 图1
取代苯并噁嗪
Figure1.
Bioactive substituted benzoxazines
图1
取代苯并噁嗪
Figure1.
Bioactive substituted benzoxazines

Citation: Wang Yufeng, Yang Yajie, Huang Ling, Jie Kun, Guo Shengmei, Cai Hua. Iodine Catalyzed Kabachnik-Fields Reaction of Trialkyl Phosphites: Facile Access to Benzoxazine Containing Phosphorus[J]. Chinese Journal of Organic Chemistry, 2017, 37(12): 3220-3228. doi: 10.6023/cjoc201705023

碘催化亚磷酸三乙酯参与的Kabachnik-Fields反应:便捷合成含磷苯并噁嗪
-
关键词:
- 亚磷酸三烷基酯
- / 苯并噁嗪
- / Kabachnik-Fields反应
- / 碘
English
Iodine Catalyzed Kabachnik-Fields Reaction of Trialkyl Phosphites: Facile Access to Benzoxazine Containing Phosphorus
-
Key words:
- trialkyl phosphite
- / benzoxazineis
- / Kabachnik-Fields reaction
- / iodine
-
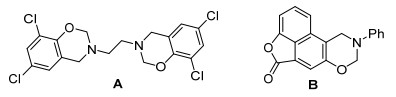
苯并噁嗪在天然产物中大量存在, 是有机材料和药物中重要的骨架.例如, 带有苯并噁嗪杂环的化合物A是抗微生物剂, 化合物B具有细胞毒性(图 1)[1].此外, 苯并噁嗪是新型酚醛树脂的前体, 具有良好耐热性和耐火性[2], 因此, 含有不同官能团的这类化合物被广泛地关注和合成[3].然而, 作为具有广谱阻燃性的磷酸酯引入到该骨架的报道较少[4], 且目前磷试剂局限于DOPO (9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide), 因此, 拓展含磷官能团的这类骨架的范围是有必要的.
Kabachnik-Fields反应始于1952年.胺、羰基化合物和亚磷酸二乙酯通过缩合加成形成α-氨基磷酸盐[5].近几年来, 许多催化剂被应用于这类反应中, 例如铁盐、Mg(ClO4)2、CeCl3、GO、NBS (CCl4)、稀土和沸石、碘.无催化剂条件下也能实现这类反应[6~15].然而, 使用亚磷酸三烷基酯作为亲核试剂来实现这类反应的报道不多. Chakraborti等通过使用磷酸盐作为亲核试剂, 在Mg(ClO4)2或锆盐催化下实现了Kabachnik-Fields反应[16]. Xu等[17]报道了一种基于DABCO的季铵盐, 并在温和条件下应用于Kabachnik-Fields反应. Lee等[18]利用Sc(OTf)3作为催化剂, 亚磷酸三烷基酯为磷源进行此反应. Singh等[19]报道了一种超声波促进α-氨基磷酸酯的反应. Bommena等[20]则使用一种溴盐为催化剂成功地实现了这类反应的进行.在这里, 我们报道了一种碘催化的Kabachnik-Fields反应, 使用亚磷酸三烷基酯为磷源, 并在温和的条件下实现了磷酸酯衍生物到苯并噁嗪的衍生物的转化.[21]
首先, 我们选择4-溴苯甲醛(1a)、亚磷酸三乙酯(2a)和对甲苯胺(3a)作为反应模板来优化反应条件.在N2氛围下, 四氢呋喃(THF)作为溶剂40 ℃下搅拌30 min, 反应并没有发生.当加入20 mol%的碘作为催化剂时, 得到所需产物, 收率有84%, 表明碘在该反应中是一个很好的催化剂(表 1, Entry 1).受此启发, 我们以碘作为催化剂进一步探索反应的条件.当催化剂的量增加到30 mol%时, 反应的收率并没有提高(表 1, Entry 3).接下来我们筛选了一系列的溶剂, 发现除了二氯甲烷(DCM)只有65%的收率之外, 其他的溶剂都具有良好的收率, 其中甲基环戊基醚(CPME)作为溶剂时, 反应收率达到了94%(表 1, Entries 4~12).当亚磷酸三乙酯的量减少时, 产率也会相应的降低.当将反应时间缩短到15 min时, 收率也可以达到95%.经过一系列研究之后, 我们确定了反应的最优条件如下:在40 ℃条件下, 1a (0.5 mmol), 2a(1.5 mmol), 3a (0.6 mmol), I2 (20 mol%), CPME (2 mL)反应15 min.

Entry Cat. n(1a):n(2a):n(3a) Solvent Yieldb/% 1 — 1:3:1.2 THF — 2 I2 1:3:1.2 THF 84 3c I2 1:3:1.2 THF 86 4 I2 1:3:1.2 Toluene 83 5 I2 1:3:1.2 DCE 83 6 I2 1:3:1.2 DMSO 84 7 I2 1:3:1.2 CH3CN 87 8 I2 1:3:1.2 DMF 88 9 I2 1:3:1.2 DCM 65 10 I2 1:3:1.2 Dioxane 83 11 I2 1:3:1.2 EtOAc 85 12 I2 1:3:1.2 CPME 97 13 I2 1:2:1.2 CPME 78 14 I2 1:1.2:1.2 CPME 77 15d I2 1:3:1.2 CPME Trace 16e I2 1:3:1.2 CPME 87 17f I2 1:3:1.2 CPME 95 a Reaction conditions: aldehyde (0.5 mmol), amine (0.6 mmol), cat. (20 mol%), solvents (2 mL), phosphite (1.5 mmol), 40 ℃ for 12 h under nitrogen; b isolated yield; c I2 (30 mol%); d 20 ℃; e80 ℃; f15 min. 在最优条件下, 我们对胺、醛以及亚磷酸酯的适应范围进行了拓展. 4-甲基苯胺以93%的收率得到产物4b, 苯胺的产物4c收率有90%.芳香环上具有官能团的胺(如4-溴苯胺)在此催化体系下也能很好地反应, 得到产物4d, 这类产物可以继续进一步转化.但是, 缺电子的胺如4-硝基苯胺得到痕量的产物4e.具有空间位阻的2-甲氧基苯胺和2-氯苯胺都以良好的产率得到目标产物4f, 4g, 甚至2, 6二甲基苯胺也可得到65%收率的目标产物4h.与芳香胺相比, 脂肪胺没有得到相应的产物4i.随后, 我们对醛进行了拓展.缺电子和富电子基团的醛, 均可得到目标产物4j~4m, 产率中等.但是4-羟基苯甲醛, 以90%的产率提供所需产物4n.我们预测水杨醛和2-氨基苯酚的加成产物可以有效制备六元杂环化合物或五元杂环化合物, 因此, 将水杨醛及其衍生物作为底物应用于反应中. 2-羟基-1-萘甲醛得到相应的产物4o, 产率为78%; 4-羟基-5-硝基苯甲醛得到目标产物4g, 产率为76%; 5-溴-2-羟基苯甲醛生成4r, 收率82%.此外, 产率62%[22]; 2-羟基-3-甲氧基苯甲醛得到目标产物4p, 我们也研究了含有不同官能团的2-氨基苯酚, 发现底物4s, 4t, 4u, 4v能以较高产率地得到目标产物, 然而, 1, 2-二氨基苯没有得到目标产物4w.此外, 在该反应中考查了亚磷酸酯的范围.我们发现亚磷酸酯如3x, 3y, 3z最后也得到相应的产物4x, 4y, 4z.遗憾的是苯乙酮在该催化体系下不发生反应.
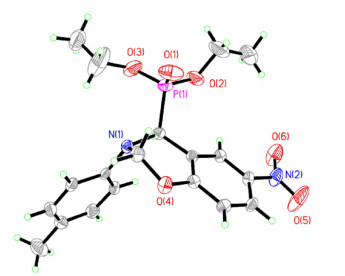
我们对这些α-氨基磷酸酯进行转化. α-氨基磷酸酯和甲醛能有效的制备苯并噁嗪(表 3).转化如下:产物4用甲醛(38%水溶液)在1, 4-二氧六环为溶剂, 100 ℃下反应12 h.如表 4所示, 底物4q以96%的收率转化为5q, 产物的结构用X单晶衍射进行了确定(图 2).[22] 4p得到所需产物5p, 产率为90%. 2-萘酚反应Kabachnik-Fields的产物4o也能成功转化为5o, 产率为78%, 4r得到5r, 收率88%.此外, 4x, 4y, 4z以良好的产率得到5x, 5y, 5z.其他醛如乙醛和肉桂醛在此反应条件下不发生反应.我们还尝试通过依次添加胺, 醛, 亚磷酸酯和甲醛一锅法合成苯并噁嗪, 但是并没有成功.可能是由于溶剂对反应产生了影响.
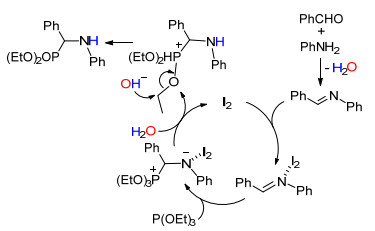
反应的可能机理如Scheme 1所示[23].醛与胺缩合形成亚胺和一分子的水; 碘单质作为路易斯酸作用于亚胺的氮原子, 使得亚胺的碳更易受到亲核试剂的进攻; 三烷基膦进攻亚胺形成了膦正离子, 该中间体在水的作用下脱去催化剂碘, 同时催化剂进入下一个循环.而水分解的羟基则经历了类似于阿布佐夫反应的过程, 即进攻磷上的烷基形成了目标产物和乙醇.
2 结论
综上所述, 我们发现了一种在温和条件下碘催化的亚磷酸三烷基酯作为磷源的Kabachnik-Fields反应, 反应速度快, 底物的适用性较广.该反应为合成α-氨基磷酸酯提供了高效简洁的方法.此外, 该反应中水杨醛的相应产物可以很好地转化为含有磷酸酯的苯并噁嗪, 收率良好.
3 实验部分
3.1 仪器与试剂
实验所用溶剂使用前均按照处理溶剂的标准方法进行, 胺、醛、溶剂、磷酸酯和酮酯均为市售.板层析使用GF254硅胶, 柱层析使用200~300目硅胶, 吸附样品使用60~100目粗硅胶, 展开剂为石油醚(60~90 ℃)和乙酸乙酯(V:V=100:15). 1H NMR及13C NMR用Bruker DRX-600及Agilent 400核磁共振仪, TMS作为内标, CDCl3作溶剂.
3.2 碘催化的Kabachnik-Fields反应步骤
将醛(0.5 mmol)、亚磷酸三烷基酯(1.5 mmol)、胺(0.6 mmol)、碘(20 mol%)、CPME (2 mL)分别加入到反应管中, 40 ℃下反应15 min, 薄层色谱(TLC)监测.待反应完毕后反应液减压下浓缩, 留下粗产品, 将其通过硅胶柱色谱纯化.
3.3 含膦酸酯苯并噁嗪的合成步骤
将4a (0.5 mmol)、甲醛溶液(5 mmol)、二氧六环(2 mL)分别加入到反应管中, 100 ℃下反应12 h, TLC监测.待反应完毕后反应液水洗三次, 二氯甲烷萃取, Na2SO4干燥, 合并有机相, 减压下浓缩, 留下粗产品, 将其通过硅胶柱色谱纯化.
[(4-溴苯基)(对甲苯基氨基)甲基]膦酸二乙酯(4a)[24]:黄色固体, 产率94%. m.p. 106~110 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.45 (d, J=8.3 Hz, 2H), 7.34 (dd, J=8.5, 2.2 Hz, 2H), 6.91 (d, J=8.3 Hz, 2H), 6.47 (d, J=8.4 Hz, 2H), 4.68 (d, J=24.4 Hz, 1H), 4.18~4.05 (m, 2H), 4.03~3.94 (m, 1H), 3.84~3.74 (m, 1H), 2.19 (s, 3H), 1.28 (t, J=7.1 Hz, 3H), 1.16 (t, J=7.1 Hz, 3H).
二乙基((4-溴苯基)((4-甲氧基苯基)氨基)甲基)膦酸酯(4b)[24]:无色液体, 产率93%. 1H NMR (400 MHz, CDCl3) δ: 7.22 (d, J=8.5 Hz, 2H), 6.91 (d, J=7.1 Hz, 2H), 6.67 (d, J=7.1 Hz, 2H), 6.51 (d, J=6.7 Hz, 2H), 4.67 (d, J=25.2 Hz, 1H), 4.54 (s, 1H), 4.11 (dd, J=18.9, 8.8 Hz, 2H), 4.02~3.92 (m, 1H), 3.77 (dd, J=12.1, 4.5 Hz, 1H), 2.18 (s, 3H), 1.66 (s, 3H), 1.28 (t, J=6.2 Hz, 3H), 1.14 (s, 3H).
二乙基[(4-溴苯基)(苯基氨基)甲基]膦酸酯(4c)[25]:无色液体, 产率87%. 1H NMR (400 MHz, CDCl3) δ: 7.45 (d, J=7.9 Hz, 2H), 7.35 (d, J=8.0 Hz, 2H), 7.09 (t, J=7.5 Hz, 2H), 6.70 (t, J=6.8 Hz, 1H), 6.56 (d, J=7.6 Hz, 2H), 4.72 (d, J=24.4 Hz, 1H), 4.10 (dt, J=16.4, 8.3 Hz, 2H), 3.97 (dt, J=16.5, 8.3 Hz, 1H), 3.78 (t, J=11.5 Hz, 1H), 1.28 (t, J=6.9 Hz, 3H), 1.15 (d, J=13.8 Hz, 3H).
二乙基((4-溴苯基)((4-溴苯基)氨基)甲基)膦酸酯(4d)[26]:无色液体, 产率90%.1H NMR (400 MHz, CDCl3) δ: 7.46 (d, J=6.5 Hz, 2H), 7.33 (s, 2H), 7.18 (d, J=6.6 Hz, 2H), 6.43 (d, J=6.7 Hz, 2H), 4.89 (d, J=5.4 Hz, 1H), 4.71~4.58 (m, 1H), 4.20~4.06 (m, 2H), 3.97 (dd, J=14.1, 6.9 Hz, 1H), 3.85~3.70 (m, 1H), 1.34~1.24 (m, 3H), 1.20~1.09 (m, 3H).
二乙基((4-溴苯基)((2-甲氧基苯基)氨基)甲基)膦酸酯(4f):无色固体, 产率89%. m.p. 104~108 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.44 (d, J=5.8 Hz, 2H), 7.35 (s, 2H), 6.80~6.63 (m, 3H), 6.38~6.29 (m, 1H), 5.30 (s, 1H), 4.72 (d, J=24.2 Hz, 1H), 4.19~3.90 (m, 4H), 3.87 (s, 3H), 1.33~1.11 (m, 6H); 13C NMR (101 MHz, CDCl3) δ: 147.16 (s), 135.82 (d, J=14.2 Hz), 135.23 (d, J=2.9 Hz), 131.42 (d, J=2.7 Hz), 129.32 (d, J=5.4 Hz), 121.53 (d, J=4.1 Hz), 120.79 (s), 117.76 (s), 111.01 (s), 109.56 (s), 63.25 (d, J=7.0 Hz), 62.96 (d, J=7.0 Hz), 56.11 (s), 55.34 (s), 54.61 (s), 16.13 (dd, J=15.8, 5.9 Hz); 31P NMR (162 MHz, CDCl3) δ: 21.89 (dd, J=15.9, 6.0 Hz); HRMS calcd for C18H24BrNO4P [M+H]+ 428.0626, found 428.0631.
二乙基((4-溴苯基)((2-氯苯基)氨基)甲基)膦酸酯(4g):无色液体, 产率81%. 1H NMR (400 MHz, CDCl3) δ: 7.46 (d, J=8.2 Hz, 2H), 7.34 (d, J=7.2 Hz, 2H), 7.25 (d, J=7.9 Hz, 1H), 6.97 (t, J=7.7 Hz, 1H), 6.63 (t, J=7.6 Hz, 1H), 6.39 (d, J=8.1 Hz, 1H), 5.42~5.31 (m, 1H), 4.73 (dd, J=24.4, 7.1 Hz, 1H), 4.17~3.82 (m, 4H), 1.24 (dt, J=28.4, 7.0 Hz, 6H; 13C NMR (101 MHz, CDCl3) δ: 142.05 (d, J=14.1 Hz), 134.56 (d, J=3.3 Hz), 131.71 (d, J=2.7 Hz), 129.39~129.06 (m), 127.58 (s), 121.94 (d, J=4.2 Hz), 120.04 (s), 118.69 (s), 112.48 (s), 63.44 (dd, J=26.1, 7.0 Hz), 56.29 (s), 54.80 (s), 16.26 (dd, J=14.8, 5.7 Hz); 31P NMR (162 MHz, CDCl3) δ: 21.01 (dd, J=16.2, 7.9 Hz); HRMS calcd for C18H24BrClNO3P [M+H]+ 447.0366, found 447.0366.
((4-溴苯基)((2, 6-二甲基苯基)氨基)甲基)膦酸二乙酯(4h):无色液体, 收率65%. 1H NMR (400 MHz, CDCl3) δ: 7.44 (d, J=8.3 Hz, 2H), 7.35~7.29 (m, 2H), 6.91 (d, J=7.4 Hz, 2H), 6.76 (t, J=7.4 Hz, 1H), 4.45 (d, J=22.5 Hz, 1H), 4.17~4.07 (m, 3H), 3.93 (dt, J=14.1, 7.1 Hz, 1H), 3.73~3.64 (m, 1H), 2.20 (s, 6H), 1.27 (t, J=7.0 Hz, 3H), 1.07 (t, J=7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 136.23 (s), 131.42 (d, J=1.5 Hz), 129.88 (d, J=6.6 Hz), 129.00 (s), 128.58 (s), 121.91 (s), 110.01 (s), 62.97 (d, J=7.2 Hz), 59.23 (s), 57.76 (s), 18.84 (s), 16.22 (dd, J=24.4, 5.9 Hz); 31P NMR (162 MHz, CDCl3) δ: 23.59 (s); HRMS calcd for C19H26BrNO3P [M+H]+ 426.0834, found 426.0841.
[(4-甲氧基苯基)(对甲苯基氨基)甲基]膦酸二乙酯(4j)[27]:无色液体, 收率65%. 1H NMR (400 MHz, CDCl3) δ: 7.46 (d, J=8.2 Hz, 2H), 7.34 (d, J=7.2 Hz, 2H), 7.25 (d, J=7.9 Hz, 1H), 6.97 (t, J=7.7 Hz, 1H), 6.63 (t, J=7.6 Hz, 1H), 6.39 (d, J=8.1 Hz, 1H), 5.42~5.31 (m, 1H), 4.73 (dd, J=24.4, 7.1 Hz, 1H), 4.17~3.82 (m, 4H), 1.24 (dt, J=28.4, 7.0 Hz, 6H)
二乙基(((4-(二甲基氨基)苯基)氨基)(对-甲苯基)甲基)膦酸酯(4k)[28]:白色固体, 收率64%. m.p. 86~90 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.29 (dd, J=8.6, 1.8 Hz, 2H), 6.90 (d, J=8.1 Hz, 2H), 6.67 (d, J=8.5 Hz, 2H), 6.52 (d, J=8.3 Hz, 2H), 4.64 (d, J=23.4 Hz, 1H), 4.17~4.03 (m, 2H), 3.93 (dd, J=15.7, 8.6 Hz, 1H), 3.74~3.63 (m, 1H), 2.91 (s, 6H), 2.17 (s, 3H), 1.28 (t, J=7.1 Hz, 3H), 1.14 (t, J=7.1 Hz, 3H).
二乙基[(4-硝基苯基)(对甲苯基氨基)甲基]膦酸酯(4l)[27]:黄色固体, 收率62%. m.p. 152~154 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.19 (d, J=8.6 Hz, 2H), 7.65 (dd, J=8.5, 1.7 Hz, 2H), 6.92 (d, J=8.1 Hz, 2H), 6.44 (d, J=8.2 Hz, 2H), 4.83 (dd, J=24.9, 6.6 Hz, 1H), 4.73~4.65 (m, 1H), 4.08 (ddd, J=24.5, 13.7, 6.5 Hz, 4H), 2.18 (s, 3H), 1.29 (t, J=7.1 Hz, 3H), 1.18 (t, J=7.0 Hz, 3H).
二乙基[(3-硝基苯基)(对甲苯基氨基)甲基]膦酸酯(4m)[27]:黄色液体, 收率63%.1H NMR (400 MHz, CDCl3) δ: 8.33 (s, 1H), 8.13 (d, J=8.1 Hz, 1H), 7.82 (d, J=7.4 Hz, 1H), 7.51 (t, J=7.9 Hz, 1H), 6.92 (d, J=8.0 Hz, 2H), 6.48 (d, J=7.8 Hz, 2H), 4.83 (d, J=24.7 Hz, 1H), 4.73 (s, 1H), 4.15 (dt, J=15.2, 6.7 Hz, 2H), 4.04 (dd, J=16.9, 7.5 Hz, 1H), 3.91 (dt, J=15.7, 7.9 Hz, 1H), 2.19 (s, 3H), 1.30 (t, J=6.8 Hz, 3H), 1.18 (t, J=6.8 Hz, 3H).
二乙基[(4-羟基苯基)(对甲苯基氨基)甲基]膦酸酯(4n)[29]:无色液体, 收率90%. 1H NMR (400 MHz, CDCl3) δ: 8.23 (s, 1H), 7.21 (d, J=6.1 Hz, 2H), 6.90 (d, J=6.4 Hz, 2H), 6.70 (d, J=7.7 Hz, 2H), 6.52 (d, J=7.8 Hz, 2H), 4.68 (d, J=23.7 Hz, 1H), 4.18~4.03 (m, 3H), 4.01~3.90 (m, 1H), 3.79~3.70 (m, 1H), 2.17 (s, 3H), 1.27 (s, 3H), 1.14 (t, J=6.6 Hz, 3H).
二乙基[(1-羟基苯基2-萘基)(对甲苯氨基)甲基]膦酸酯(4o):白色固体, 产率62%, m.p. 122~125 ℃; 1HNMR (400 MHz, CDCl3) δ: 10.39 (s, 1H), 8.00 (d, J=7.9 Hz, 1H), 7.83~7.64 (m, 2H), 7.53 (d, J=6.6 Hz, 1H), 7.35 (d, J=7.5 Hz, 1H), 7.13 (t, J=8.4 Hz, 1H), 6.89 (d, J=7.7 Hz, 2H), 6.65 (s, 2H), 5.61 (dd, J=20.8, 9.0 Hz, 1H), 4.06 (dd, J=62.0, 6.7 Hz, 4H), 3.80~3.66 (m, 1H), 2.17 (d, J=9.0 Hz, 3H), 1.27 (d, J=7.0 Hz, 3H), 1.02 (d, J=7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 156.02 (d, J=5.2 Hz), 143.93 (d, J=13.9 Hz), 130.67~129.94 (m), 129.79 (s), 129.18 (s), 128.90 (s), 126.82 (s), 123.07 (s), 121.76 (s), 120.48 (s), 115.81 (s), 63.80 (dd, J=16.1, 7.2 Hz), 53.72 (s), 52.18 (s), 20.38 (s), 16.37 (d, J=5.5 Hz), 16.06 (d, J=5.6 Hz); 31P NMR (162 MHz, CDCl3) δ: 26.05 (s); HRMS calcd for C22H27NO4P [M+H]+ 400.1678, found 400.1683.
[(2-羟基-3-甲基苯基)(对甲苯氨基)甲基]膦酸二乙酯(4p):黄色液体, 产率78%. 1HNMR (400 MHz, CDCl3) δ: 7.46 (d, J=8.2 Hz, 2H), 7.34 (d, J=7.2 Hz, 2H), 7.25 (d, J=7.9 Hz, 1H), 6.97 (t, J=7.7 Hz, 1H), 6.63 (t, J=7.6 Hz, 1H), 6.39 (d, J=8.1 Hz, 1H), 5.42~5.31 (m, 1H), 4.73 (dd, J=24.4, 7.1 Hz, 1H), 4.17~3.82 (m, 4H), 1.24 (dt, J=28.4, 7.0 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 129.68 (d, J=13.6 Hz), 127.75 (s), 122.22 (s), 120.52 (s), 120.04 (s), 117.46 (d, J=6.9 Hz), 114.05 (s), 110.33 (d, J=2.5 Hz), 55.99 (s), 51.16 (s), 49.63 (s), 29.65 (s), 20.32 (s), 16.39 (d, J=5.8 Hz), 16.10 (s); 31P NMR (162 MHz, CDCl3) δ: 24.15; HRMS calcd for C19H27NO5P [M+H]+ 380.1627, found 380.1625.
[(2-羟基-5-硝基苯基)(对甲苯基氨基)甲基]膦酸二乙酯(4q):白色固体, 产率76%. m.p. 170~172 ℃; 1H NMR (400 MHz, CDCl3) δ: 10.65 (s, 1H), 8.19 (s, 1H), 7.97 (d, J=8.8 Hz, 1H), 6.95 (d, J=8.0 Hz, 2H), 6.87 (d, J=8.9 Hz, 1H), 6.57 (d, J=8.0 Hz, 2H), 5.15 (dd, J=24.2, 5.5 Hz, 1H), 4.69~4.59 (m, 1H), 4.18 (tt, J=18.6, 9.3 Hz, 4H), 2.20 (s, 3H), 1.40~1.28 (m, 6H); 13C NMR (101 MHz, CDCl3) δ: 162.11 (d, J=4.4 Hz), 143.06 (d, J=13.9 Hz), 140.77 (s), 129.89 (s), 129.33 (s), 125.30 (s), 124.79 (d, J=5.6 Hz), 122.38 (d, J=2.2 Hz), 116.81 (s), 114.56 (s), 64.31 (d, J=7.4 Hz), 63.93 (d, J=7.2 Hz), 20.33 (s), 16.32 (dd, J=10.4, 5.6 Hz); 31P NMR (162 MHz, CDCl3) δ: 21.63; HRMS calcd for C18H24N2O6P [M+H]+ 395.1372, found 395.1376.
[(5-溴-2-羟基苯基)(对甲苯基氨基)甲基]膦酸二乙酯(4r):无色液体, 产率82%. 1H NMR (400 MHz, CDCl3) δ: 9.34 (s, 1H), 7.37 (t, J=2.1 Hz, 1H), 7.18 (dt, J=8.6, 2.0 Hz, 1H), 6.94 (d, J=8.2 Hz, 2H), 6.75 (d, J=8.6 Hz, 1H), 6.58 (d, J=8.4 Hz, 2H), 5.04 (dd, J=23.8, 6.6 Hz, 1H), 4.62 (t, J=7.7 Hz, 1H), 4.18~4.02 (m, 4H), 2.21 (s, 3H), 1.28 (dt, J=26.1, 7.1 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 155.00 (d, J=4.9 Hz), 143.55 (d, J=13.9 Hz), 131.86 (d, J=3.0 Hz), 131.07 (d, J=5.9 Hz), 129.70 (s), 128.69 (s), 123.81 (s), 119.06 (s), 114.44 (s), 111.93 (d, J=2.6 Hz), 63.99 (d, J=7.3 Hz), 63.56 (d, J=7.0 Hz), 53.66 (s), 52.15 (s), 20.29 (s), 16.22 (dd, J=14.1, 5.6 Hz); 31P NMR (162 MHz, CDCl3) δ: 23.08 (dd, J=15.4, 7.3 Hz); HRMS calcd for C18H24BrNO4P [M+H]+ 428.0626, found 428.0629.
((4-溴苯基)((2-羟基-5-甲基苯基)氨基)甲基)膦酸二乙酯(4s):白色固体, 产率96%. m.p. 148~150 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.30 (s, 4H), 6.60 (s, 1H), 6.45 (d, J=8.0 Hz, 1H), 6.34 (d, J=7.9 Hz, 1H), 5.48 (s, 1H), 4.81 (d, J=25.3 Hz, 1H), 4.25 (dd, J=14.2, 7.0 Hz, 2H), 4.20~3.94 (m, 2H), 2.12 (s, 3H), 1.31 (t, J=6.9 Hz, 3H), 1.16 (t, J=6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 145.13 (s), 135.02 (s), 131.98 (d, J=16.7 Hz), 131.54 (d, J=2.5 Hz), 129.65 (d, J=5.6 Hz), 128.23 (s), 121.74 (d, J=4.0 Hz), 120.05 (s), 115.32 (s), 112.12 (s), 64.39 (d, J=7.0 Hz), 63.52 (d, J=7.2 Hz), 56.55 (s), 55.01 (s), 20.49 (s), 16.40 (d, J=5.8 Hz), 16.13 (d, J=5.8 Hz); 31P NMR (162 MHz, CDCl3) δ: 23.93 (dd, J=17.1, 7.6 Hz); HRMS calcd for C18H24BrNO4P [M+H]+ 428.0626, found 428.0633.
二乙基((4-溴苯基)((2-羟基苯基)氨基)甲基)膦酸酯(4t):白色固体, 产率97%. m.p. 144~146 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.46 (d, J=8.2 Hz, 2H), 7.34 (d, J=7.2 Hz, 2H), 7.25 (d, J=7.9 Hz, 1H), 6.97 (t, J=7.7 Hz, 1H), 6.63 (t, J=7.6 Hz, 1H), 6.39 (d, J=8.1 Hz, 1H), 5.42~5.31 (m, 1H), 4.73 (dd, J=24.4, 7.1 Hz, 1H), 4.17~3.82 (m, 4H), 1.24 (dt, J=28.4, 7.0 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 145.03 (s), 134.88 (s), 131.59 (d, J=2.5 Hz), 129.65 (d, J=5.6 Hz), 119.99 (s), 118.53 (s), 114.37 (s), 111.95 (s), 64.34 (d, J=7.0 Hz), 63.68 (d, J=7.3 Hz), 56.22 (s), 54.68 (s), 16.30 (dd, J=24.6, 5.8 Hz); 31P NMR (162 MHz, CDCl3) δ: 23.33; HRMS calcd for C17H22BrNO4P [M+H]+ 414.0470, found 414.0468.
((4-溴苯基)((3-氯-2-羟基苯基)氨基)甲基)膦酸二乙酯(4u):白色固体, 产率86%. m.p. 140~142 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.41 (d, J=7.7 Hz, 2H), 7.33 (d, J=6.9 Hz, 2H), 6.67 (d, J=8.2 Hz, 1H), 6.60 (t, J=8.0 Hz, 1H), 6.26 (d, J=7.9 Hz, 1H), 5.37 (s, 1H), 4.73 (d, J=24.6 Hz, 1H), 4.22~3.96 (m, 4H), 3.88~3.77 (m, 1H), 1.30 (t, J=7.0 Hz, 3H), 1.19 (d, J=14.0 Hz, 3H); 13C NMR (101 MHz, DMSO) δ: 177.46 (s), 158.77 (s), 134.34 (s), 51.43 (s), 43.87 (s), 42.08 (s), 40.34 (s), 40.13 (s), 39.93 (s), 39.72 (s), 39.51 (s); 31P NMR (162 MHz, CDCl3) δ: 21.96; HRMS calcd for C17H21BrClNO4P [M+H]+: 448.0080, found 448.0086.
二乙基((4-溴苯基)((2-羟基-3-甲基苯基)氨基)甲基)膦酸酯(4v):黑色固体, 产率91%. m.p. 144~149 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.46 (d, J=8.2 Hz, 2H), 7.34 (d, J=7.2 Hz, 2H), 7.25 (d, J=7.9 Hz, 1H), 6.97 (t, J=7.7 Hz, 1H), 6.63 (t, J=7.6 Hz, 1H), 6.39 (d, J=8.1 Hz, 1H), 5.42~5.31 (m, 1H), 4.73 (dd, J=24.4, 7.1 Hz, 1H), 4.17~3.82 (m, 4H), 1.24 (dt, J=28.4, 7.0 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 143.37 (s), 135.21 (s), 134.79 (d, J=15.2 Hz), 132.20 (s), 131.61 (d, J=2.2 Hz), 129.62 (d, J=5.6 Hz), 128.99 (s), 121.30 (s), 120.13 (s), 111.31 (s), 63.98 (d, J=7.5 Hz), 63.47 (d, J=7.5 Hz), 56.89 (s), 55.36 (s), 29.68 (s), 16.03 (s); 31P NMR (162 MHz, CDCl3) δ: 22.70 (s); HRMS calcd for C18H24BrNO4P [M+H]+ 428.0626, found 428.0620.
二甲基((2-羟基-5-硝基苯基)(氨基)甲基)膦酸酯(4x):白色固体, 产率77%. m.p. 166~169 ℃; 1H NMR (400 MHz, CDCl3) δ: 10.61 (s, 1H), 8.21 (s, 1H), 7.97 (d, J=8.5 Hz, 1H), 6.91 (dd, J=18.9, 8.4 Hz, 3H), 6.57 (d, J=8.2 Hz, 2H), 5.28 (d, J=24.8 Hz, 1H), 3.86~3.78 (m, 6H), 2.19 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 161.90 (d, J=4.4 Hz), 142.80 (d, J=14.2 Hz), 140.75 (d, J=2.4 Hz), 129.87 (s), 129.18 (s), 125.43 (d, J=2.4 Hz), 124.67 (d, J=5.7 Hz), 122.19 (s), 116.46 (d, J=1.8 Hz), 114.33 (s), 54.48 (d, J=7.3 Hz), 54.12 (d, J=7.1 Hz), 52.19 (s), 50.66 (s), 20.33 (s); 31P NMR (162 MHz, CDCl3) δ: 24.70; HRMS calcd for C16H20N2O6P [M+H]+ 367.1059, found 367.1059.
[(2-羟基-5-硝基苯基)(对甲苯氨基)甲基]膦酸二丁酯(4y):白色固体, 产率90%. m.p. 160~162 ℃; 1H NMR (400 MHz, CDCl3) δ: 10.72 (s, 1H), 8.19 (s, 1H), 7.95 (d, J=8.8 Hz, 1H), 6.95 (d, J=7.6 Hz, 2H), 6.85 (d, J=8.9 Hz, 1H), 6.57 (d, J=7.9 Hz, 2H), 5.18 (d, J=24.3 Hz, 1H), 4.63 (s, 1H), 4.13 (dd, J=13.6, 6.7 Hz, 4H), 2.20 (s, 3H), 1.72~1.60 (m, 4H), 1.44~1.31 (m, 4H), 0.92 (dt, J=14.3, 7.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 160.05 (d, J=5.0 Hz), 146.33 (d, J=11.6 Hz), 132.94 (s), 129.92 (s), 124.89 (d, J=3.3 Hz), 124.31 (d, J=2.4 Hz), 119.23 (s), 118.08 (s), 116.62 (d, J=2.2 Hz), 77.98 (s), 68.14 (d, J=7.3 Hz), 66.33 (d, J=7.9 Hz), 59.09 (s), 57.44 (s), 32.63 (d, J=5.5 Hz), 32.33 (d, J=6.0 Hz), 20.45 (s), 18.61 (d, J=8.7 Hz), 13.45 (d, J=5.7 Hz); 31P NMR (162 MHz, CDCl3) δ: 21.66; HRMS calcd for C22H32N2O6P [M+H]+ 451.1998, found 451.2001.
[(2-羟基-5-硝基苯基)(对甲苯基氨基)甲基]膦酸二异丙酯(4z):白色固体, 产率80%. m.p. 157~160 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.26 (s, 1H), 8.03 (d, J=9.1 Hz, 1H), 7.06 (q, J=8.6 Hz, 4H), 6.91 (d, J=9.1 Hz, 1H), 5.95 (d, J=11.0 Hz, 1H), 5.41 (d, J=11.0 Hz, 1H), 4.92~4.75 (m, 3H), 2.26 (s, 3H), 1.43 (dd, J=9.9, 6.2 Hz, 6H), 1.32 (dd, J=15.0, 6.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 160.12 (d, J=5.0 Hz), 146.39 (d, J=12.1 Hz), 140.69 (d, J=2.6 Hz), 132.78 (s), 129.87 (s), 125.33 (d, J=3.3 Hz), 124.25 (d, J=2.3 Hz), 119.27 (s), 117.97 (s), 116.85 (d, J=2.3 Hz), 78.06 (s), 73.14 (d, J=7.4 Hz), 71.70 (d, J=8.0 Hz), 59.10 (s), 57.43 (s), 24.50 (d, J=3.1 Hz), 24.03 (d, J=3.3 Hz), 23.69 (dd, J=5.7, 3.2 Hz); 31P NMR (162 MHz, CDCl3) δ: 20.54; HRMS calcd for C20H28N2O6P [M+H]+ 423.1685, found 423.1681.
(6-硝基-3-对甲苯基-3, 4-二氢-2H-苯并[e][1,3]噁嗪-4-基)膦酸二乙酯(5q):白色固体, 产率96%. m.p. 119~121 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.23 (s, 1H), 8.05 (d, J=9.0 Hz, 1H), 7.08 (dd, J=16.7, 7.4 Hz, 4H), 6.94 (d, J=9.1 Hz, 1H), 5.91 (d, J=11.1 Hz, 1H), 5.42 (d, J=11.1 Hz, 1H), 4.84 (d, J=24.5 Hz, 1H), 4.37~4.18 (m, 4H), 2.28 (s, 3H), 1.46~1.35 (m, 6H); 13C NMR (101 MHz, DMSO-d6) δ: 165.29 (s), 134.35 (s), 129.69 (s), 122.65 (s), 61.73 (s), 60.71 (s), 40.35 (s), 40.14 (s), 39.93 (s), 39.82 (d, J=21.0 Hz), 39.47 (s), 38.47 (s), 26.40 (s); 31P NMR (162 MHz, CDCl3) δ: 19.56; HRMS calcd for C19H24N2O6P [M+H]+ 407.1372, found 407.1377
(6-硝基-3-对甲苯基-3, 4-二氢-2H-苯并[e][1,3]噁嗪-4-基)膦酸二乙酯(5p):白色固体, 产率90%. m.p. 96~99 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.46 (d, J=8.2 Hz, 2H), 7.34 (d, J=7.2 Hz, 2H), 7.25 (d, J=7.9 Hz, 1H), 6.97 (t, J=7.7 Hz, 1H), 6.63 (t, J=7.6 Hz, 1H), 6.39 (d, J=8.1 Hz, 1H), 5.42~5.31 (m, 1H), 4.73 (dd, J=24.4, 7.1 Hz, 1H), 4.17~3.82 (m, 4H), 1.24 (dt, J=28.4, 7.0 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 132.06 (s), 129.79 (s), 120.24 (d, J=2.7 Hz), 119.83 (d, J=2.6 Hz), 119.15 (s), 110.22 (d, J=2.7 Hz), 77.16 (s), 64.05 (s), 62.63 (d, J=7.9 Hz), 59.46 (s), 57.82 (s), 55.88 (s), 20.52 (s), 16.53 (dd, J=14.2, 5.6 Hz); 31P NMR (162 MHz, CDCl3) δ: 20.33; HRMS calcd For C20H27NO5P [M+H]+ 392.1627, found 392.1623.
(2-对甲苯基-2, 3-二氢-1H-萘并[1, 2-e][1,3]噁嗪-1-基)膦酸二乙酯(5o):白色固体, 产率78%. m.p. 70~74 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.02~7.91 (m, 1H), 7.68 (dd, J=18.7, 8.3 Hz, 2H), 7.50~7.37 (m, 1H), 7.30 (dd, J=13.0, 6.0 Hz, 1H), 7.17~6.91 (m, 5H), 6.23 (dd, J=21.2, 10.9 Hz, 1H), 5.50~5.32 (m, 2H), 4.32~4.11 (m, 2H), 3.99 (td, J=20.3, 10.5 Hz, 1H), 3.70 (dd, J=9.9, 4.6 Hz, 1H), 2.20 (dd, J=11.0, 7.9 Hz, 3H), 1.37~1.21 (m, 3H), 1.04 (dd, J=12.9, 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 152.40 (d, J=6.1 Hz), 147.32 (d, J=13.2 Hz), 132.39 (s), 129.92~129.44 (m), 129.10 (d, J=1.5 Hz), 128.28 (s), 125.97 (s), 123.23 (d, J=4.7 Hz), 119.90 (s), 118.96 (s), 77.51 (s), 63.43 (d, J=7.2 Hz), 62.73 (d, J=7.2 Hz), 57.49 (s), 55.85 (s), 20.45 (s), 16.46 (d, J=5.4 Hz), 16.10 (d, J=5.7 Hz); 31P NMR (162 MHz, CDCl3) δ: 20.33; HRMS calcdf or C23H27NO4P [M+H]+ 412.1678, found 412.1681.
(6-溴-3-对甲苯基-3, 4-二氢-2H-苯并[e][1,3]噁嗪-4-基)膦酸二乙酯(5r):白色固体, 产率88%. m.p. 88~92 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.40 (s, 1H), 7.26~7.21 (m, 1H), 7.10~7.02 (m, 4H), 6.73 (d, J=8.8 Hz, 1H), 5.75 (d, J=11.1 Hz, 1H), 5.31 (d, J=11.1 Hz, 1H), 4.76 (d, J=24.3 Hz, 1H), 4.32~4.15 (m, 4H), 2.26 (s, 3H), 1.43~1.30 (m, 6H); 13C NMR (101 MHz, CDCl3) δ: 153.83 (d, J=5.4 Hz), 132.48 (s), 131.48 (d, J=2.8 Hz), 131.03 (d, J=3.0 Hz), 129.86 (s), 119.30 (s), 77.16 (s), 64.21 (d, J=7.1 Hz), 62.69 (d, J=7.7 Hz), 59.06 (s), 57.41 (s), 20.52 (s), 16.47 (dd, J=24.9, 5.7 Hz); 31P NMR (162 MHz, CDCl3) δ: 19.67; HRMS calcd for C19H24BrN-O4P [M+H]+ 440.0626, found 440.0626.
(6-硝基-3-对甲苯基-3, 4-二氢-2H-苯并[e][1,3]噁嗪-4-基)膦酸二甲酯(5x):白色固体, 产率89%. m.p. 148~151 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.17 (s, 1H), 8.05 (d, J=8.5 Hz, 1H), 7.07 (dd, J=20.7, 7.6 Hz, 4H), 6.95 (d, J=9.0 Hz, 1H), 5.89 (d, J=11.0 Hz, 1H), 5.41 (d, J=11.1 Hz, 1H), 4.87 (d, J=24.5 Hz, 1H), 3.96 (d, J=10.4 Hz, 3H), 3.86 (d, J=9.5 Hz, 3H), 2.27 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 160.06 (d, J=5.2 Hz), 146.27 (d, J=11.8 Hz), 133.25 (s), 130.07 (s), 124.60 (dd, J=18.7, 2.8 Hz), 119.38 (s), 118.24 (s), 116.33 (d, J=2.3 Hz), 78.03 (s), 59.10 (s), 57.44 (s), 55.14 (d, J=7.2 Hz), 53.26 (d, J=7.8 Hz), 20.51 (s); 31P NMR (162 MHz, CDCl3) δ: 21.96; HRMS calcd for C17H20N2O6P [M+H]+ 379.1059, found 379.1053.
(6-硝基-3-对甲苯基-3, 4-二氢-2H-苯并[e][1,3]噁嗪-4-基)膦酸二丁酯(5y):白色固体, 产率93%. m.p. 116~119 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.20 (s, 1H), 8.04 (d, J=8.9 Hz, 1H), 7.06 (dd, J=19.7, 7.9 Hz, 4H), 6.92 (d, J=9.0 Hz, 1H), 5.90 (d, J=10.9 Hz, 1H), 5.39 (d, J=10.8 Hz, 1H), 4.83 (d, J=24.2 Hz, 1H), 4.19 (ddd, J=32.6, 19.0, 6.8 Hz, 4H), 2.26 (s, 3H), 1.70 (d, J=5.8 Hz, 4H), 1.49~1.35 (m, 4H), 0.93 (d, J=7.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 160.08 (d, J=5.0 Hz), 146.36 (d, J=11.6 Hz), 132.97 (s), 129.95 (s), 124.92 (d, J=3.3 Hz), 124.34 (d, J=2.4 Hz), 119.26 (s), 118.11 (s), 78.01 (s), 68.17 (d, J=7.3 Hz), 66.36 (d, J=7.9 Hz), 59.12 (s), 57.47 (s), 32.66 (d, J=5.5 Hz), 32.36 (d, J=6.0 Hz), 20.48 (s), 18.64 (d, J=8.7 Hz), 13.48 (d, J=5.7 Hz); 31P NMR (162 MHz, CDCl3) δ: 19.41; HRMS calcd for C23H32N2O6P [M+H]+ 463.1998, found 463.1995.
(6-硝基-3-对甲苯基-3, 4-二氢-2H-苯并[e][1,3]噁嗪-4-基)膦酸二异丙酯(5z):白色固体, 产率91%. m.p. 144~146 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.26 (s, 1H), 8.03 (d, J=9.1 Hz, 1H), 7.06 (q, J=8.6 Hz, 4H), 6.91 (d, J=9.1 Hz, 1H), 5.95 (d, J=11.0 Hz, 1H), 5.41 (d, J=11.0 Hz, 1H), 4.96~4.75 (m, 3H), 4.20 (ddd, J=138.3, 109.5, 80.3 Hz, 2H), 2.26 (s, 3H), 1.43 (dd, J=9.9, 6.2 Hz, 6H), 1.32 (dd, J=15.0, 6.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 160.12 (d, J=5.0 Hz), 146.39 (d, J=12.1 Hz), 132.78 (s), 129.87 (s), 125.33 (d, J=3.3 Hz), 124.25 (d, J=2.3 Hz), 119.27 (s), 117.97 (s), 78.06 (s), 73.14 (d, J=7.4 Hz), 59.10 (s), 57.43 (s), 24.50 (d, J=3.1 Hz), 24.03 (d, J=3.3 Hz), 23.69 (dd, J=5.7, 3.2 Hz), 20.47 (s); 31P NMR (162 MHz, CDCl3) δ: 17.41; HRMS calcd for C21H28N2O6P [M+H]+ 435.1685, found 435.1685.
辅助材料(Supporting Information)产物的核磁谱图及高分辨数据.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
(a) Schmidt, R. R. Synthesis 1972, 7, 333.
(b) Burke, W. J. J. Am. Chem. Soc. 1949, 71, 609.
(c) Mueller, R.; Li, Y. X.; Hampson, A.; Zhong, S.; Harris, C.; Marrs, C.; Rachwal, R.; Ulas, J.; Nielsson, L.; Rogers, G. Bioorg. Med. Chem. Lett. 2011, 21, 3923.
(d) Dutta, A. K.; Gogoi, P.; Saikia, M.; Borah, P. R. Catal. Lett. 2016, 146, 902. -
[2]
(a) Bouaziz, Z.; Riondel, J.; Mey, A.; Berlion, M.; Villard, J.; Fillion, H. Eur. J. Med. Chem. 1991, 26, 469.
(b) Chylinska, J. B.; Urbanski, T.; Mordarski, M. J. Med. Chem. 1963, 6, 484.
(c) Benameur, L.; Bouaziz, Z.; Nebois, P.; Bartoli, M. H.; Boitard, M.; Fillion, H. Chem. Pharm. Bull. 1996, 44, 605.
(d) Mathew, B. P.; Kumar, A.; Sharma, S.; Shula, P. K.; Nath, M. Eur. J. Med. Chem. 2010, 45, 1502.
(e) Petrlkov, E.; Waisser, K.; DiviSova, H.; Husakov, P.; Vrabcova, P.; Kunes, J.; Kolr, K.; Stolarikov, J. Bioorg. Med. Chem. 2010, 18, 8178.
(f) Waghmode, N. A.; Kalbandhe, A. H.; Thorat, P. B.; Karade, N. N. Tetrahedron Lett. 2016, 57, 680. -
[3]
(a) Froimowicz, P.; Zhang, K.; Ishida, H. Chem.-Eur. J. 2016, 22, 2691.
(b) Liu, Y.-X.; Ma, H.-M.; Liu, Y.; Qiu, J.-J.; Liu, C.-M. Polymer 2016, 82, 32.
(c) Huang, C. C.; Lin, C. S.; Dai, S. A. RSC Adv. 2015, 5, 74874.
(d) Zhang, Q.; Yang, P.; Deng, Y.; Zhang, C.; Zhu, R.; Gu, Y. RSC Adv. 2015, 5, 103203.
(e) Gupta, K. S. V.; Ramana, D. V.; Vinayak, B.; Sridhar, B.; Chandrasekharam, M. New J. Chem. 2016, 40, 6389.
(f) Barta, P.; Szatmári, I.; Fülö p, F.; Heydenreich, M.; Koch, A.; Kleinpeter, E. Tetrahedron 2016, 72, 2402.
(g) Dumas, L.; Bonaud, L.; Olivier, M.; Poorteman, M.; Dubois, P. Eur. Polym. J. 2016, 75, 486.
(h) Wipt, P.; Hayes, G. B. Tetrahedron 1998, 54, 6987. -
[4]
(a) Su, H.; Liu, Z. J. Therm. Anal. Calorim 2013, 114, 1207.
(b) Lin, C. H.; Lin, H. T.; Sie, J. W.; Hwang, K. Y.; Tu, A. P. J. Polym. Sci.: Part A: Polym. Chem. 2010, 4555. -
[5]
(a) Kabachnik, M. I. Dokl. Akad. Nauk SSSR 1952, 83, 689.
(b) Fields, E. K. J. Am. Chem. Soc. 1952, 74, 1528 -
[6]
(a) Wu, J.; Sun, W.; Wang, W.-Z.; Xia, H.-G. Chin. J. Chem. 2006, 24, 1054.
(b) Reddy, B. V. S.; Krishna, A. S.; Ganesh, A. V.; Kumar, J. J. S. N.Tetrahedron Lett. 2011, 52, 1369. -
[7]
Wu, J.; Sun, W.; Xia, H.-G.; Sun, X. Org. Biomol. Chem. 2006, 4, 1663. doi: 10.1039/B602536F
-
[8]
Jafari, A. A.; Nazarpour, M.; Abdollahi-Alibeik, M. Heteroat. Chem. 2010, 21, 397. doi: 10.1002/hc.20635
-
[9]
Bhattacharya, T.; Majumdar, B.; Dey, D.; Sarma, T. K. RSC Adv. 2014, 4, 45831. doi: 10.1039/C4RA08533G
-
[10]
Wu, J.; Sun, W.; Sun, X.; Xia, H.-G. Green Chem. 2006, 8, 365. doi: 10.1039/b517488k
-
[11]
(a) Ambica; Kumar, S.; Taneja, S. C.; Hundal, M. S.; Kapoor, K. K. Tetrahedron Lett. 2008, 49, 2208.
(b) Li, X.-C.; Gong, S.-S.; Zeng, D.-Y.; You, Y.-H.; Sun, Q. Tetrahedron Lett. 2016, 57, 1782.
(c) Manabe, K.; Kobayashi, S. Chem. Commun. 2000, 669.
(d) Qian, C.; Huang, T. J. Org. Chem. 1998, 63, 4125.
(e) Ranu, B. C.; Hajra, A.; Jana, U. Org. Lett. 1999, 1, 1141. -
[12]
(a) Tillu, V. H.; Dumbre, D. K.; Wakharkar, R. D.; Choudhary, V. R. Tetrahedron Lett. 2011, 52, 863.
(b) Kaboudin, B.; Nazari, R. Tetrahedron Lett. 2001, 42, 8211. -
[13]
(a) Mu, X.-J.; Lei, M.-Y.; Zou, J.-P.; Zhang, W. Tetrahedron Lett. 2006, 47, 1125.
(b) Bhattacharya, A. K.; Rana, K. C. Tetrahedron Lett. 2008, 49, 1782. -
[14]
(a) Ouahrouch, A.; Taourirte, M.; Schols, D.; Snoeck, R.; Andrei, G.; Angel, J. W.; Lazrek, H. B. Arch. Phram. Chem. Life Sci. 2016, 349, 30.
(b) Ouahrouch, A.; Krim, J.; Taourirte, M.; Lazrek, H. B.; Engels, J. W.; Bats, J. W. Acta Crystallogr. 2013, C69, 1157. -
[15]
(a) Thirumurugan, P.; Nandakumar, A.; SudhaPriya, N.; Muralidaran, D.; Perumal, P. T. Tetrahedron Lett. 2010, 51, 15708.
(b) Yadava, J. S.; Reddy, B. V. S.; Sreedhar, P. Green Chem. 2002, 4, 436.
(c) Disale, S. T.; Kale, S. R.; Kahandal, S. S.; Srinivasan, T. J.; Jayaram, R. V. Tetrahedron Lett. 2012, 53, 2277.
(d) Ordóñ nez, M.; Sayago, F. J.; Cativiela, C. Tetrahedron 2012, 68, 6369. -
[16]
(a) Bhagat, S.; Chakraborti, A. K. J. Org. Chem. 2008, 73, 6029.
(b) Bhagat, S.; Chakraborti, A. K. J. Org. Chem. 2007, 72, 1263. -
[17]
Yu, Y.-Q.; Xu, D.-Z. Synthesis 2015, 47, 1869. doi: 10.1055/s-00000084
-
[18]
Lee, S.; Park, J. H.; Kang, J.; Lee, J. K. Chem. Commun. 2001, 1698. http://www.ncbi.nlm.nih.gov/pubmed/12240450
-
[19]
Dar, B.; Singh, A.; Sahu, A.; Patida, P.; Chakraborty, A.; Sharma, M.; Singh, B. Tetrahedron Lett. 2012, 53, 5497. doi: 10.1016/j.tetlet.2012.07.123
-
[20]
Kudrimoti, S.; Bommena, V. R. Tetrahedron Lett. 2005, 46, 1209 doi: 10.1016/j.tetlet.2004.12.070
-
[21]
(a) Huang, L.; Gong, J.; Zhu, Z.; Wang, Y.; Guo, S.; Cai, H. Org. Lett. 2017, 29, 2242.
(b) Huang, L.; Zhu, Z.; Cao, T.; Lei, X.; Gong, J.; Guo, S.; Cai, H. Chin. J. Org. Chem. 2017, 37, 1571 (in Chinese).
(c) Gong, J.; Zhu, Z.; Lu, L.; Guo, S.; Cai, H. Chin. J. Org. Chem. 2015, 35, 1917 (in Chinese).
(d) Gong, J.; Huang, L.; Deng, Q.; Jie, K.; Wang, Y.; Guo, S.; Cai, H. Org. Chem. Front. 2017, 4, DOI: 10. 1039/C7QO00318H. -
[22]
Cambridge Crystallographic Data Centre (CCDC) for 4o (1509069) and 5q (1509068).
-
[23]
(a) Sun, J.; Qiu, J.-K.; Jiang, B.; Hao, W.-J.; Guo, C.; Tu, S.-J. J. Org. Chem. 2016, 81, 3321.
(b) Ji, S.-J.; Wang, S.-Y.; Zhang Y.; Loh, T.-P. Tetrahedron 2004, 60, 2051.
(c) Zhang, H.; Wei, Q.; Zhu, G.; Qu, J.; Wang, B. Tetrahedron Lett. 2016, 57, 2633. -
[24]
Zhang, Y.; Zhu, C. Catal. Commun. 2011, 28, 134. http://www.ncbi.nlm.nih.gov/pubmed/21541418
-
[25]
Li, N.; Qiu, R.; Xu, X.; Chen, J.; Zhang, X.; Chen, S.; Yin, S. Catal. Commun. 2014, 43, 184. doi: 10.1016/j.catcom.2013.10.013
-
[26]
Thirmurugan, P.; Nandakumar, A.; Sudha, N.; Muralidaran, D.; Perumal, P. Tetrahedron. Lett. 2010, 51, 5708 doi: 10.1016/j.tetlet.2010.08.066
-
[27]
Song, L.; Yang, C.; Yu, Y.; Xu, D. Synthesis 2017, 49, 1641.
-
[28]
Das, B.; Satyalakshmi, G.; Suneel, K.; Damodar, K. J. Org. Chem. 2009, 74, 8400. doi: 10.1021/jo901765s
-
[29]
Shinde, p.; Kategaonkar, A.; Shingate, B.; Shingare, M. Tetrahedron Lett. 2011, 52, 2889. (Li, L.; Fan, Y.) doi: 10.1016/j.tetlet.2011.03.138
-
[1]
-
表 1 α-氨基磷酸酯合成的条件优化
Table 1. Screening for optimal conditionsa

Entry Cat. n(1a):n(2a):n(3a) Solvent Yieldb/% 1 — 1:3:1.2 THF — 2 I2 1:3:1.2 THF 84 3c I2 1:3:1.2 THF 86 4 I2 1:3:1.2 Toluene 83 5 I2 1:3:1.2 DCE 83 6 I2 1:3:1.2 DMSO 84 7 I2 1:3:1.2 CH3CN 87 8 I2 1:3:1.2 DMF 88 9 I2 1:3:1.2 DCM 65 10 I2 1:3:1.2 Dioxane 83 11 I2 1:3:1.2 EtOAc 85 12 I2 1:3:1.2 CPME 97 13 I2 1:2:1.2 CPME 78 14 I2 1:1.2:1.2 CPME 77 15d I2 1:3:1.2 CPME Trace 16e I2 1:3:1.2 CPME 87 17f I2 1:3:1.2 CPME 95 a Reaction conditions: aldehyde (0.5 mmol), amine (0.6 mmol), cat. (20 mol%), solvents (2 mL), phosphite (1.5 mmol), 40 ℃ for 12 h under nitrogen; b isolated yield; c I2 (30 mol%); d 20 ℃; e80 ℃; f15 min. 表 3 苯并噁嗪的合成
Table 3. Synthesis of the benzoxazines

表 2 底物拓展
Table 2. Exploration of substrate scope

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 10
- 文章访问数: 1905
- HTML全文浏览量: 411


 下载:
下载:


 下载:
下载:

