inhibitory activity [IC50/(μmol•L-1)] of selected compounds against related PTPs
Compd. PTP1B TCPTP CDC25B LAR SHP-1 SHP-2 9g 6.30±0.24 13.27±0.52 NAb NA NA NA 9i 8.05±0.39 20.72±0.35 NA NA NA NA 10h 4.30±0.21 NA 42.93±6.99 NA NA NA 10i 2.98±0.04 10.43±0.04 NA NA NA NA a The pNPP assay. IC50 values were determined by regression analyses and expressed. b Not active at 20 μg/mL concentration. -

计量
- PDF下载量: 10
- 文章访问数: 987
- HTML全文浏览量: 135

 下载:
下载:
 下载:
下载:
 下载:
下载:

