 图Scheme 1
Functionalization at the C5 position of quinoline amides.
Scheme1.
Functionalization at the C5 position of quinoline amides.
图Scheme 1
Functionalization at the C5 position of quinoline amides.
Scheme1.
Functionalization at the C5 position of quinoline amides.

无金属催化的8-氨基喹啉C5位上的卤化反应
English
Catalyst-Free Selective C5-H Bromination and Chlorination of 8-Amido Quinolines
-
Key words:
- 8-amidoquinoline
- / selective
- / catalyst-free
- / C5-H bond
- / halogenation
-
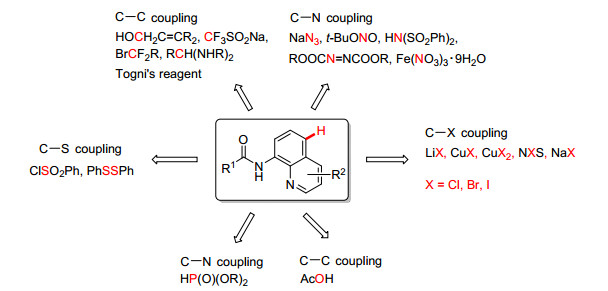
As a class of important heterocyclic compounds, quinolines have widespread applications in materials science, drug discovery and organic synthesis.[1] Inspired by the important biological significance of the quinoline skeleton, the synthetic methods in the construction and derivation of quinolines have attracted considerable attention from organic and medicinal researchers.[2~5] Many examples in the synthesis of diverse quinoline derivatives via quinoline elaboration are present available. Although great progress has been made for functionalization at the C2, C3, C4 and C8 positions of the quinolines, [6~8] it is note that the status of quinoline functionalization at the C5 is gradually rising. As show in the Scheme 1, some approaches for the direct construction of C—C[9] and C—heteroatom bonds (such as C—N[10], C—S[11], C—X[12], C—P[13] and C—O[14]) at the C5 position of quinolines have been developed. However, all these transformations required use of the metal catalysts and introduced large number of metal impurities into the target products. Thus, the development of the new synthetic methodology on these skeletons still remains a challenge.
 图Scheme 1
Functionalization at the C5 position of quinoline amides.
Scheme1.
Functionalization at the C5 position of quinoline amides.
图Scheme 1
Functionalization at the C5 position of quinoline amides.
Scheme1.
Functionalization at the C5 position of quinoline amides.
On the other hand, among the numerous quinoline derivatives, halogenated quinolines have been constantly found in many natural products and bioactive molecules. Many halogenated quinolines with versataile biological and pharmacological activities have been identified in natural products and marketed drugs.[15, 16] Moreover, they are also practically employed as key intermediates in the synthesis of substituted quinolines by generating increased molecular diverstiy. Hence, the synthesis of C5 halogenated quinolines has attracted broad attention. Recently, the transition-metal-catalyzed transformation of the C—H bond on the C5 position of quinolines into C—X (X=halogen) has been achieved with different catalytic conditions. In 2013, Stahl and co-workers for the first time reported the C5 chlorination of quinolines catalyzed by Copper(Ⅱ) salt.[12a] Late on, Xu and co-workers reported equivalent C5-bromination and iodination via Cu(OAc)2/ PhI(OAc)2 catalysis wherein NaBr and NaI were utilized as the halogen sources.[12d] Very recently, Zhu et al reported the copper(Ⅰ) salt and N-fluorobenzenesulfo-nimide mediating regioselective halogenation of 8-aminoquinoline on the C5 position, [12h] to name but a few. Although different approaches have been developed, most of these known methods rely on the employment of transition metal catalyst.[17] Herein, based on our recent research concern in aryl C—H halogenation reactions, [18] we report an efficient method for the direct synthesis of 5-chloro/bromo-8-ami-doquinolines via catalyst-free C5-H holagenation without using any additive under aerobic atmosphere.
1 Results and discussion
We began our studies by using amide 1a and NBS (2a) as substrates to probe reaction conditions. The relevant findings of the conditions screening are summarized in Table 1. Initially, the reaction was carried out in the presence of NiCl2 (20 mol%) in MeCN at 100 ℃ under O2. To our delight, brominated amide (3a) was successfully isolated in 65% yield (Table 1, Entry 1). In light of this result, we investigate different catalyst such as NiBr2, Ni(acac)2 and Ni(NO3)2•6H2O which gave only moderate yields for the highly site-selective C5 bromination of N-(8-quinolinyl)benzamide (Table 1, Entries 2~4). Interestingly, an almost similar yield was obtained without the addition of nickel salt (Table 1, Entry 5). Other solvent in the reaction were then screened and 1, 2-dichloromethane (DCM) was found to be the best choice which provides the corresponding product in 85% yield (Table 1, Entries 6~10). It was found that the product yields can not be improved by changing the volume of DCM from 0.5 mL to 4 mL (Entries 11~12). A lower yield of 45% was obtained when the reaction was performed at 80 ℃ (Table 1, Entry 14), while only 10% yield product could be generated when the tempature was lower than 60 ℃ (Table 1, Entries 15). However, when extending the reaction time at room temperature to 24 h, we can also get the target product with 82% yield (Table 1, Entries 16).
Entry Catalyst T/℃ Sovent Yieldb/% 1 NiBr2 100 MeCN 65 2 NiCl2 100 MeCN 50 3 Ni(acac)2 100 MeCN 51 4 Ni(NO3)2·6H2O 100 MeCN 55 5 — 100 MeCN 65 6 — 100 PET 25 7 — 100 Toluene 40 8 — 100 DCE 45 9 — 100 DCM 85 10 — 100 DMSO N.D. 11c — 100 DCM 67 12d — 100 DCM 63 13 — 110 DCM 84 14 — 80 DCM 45 15 — 60 DCM 10 16e — 40 DCM 82 aStandard reaction: 1 (0.25 mmol), NBS (1.1 equiv.), solvent (2 mL) in sealed tube. N.D.=not detected. bIsolated yield based on 1. cThe volume of DCM is 0.5 mL. dThe volume of DCM is 4 mL. eWhen the reaction time was change to 24 h at 40℃, the yield was 82%. With the optimized conditions in hand, the bromination of a series of 8-aminoquinoline amides (1) with NBS (2a) was investigated as shown in Table 2. The reaction could tolerate various functional groups. Both electron-withdrawing and electron-donating benzamides were turned into the corresponding products in good yields (3b~3j). Notably, it shows that the reactants with electron-donating substituents afforded the desired products in higher yields relative to those with electron-withdrawing groups (3e and 3j, Table 2). Moreover, heteroarene-fused product such as N-(5-bromoquinolin-8-yl)thiophene-2-carboxamide (3k) and N-(5-bromoquinolin-8-yl)furan-2-carboxamide (3l) were also generated in 88% and 87% yields, respectively. Alkyl amides were also occurred smoothly in high yields. For instance, N-(quinolin-8-yl)-cyclohexane carboxamide and non-cyclic alkyl amides such as N-(quinolin-8-yl)acetamide (1n) and N-(quinolin-8-yl) propionamide(1o) could act as good reaction partners under the optimized conditions, which produce the corresponding products (3m, 3n and 3o) in good yields. In the further study, we found that chlorination could also be achieved and provide the chlorination products in good yield. As for the bromination at the C5 position of quinolines, both electron-withdrawing and electron-donating benzamides can also turn into the corresponding chlorination products in good yields (3p~3r).
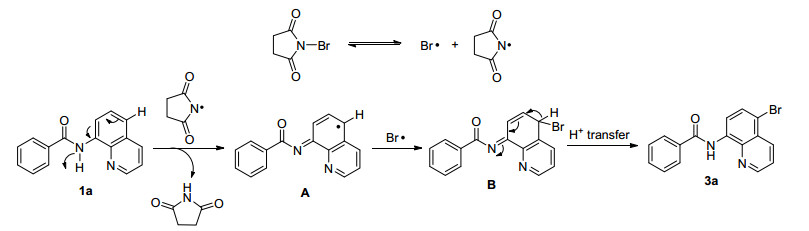
In order to clarificate the reaction mechanism, we examined the reaction between 1a with NBS (2a) in the presence of a radical scavenge 2, 2, 6, 6-tetramethyl-1-pi-peridinyloxy (TEMPO) (Eq. 1). The reaction was completely suppressed probably indicating that the reaction might proceed through a radical mechanism (Scheme 1).
Based on above results and previous reports, [19] a plausible mechanism for the C5 C—H halogenation of 8-amino-quinoline amides (1a) was presented in Scheme 2. First, a succinimide radical was generated from NBS under the high temperature, which then reacted with 8-aminoqui-noline amides (1a) to generate the intermediate A. Next, intermediate A was trapped by Br• in the system to form the intermediate B. Finally, intermediate B underwent a proton-transfer process to give the C5 halogenation product 3a.
2 Conclusions
In summary, a high efficient oxidative C—H halogenation (Cl, Br) of 8-aminoquinoline amides on C5 position has been developed. This method avoided the use of transition metal catalysts as well as transition-metal or organic oxidants. It shows air and moisture tolerance, good functional groups compatibility, and gave the highly selective C5-halogenated products in good to excellent yields. It provides a simple and efficient synthetic strategy for the construction of C5 functionalized quinolines and has good application foreground as it is easily-handled, economical and environment friendly.
3 Experimental section
The general procedure for the synthesis of products 3. In an oven-dried tube with a magnetic stir bar was charged with the quinoline 1 (0.25 mmol) and NXS (2) (X=Br or Cl, 0.275 mmol), and DCM (2.0 mL). The tube was sealed and stirred at 100 ℃ for 2 h. After completion, the mixture was cooled to room temperature and diluted with water (10 mL), and then extracted with EtOAc (10 mL×3). The combined organic phase was dried over Na2SO4. The mixture was concentrated in vacuum, and the residues were purified by silica gel column chromatography (hexane/ethyl acetate, V:V=25:1) to afford the desired product 3.
N-(5-Bromoquinolin-8-yl)benzamide (3a): White solid; m.p. 122~124 ℃ (Lit.[20]120~122 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.73 (s, 1H), 8.89~8.83 (m, 2H), 8.58 (dd, J=8.4, 1.2 Hz, 1H), 8.10~8.06 (m, 2H), 7.87 (d, J=8.4 Hz, 1H), 7.62~7.55 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 165.5, 148.7, 139.4, 136.2, 134.9, 134.5, 132.1, 131.1, 128.9, 127.3, 122.7, 117.3, 114.5.
N-(5-Bromoquinolin-8-yl)-4-(trifluoromethyl)benzamid-e (3b): White solid, m.p. 144~146 ℃(Lit.[21]141~143 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.71 (s, 1H), 8.85 (dd, J=4.2, 1.6 Hz, 1H), 8.78 (d, J=8.4 Hz, 1H), 8.53 (dd, J=8.4, 1.6 Hz, 1H), 8.15 (d, J=8.0 Hz, 2H), 7.82 (t, J=7.8 Hz, 3H), 7.58 (q, J=4.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 163.9, 148.9, 139.3, 138.0, 136.1, 134.0, 133.8, 130.9, 127.7, 127.3, 125.9 (q, JCF3=4 Hz), 125.0, 122.9, 117.2, 115.0.
N-(5-Bromoquinolin-8-yl)-4-chlorobenzamide (3c): White solid; m.p. 145~146 ℃ (Lit.[20]145~146 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.60 (s, 1H), 8.79 (dd, J=4.2, 1.6 Hz, 1H), 8.72 (d, J=8.4 Hz, 1H), 8.48 (dd, J=8.4, 1.6 Hz, 1H), 7.93 (d, J=8.4 Hz, 2H), 7.77 (d, J=8.4 Hz, 1H), 7.53 (q, J=4.4 Hz, 1H), 7.45 (d, J=8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 163.3, 147.8, 138.3, 137.3, 135.1, 133.2, 132.2, 130.0, 128.1, 127.7, 126.3, 121.8, 116.1, 113.7.
N-(5-Bromoquinolin-8-yl)-4-methylbenzamide (3d): White solid; m.p. 158~160 ℃ (Lit.[20]156~158 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.64 (s, 1H), 8.82 (dd, J=4.0, 1.2 Hz, 1H), 8.80 (d, J=8.4 Hz, 1H), 8.49 (dd, J=8.4, 1.6 Hz, 1H), 7.94 (d, J=8.0 Hz, 2H), 7.80 (d, J=8.4 Hz, 1H), 7.54 (q, J=4.4 Hz, 1H), 7.32 (d, J=8.0 Hz, 2H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.3, 148.7, 142.6, 139.4, 136.0, 134.6, 132.0, 131.0, 129.5, 127.3, 127.2, 122.7, 117.0, 114.2, 21.6.
N-(5-Bromoquinolin-8-yl)-4-(tert-butyl)benzamide (3e): White liquid; 1H NMR (400 MHz, CDCl3) δ: 10.64 (s, 1H), 8.80~8.78 (m, 2H), 8.47 (dd, J=8.4, 1.6 Hz, 1H), 7.99 (d, J=8.4 Hz, 2H), 7.78 (d, J=8.4 Hz, 1H), 7.55 (d, J=8.4 Hz, 2H), 7.52 (q, J=4.4 Hz, 1H), 1.37 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 165.3, 155.6, 148.7, 139.4, 135.9, 135.8, 134.6, 132.0, 130.9, 127.2, 125.8, 122.7, 116.9, 114.2, 35.1, 31.2. HRMS (ESI+) calcd for C20H20BrN2O [M+H]+ 383.0754, found 383.0752.
3-Bromo-N-(5-bromoquinolin-8-yl)benzamide (3f): White solid; m.p. 145~147 ℃; 1H NMR (400 MHz, CDCl3) δ: 10.57 (s, 1H), 8.83 (dd, J=4.2, 1.6 Hz, 1H), 8.73 (d, J=8.4 Hz, 1H), 8.48 (dd, J=8.4, 1.6 Hz, 1H), 8.16 (t, J=1.8 Hz, 1H), 7.93 (d, J=7.6 Hz, 1H), 7.78 (d, J=8.4 Hz, 1H), 7.68 (d, J=8.0 Hz, 1H), 7.55 (q, J=4.4 Hz, 1H), 7.39 (t, J=7.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 163.7, 148.9, 139.2, 136.7, 136.0, 135.0, 134.1, 130.9, 130.6, 130.3, 127.2, 125.6, 123.1, 122.8, 117.1, 114.8. HRMS (ESI+) calcd for C16H11Br2N2O [M+H]+ 404.9233, found 404.9232.
N-(5-Bromoquinolin-8-yl)-3-fluorobenzamide (3g): White solid; m.p. 142~144 ℃; 1H NMR (400 MHz, CDCl3) δ: 10.61 (s, 1H), 8.81 (dd, J=4.2, 1.6 Hz, 1H), 8.73 (d, J=8.0 Hz, 1H), 8.47 (dd, J=8.4, 1.6 Hz, 1H), 7.81~7.72 (m, 3H), 7.55~7.47 (m, 2H), 7.24~7.29 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 163.8, 162.9 (q, 1JCF=246 Hz), 161.7, 148.8, 139.2, 137.0 (q, 3JCF=6 Hz), 136.0, 134.1, 130.8, 130.5 (d, 3JCF=8 Hz), 127.2, 122.8, 122.7 (d, 3JCF=3 Hz), 119.0 (d, 2JCF=21 Hz), 114.7, 114.6 (d, 2JCF=23 Hz); HRMS (ESI+) calcd for C16H11BrFN2O [M+H]+ 345.0033, found 345.0037.
N-(5-Bromoquinolin-8-yl)-2-chlorobenzamide (3h): White solid; m.p. 171~173 ℃; 1H NMR (400 MHz, CDCl3) δ: 10.49 (s, 1H), 8.84 (d, J=8.4 Hz, 1H), 8.80 (dd, J=4.0, 1.2 Hz, 1H), 8.52 (dd, J=8.4, 1.2 Hz, 1H), 7.86~7.80 (m, 2H), 7.56 (q, J=4.4 Hz, 1H), 7.50 (dd, J=7.6, 1.8 Hz, 1H), 7.46~7.40 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 164.8, 148.9, 139.3, 136.0, 135.5, 134.4, 131.7, 131.2, 130.9, 130.6, 130.2, 127.3, 127.2, 122.8, 117.4, 114.9; HRMS (ESI+) calcd for C16H11BrClN2O [M+H]+ 360.9738, found 360.9736.
N-(5-Bromoquinolin-8-yl)-3-methylbenzamide1 (3i): White solid; m.p. 91~93 ℃ (Lit.[12c] 90~93 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.47 (s, 1H), 8.66 (m, 2H), 8.32 (d, J=8.4 Hz, 1H), 7.72~7.69 (m, 2H), 7.65 (d, J=8.4 Hz, 1H), 7.38 (q, J=4.4 Hz, 1H), 7.31~7.24 (m, 2H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.5, 148.7, 139.3, 138.7, 135.9, 134.7, 134.5, 132.8, 130.9, 128.7, 128.0, 127.1, 124.2, 122.7, 116.9, 114.3, 21.5.
N-(5-Bromoquinolin-8-yl)-3, 5-dimethylbenzamide (3j): White solid; m.p. 142~144 ℃; 1H NMR (400 MHz, CDCl3) δ: 10.64 (s, 1H), 8.89 (dd, J=4.4, 1.6 Hz, 1H), 8.84 (d, J=8.4 Hz, 1H), 8.56 (dd, J=8.8, 1.6 Hz, 1H), 7.85 (d, J=8.4 Hz, 1H), 7.65 (s, 2H), 7.60 (q, J=4.4 Hz, 1H), 7.22 (s, 1 H), 2.45 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 165.9, 148.8, 139.5, 138.6, 136.0, 135.0, 134.7, 133.7, 131.0, 127.3, 125.0, 122.7, 117.1, 114.3, 21.4. HRMS (ESI+) calcd for C18H16BrN2O [M+H]+355.0441, found 355.0439.
N-(5-Bromoquinolin-8-yl)thiophene-2-carboxamide (3k): White solid; m.p. 134~136 ℃ (Lit.[12c] 134~136 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.45 (s, 1H), 8.79 (dd, J=4.0, 1.2 Hz, 1H), 8.66 (d, J=8.4 Hz, 1H), 8.45 (dd, J=8.4, 1.6 Hz, 1H), 7.78~7.74 (m, 2H), 7.57 (d, J=4.8 Hz, 1H), 7.51 (q, J=4.4 Hz, 1H), 7.16 (t, J=4.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 159.9, 148.8, 139.7, 139.0, 135.9, 134.2, 131.2, 130.9, 128.6, 127.9, 127.1, 122.7, 116.9, 114.4.
N-(5-Bromoquinolin-8-yl)furan-2-carboxamide (3l): White solid; m.p. 194~196 ℃ (Lit.[12d]191~192 ℃); 1HNMR (400 MHz, CDCl3) δ: 10.73 (s, 1H), 8.89 (dd, J=4.0, 1.2 Hz, 1H), 8.76 (d, J=8.4 Hz, 1H), 8.53 (dd, J=8.4, 1.2 Hz, 1H), 7.81 (d, J=8.4 Hz, 1H), 7.63 (s, 1H), 7.58 (q, J=4.4 Hz, 1H), 7.31 (d, J=3.6 Hz, 1H), 6.59 (q, J=1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 156.3, 148.9, 148.2, 144.7, 139.3, 135.9, 134.1, 130.9, 127.2, 122.8, 117.1, 115.4, 114.6, 112. 6.
N-(5-Chloroquinolin-8-yl)cyclohexanecarboxamide (3m): White solid; m.p. 93~95 ℃ (Lit.[20]92~93 ℃); 1H NMR (400 MHz, CDCl3) δ: 9.87 (s, 1H), 8.83 (dd, J=4.4, 1.6 Hz, 1H), 8.70 (d, J=8.4 Hz, 1H), 8.52 (dd, J=8.4, 1.2 Hz, 1H), 7.79 (d, J=8.4 Hz, 1H), 7.56 (q, J=4.4 Hz, 1H), 2.51~2.44 (m, 1H), 2.11~2.05 (m, 2H), 1.90~1.86 (m, 2H), 1.58~1.76 (m, 3H), 1.44~1.25 (m, 3H); 13C NMR (100 MHz, CDCl3) δ: 174.9, 148.6, 139.2, 136.0, 134.6, 131.0, 127.2, 122.6, 117.0, 113.9, 46.9, 29.7, 25.8, 25.7.
N-(5-Bromoquinolin-8-yl)acetamide (3n): Light yellow solid, m.p. 140~141 ℃ (Lit.[20]139~141 ℃); 1H NMR (400 MHz CDCl3) δ: 9.74 (s, 1H), 8.79 (d, J=4.3 Hz, 1H), 8.64 (d, J=8.4 Hz, 1H), 8.50 (d, J=8.6 Hz, 1H), 7.76 (d, J=8.4 Hz, 1H), 7.54 (dd, J=8.6, 4.2 Hz, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 168.7, 148.5, 139.0, 136.0, 134.4, 130.9, 127.1, 122.6, 116.9, 114.1, 25.1.
N-(5-Bromoquinolin-8-yl)propionamide (3o): White solid, m.p. 103~105 ℃ (Lit.[20]103~105 ℃); 1H NMR (400 MHz, CDCl3) δ: 9.78 (s, 1H), 8.79 (dd, J=4.3, 1.6 Hz, 1H), 8.66 (d, J=8.4 Hz, 1H), 8.49 (dd, J=8.6, 1.6 Hz, 1H), 7.77 (d, J=8.4 Hz, 1H), 7.54 (dd, J=8.5, 4.2 Hz, 1H), 2.59 (q, J=7.6 Hz, 2H), 1.33 (t, J=7.5 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 172.5, 148.5, 139.0, 136.0, 134.5, 131.0, 127.2, 122.6, 116.9, 114.0, 31.2, 9.6.
N-(5-Chloroquinolin-8-yl)benzamide (3p): White solid; m.p. 134~136 ℃ (Lit.[20]128~130 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.55 (s, 1H), 8.77~8.75 (m, 2H), 8.44 (dd, J=8.4, 1.6 Hz, 1H), 7.96 (dd, J=8.0, 1.2 Hz, 2H), 7.52 (d, J=8.4 Hz, 1H), 7.49~7.42 (m, 4 H); 13C NMR (100 MHz, CDCl3) δ: 165.3, 148.7, 139.2, 134.9, 133.8, 133.4, 132.0, 130.6, 128.9, 127.3, 126.0, 124.47, 122.4, 116.5.
4-Chloro-N-(5-chloroquinolin-8-yl)benzamide (3q): White solid; m.p. 160~162 ℃ (Lit.[12c]160~162 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.62 (s, 1H), 8.85 (dd, J=3.2, 16 Hz, 2H), 8.57 (d, J=8.4 Hz, 1H), 7.98 (d, J=8.0 Hz, 2H), 7.57~7.64 (m, 2H), 7.51 (d, J=8.0 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 164.2, 148.8, 139.2, 138.3, 133.6, 133.5, 133.2, 129.1, 128.7, 127.3, 126.0, 124.8, 122.5, 116.6.
N-(5-Chloroquinolin-8-yl)-4-methylbenzamide (3r): White solid; m.p. 123~125 ℃ (Lit.[20]120~122 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.57 (s, 1H), 8.83~8.79 (m, 2H), 8.51 (dd, J=8.4, 1.6 Hz, 1H), 7.80~7.76 (m, 2H), 7.57 (d, J=8.4 Hz, 1H), 7.51 (q, J=4.4 Hz, 1H), 7.38~7.31 (m, 2H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.7, 148.7, 139.3, 138.8, 134.9, 133.9, 133.5, 132.8, 128.7, 128.1, 127.4, 126.0, 124.4, 124.2, 122.4, 116.6, 21.5.
Supporting InformationThe 1H NMR and 13C NMR spectra for compound 3. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn.
-
-
[1]
(a) Solomon, V. R. ; Lee, H. Curr. Med. Chem. 2011, 18, 1488.
(b) Liu, G. ; Yi, M. ; Liu, L. ; Wang, J. ; Wang, J. Chem. Commun. 2015, 51, 2911. -
[2]
Wan, J.; Y. Jing, Y.; Wei, L. Asian J. Org. Chem. 2017, 6, 668.
-
[3]
Gharpure, S. J.; Nanda, S. K.; Adate, P. A.; Shelke, Y. G. J. Org. Chem. 2017, 82, 2067. doi: 10.1021/acs.joc.6b02896
-
[4]
Ali, W.; Dahiya, A.; Pandey, R.; Alam, T.; Patel, B. K. J. Org. Chem. 2017, 82, 2089. doi: 10.1021/acs.joc.6b02912
-
[5]
Tobisu, M.; Hyodo, I.; Chatani, N. J. Am. Chem. Soc. 2009, 131, 12070. doi: 10.1021/ja9053509
-
[6]
Sun, K.; Lv, Y.; Wang, J.; Sun, J.; Liu, L.; Jia, M.; Liu, X.; Z. Li, Z.; Wang, X. Org. Lett. 2015, 17, 4408. doi: 10.1021/acs.orglett.5b01857
-
[7]
Berman, A. M.; Lewis, J. C.; Bergman, R. G.; Ellman, J. A. J. Am. Chem. Soc. 2008, 130, 14926. doi: 10.1021/ja8059396
-
[8]
Boudet, N.; Lachs, J. R.; Knochel, P. Org. Lett. 2007, 9, 5525. doi: 10.1021/ol702494k
-
[9]
(a) Cong, X. ; Zeng, X. Org. Lett. 2014, 16, 3716.
(b) Reddy, M. D. ; Fronczek, F. R. ; Watkins, E. B. Org. Lett. 2016, 18, 5620.
(c) Chen, H. ; Li, P. ; Wang, M. ; Wang, L. Org. Lett. 2016, 18, 4794.
(d) Kuninobu, Y. ; Nishia, M. ; Kanai, M. Org. Biomol. Chem. 2016, 14, 8092.
(e) Shen, C. ; Xu, J. ; Beibei Ying, B. ; Zhang, P. ChemCatChem 2016, 8, 3557.
(f) Zhang, J. ; Hao, X. ; Wang, Z. ; Ren, C. ; Niu, J. ; Song, M. Chin. J. Org. Chem. 2017, 37, 1237.
(g) Luo, F. ; Long, Y. ; Li, Z. ; Zhou, X. Acta Chim. Sinica 2016, 74, 805. -
[10]
(a) Ji, D. ; He, X. ; Xu, Y. ; Xu, Z. ; Bian, Y. ; Liu, W. ; Zhu, Q. ; Xu, Y. Org. Lett. 2016, 18, 4478.
(b) Whiteoak, C. J. ; Planas, O. ; Company, A. ; Ribasa, X. Adv. Synth. Catal. 2016, 358, 1679.
(c) He, Y. ; Zhao, N. ; Qiu, L. ; Zhang, X. ; Fan, X. Org. Lett. 2016, 18, 6054.
(d) Dou, Y. ; Xie, Z. ; Sun, Z. ; Fang, H. ; Shen, C. ; Zhang, P. ; Qing Zhu, Q. ChemCatChem 2016, 8, 3570.
(e) Sahoo, H. ; Kesava, M. ; Ramakrishna, R. ; Baidya, M. Chem. -Eur. J. 2016, 22, 1592. -
[11]
(a) Liang, H. ; Jiang, K. ; Ding, W. ; Yuan, Y. ; Shuai, Li. ; Chen, Y. ; Ye Wei, Y. Chem. Commun. 2015, 51, 16928.
(b) Qiao, H. ; Sun, S. ; Yang, F. ; Zhu, Y. ; Zhu, W. ; Dong, Y. ; Wu, Y. ; Kong, X. ; Jiang, L. ; Wu, Y. Org. Lett. 2015, 17, 6086.
(c) Wei, J. ; Jiang, J. ; Xiao, X. ; Lin, D. ; Deng, Y. ; Ke, Z. ; Jiang, H. ; Zeng, W. J. Org. Chem. 2016, 81, 946.
(d) Xu, J. ; Shen, C. ; Zhu, X. ; Zhang, P. ; Ajitha, M. J. ; Huang, K. ; An, Z. ; Liu, X. Chem. Asian J. 2016, 11, 882.
(e) Zhu, L. ; Qiu, R. ; Cao, X. ; Xiao, S. ; Xu, X. ; Au, C. ; Yin, S. Org. Lett. 2015, 17, 5528. -
[12]
(a) Suess, A. M. ; Mehmed Z. Ertem, M. Z. ; Cramer, C. J. Stahl, S. S. J. Am. Chem. Soc. 2013, 135, 9797.
(b) Guo, H. ; Chen, M. ; Jiang, P. ; Chen, J. ; Pan, L. ; Wang, M. ; Xie, C. ; Zhang, Y. Tetrahedron 2015, 71, 70.
(c) Liu, X. ; Wu, Z. ; Luo, X. ; He, Y. ; Zhou, X. ; Fan, Y. ; Huang, G. RSC Adv. 2016, 6, 71485.
(d) Xu, J. ; Zhu, X. ; Zhou, G. ; Ying, B. ; Ye, P. ; Su, L. ; Shen, C. ; Zhang, P. Org. Biomol. Chem. 2016, 14, 3016.
(e) Khan, B. ; Ruchir Kant, R. ; Koley, D. Adv. Synth. Catal. 2016, 358, 2352.
(f) Wu, C. ; Zhou, H. ; Wu, Q. ; He, M. ; Li, P. ; Su, Q. ; Mu, Y. Synlett. 2016, 27, A~H.
(g) Sahoo, H. ; Ramakrishna, I. ; Baidya, M. Chem. Select 2016, 1, 1949.
(h) He, X. ; Xu, Y. ; Kong, L. ; Wu, H. ; Ji, D. ; Wang, Z. ; Xu, Y. ; Zhu, Q. Org. Chem. Front. 2017, 4, 1046.
(i) Rao, N. S. ; Reddy, G. M. ; Sridhar, B. ; Sarma, M. S. Eur. J. Org. Chem. 2017, 438. -
[13]
Sun, M.; Sun, S.; Qiao, H.; Yang, F.; Zhu, Y.; Kang, J.; Wu, Y.; Wu, Y. Org. Chem. Front. 2016, 3, 1646. doi: 10.1039/C6QO00379F
-
[14]
Xia, C.; Wang, K.; Xu, J.; Shen, C.; Sun, D.; Li, H.; Wang, G.; Zhang, P. Org. Biomol. Chem., 2017, 15, 531. doi: 10.1039/C6OB02375D
-
[15]
(a) Abouelhassan, Y. ; Garrison, V. G. ; Burch, M. ; Wong, W. ; Norwood, V. M. ; Huigens, R. W. Bioorg. Med. Chem. Lett. 2014, 24, 5076.
(b) Liu, Y. -C. ; Wei, J. -H. ; Chen, Z. -F. ; Liu, M. ; Gu, Y. -Q. ; Huang, K. -B. ; Li, Z. -Q. ; Liang, H. Eur. J. Med. Chem. 2013, 69, 554.
(c) Tardito, S. ; Barilli, A. ; Bassanetti, I. ; Tegoni, M. ; Bussolati, O. ; Franchi-Gazzola, R. ; Mucchino, C. ; Marchio, L. J. Med. Chem. 2012, 55, 10448.
(d) Bhat, S. ; Shim, J. S. ; Zhang, F. ; Chong, C. R. ; Liu, J. O. Org. Biomol. Chem. 2012, 10, 2979.
(e) Madrid, P. B. ; Sherrill, J. ; Liou, A. P. ; Weisman, J. L. ; Derisi, J. L. ; Guy, R. K. Bioorg. Med. Chem. Lett. 2005, 15, 1015. -
[16]
(a) Jiang, H. ; Taggart, H. ; Zhang, X. ; Benbrook, D. M. ; Lind, S. E. ; Ding, W. -Q. Cancer Lett. 2011, 312, 11.
(b) Heidary, D. K. ; Howerton, B. S. ; Glazer, E. C. J. Med. Chem. 2014, 57, 8936. -
[17]
During the preparation of our manuscript, a water-mediated C5-H halogenations of 8-amidoquinolines has been reported. Chen, J. ; Wang, T. ; Liu, Y. ; Wang, T. ; Lin, A. ; Yao, H. ; Xu, J. Org. Chem. Front. 2017, 4, 622.
-
[18]
Hao, W.-Y.; Liu, Y.-Y. Beilstein J. Org. Chem. 2015, 11, 2132. doi: 10.3762/bjoc.11.230
-
[19]
Menini, L.; da Cruz Santos, J. C.; Gusevskaya, E. V. Adv. Synth. Catal. 2008, 350, 2052. doi: 10.1002/adsc.v350:13
-
[20]
Wang, Y.; Wang, Y.; Jiang, K.; Zhang, Q.; Li, D. Org. Biomol. Chem. 2016, 14, 10180. doi: 10.1039/C6OB02079H
-
[21]
Qiao, H. J.; Sun, S. Y.; Yang, F.; Zhu, Y.; Kang, J. X.; Wu, Y. S.; Wu, Y. J. Adv. Synth. Catal. 2017, 359, 1976. doi: 10.1002/adsc.v359.11
-
[1]
-
Table 1. Optimization of the reaction conditionsa
Entry Catalyst T/℃ Sovent Yieldb/% 1 NiBr2 100 MeCN 65 2 NiCl2 100 MeCN 50 3 Ni(acac)2 100 MeCN 51 4 Ni(NO3)2·6H2O 100 MeCN 55 5 — 100 MeCN 65 6 — 100 PET 25 7 — 100 Toluene 40 8 — 100 DCE 45 9 — 100 DCM 85 10 — 100 DMSO N.D. 11c — 100 DCM 67 12d — 100 DCM 63 13 — 110 DCM 84 14 — 80 DCM 45 15 — 60 DCM 10 16e — 40 DCM 82 aStandard reaction: 1 (0.25 mmol), NBS (1.1 equiv.), solvent (2 mL) in sealed tube. N.D.=not detected. bIsolated yield based on 1. cThe volume of DCM is 0.5 mL. dThe volume of DCM is 4 mL. eWhen the reaction time was change to 24 h at 40℃, the yield was 82%. Table 2. Substrate scope of bromination of 8-aminoquino-line amidesa, b

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 1450
- HTML全文浏览量: 135


 下载:
下载:

 下载:
下载:

