 图1
Representative drugs containing the halogenated quinolines
Figure1.
Representative drugs containing the halogenated quinolines
图1
Representative drugs containing the halogenated quinolines
Figure1.
Representative drugs containing the halogenated quinolines

镍催化的8-氨基喹啉C—H键卤化反应合成C(5) 和C(7) 双卤代喹啉
English
Nickel-Catalyzed C-H Halogenation of 8-Aminoquinolines for the Synthesis of C(5) and C(7) Di-halogenated Quinolines
-
Key words:
- Nickel-catalyze
- / C—H halogenation
- / N-bromo-succinimide
- / 8-aminoquinolines
- / dihalogenated quinoline
-
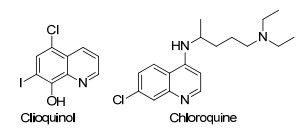
It is well documented that quinolines are very important N-heterocyclic compounds which are frequently found in biologically active natural products and pharmaceuticals.[1] Among the numerous quinoline derivatives, the halogenated quinolines are a class of important one which often act as intermediates in the synthesis of substituted quinolines.[2] Moreover, many halogenated quinolines such as clioquinol and chloroquine are frequently used as antibiotic and antimalaria agents in clinical application (Figure 1).[3] Therefore, further development of more simple method for the synthesis of halogenated quinolines remains highly appealing.
 图1
Representative drugs containing the halogenated quinolines
Figure1.
Representative drugs containing the halogenated quinolines
图1
Representative drugs containing the halogenated quinolines
Figure1.
Representative drugs containing the halogenated quinolines
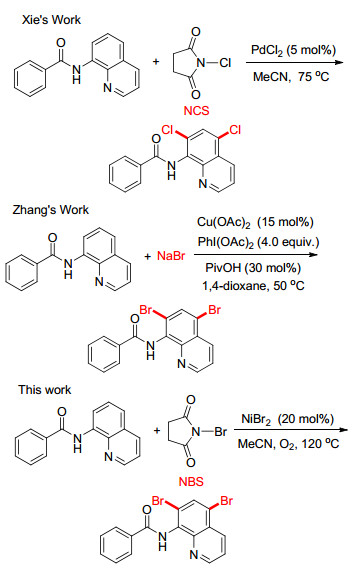
Although many methods have been reported for the synthesis of halogenated quinolines, they are often confined to the functionalization of quinoline scaffolds mainly at the C(2), C(3), C(4), and C(8) positions.[4, 5] Reports involving halogenation of quinolines at the C(5) and C(7) position are quite rare.[6] Recently, following the developments of transition metal catalyzed C—H activation stratregies, some examples on C(5) functionalized quinolines have been achieved.[7] However, to the best of our knowledge, only two published examples on the simultaneous C(5) and C(7)-dihalogenation of quinoline are known. In 2015, Xie and coworkers[6a] have developed an efficient method of Pd-catalyzed synthesis of 5, 7-dichlo-rinated quinolines utilizing quinoline C—H haogenation. More recently, Zhang et al.[6b] have developed an alternative version of such type to access 5, 7-dibrominated quinolines by means of copper catalysis (Scheme 1). While drawbacks such as narrow substrate scope, noble metal catalyst, employment of stoichiometric oxidant etc. remain with the known synthetic methods, developing complementary catalytic method to run this di-halogenation process is still highly desirable.
As a class of important first-row transition metal catalyst, nickel salts were frequently and widely employed in organic synthesis to construct various bioactive compounds due to its particular electronic properties, low cost and low toxicity. Indeed, nickel catalysts have been applied in many types of catalytic transformations such as coupling reactions, [8] carbonylation reactions[9] and heterocycle synthesis.[10] However, there have been few reports of C—H bond halogenation utilizing a nickel catalyst.[11] As our continuous interests in the development of nickel catalyzed synthesis and 8-aminoquinoline assisted C—H functionalization for the synthesis biologically relevant small molecules, [12] we report herein a C—H halogenation reaction of 8-aminoquinolines that gave the 5, 7-dihalo-generated products with cheap and easily available NiBr2 catalyst.
1 Results and discussion
We began our studies by using amide (1a) and N-bromo-succinimide as the model substrates to optimize the reaction conditions, and the results are summarized in Table 1. When the reaction was carried out in the presence of NiCl2 (20 mol%) in MeCN at 120 ℃ under O2 overnight, the di-brominated amide (3a) was successfully isolated in 55% yield (Table 1, Entry 1). The structure of 3a was further confirmed by X-ray diffraction analysis (see the Supporting Information).

Entry Catalyst Oxidant Base Solvent Yield/% of 3a/2a 1 NiCl2 O2 — MeCN 55/30 2 NiBr2 O2 — MeCN 87/— 3 Ni(acac)2 O2 — MeCN 60/20 4 Ni(NO3)2 O2 — MeCN 57/33 5 — O2 — MeCN —/85 6 NiBr2 BQ — MeCN 55/27 7 NiBr2 TBHP — MeCN 65/25 8 NiBr2 Ag2O — MeCN 60/30 9 NiBr2 O2 Cs2CO3 MeCN 30/54 10 NiBr2 O2 Na2CO3 MeCN 28/60 11 NiBr2 O2 NaHCO3 MeCN 33/59 12 NiBr2 O2 — Toluene 55/35 13 NiBr2 O2 — PET —/25 14 NiBr2 O2 — DCM —/82 15 NiBr2 O2 — DCE 15/45 16 b NiBr2 O2 — MeCN 84/15 17 c NiBr2 O2 — MeCN 87/12 18 d NiBr2 O2 — MeCN 85/14 a Reaction conditions: 1a (0.25 mmol), NiBr2 (20 mol%), NBS (2.0 equiv.), MeCN (2.0 mL), stirred at 120 ℃, under O2, overnight, isolated yields. b The reaction temperature is 100 ℃. c The reaction temperature is 110 ℃. d The reaction temperature is 130 ℃. Next, different nickel catalysts in the reaction were then screened and NiBr2 was found to be the best choice which provides the di-brominated product in 87% yield (Table 1, Entries 2~4). Meanwhile, a blank experiment indicated that the nickel catalyst was the key factor, because the desired product 3a was not obtained in the absence of nickel catalyst (Table 1, Entry 5). Furthermore, screening of different oxidants proved the O2 was the best choice, providing the desired product in 87% yield, and the other oxidants such as 1, 4-benzoquinone (BQ), tert-butyl hydroperoxide (TBHP), Ag2O gave lower yields (Table 1, Entries 6~8). Different bases such as Cs2CO3, K2CO3, K3PO4 were screened subsequently. The results indicated that they were substantially less effective (Table 1, Entries 9~11). In order to insight into the effect of solvent on the reaction, a variety of solvents such as N, N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and tetrahydrofuran (THF) were also examined. The experiment results revealed that attempts to exchange the solvent failed to improve product yield (Table 1, Entries 12~15). Finally, different temperatures on the reaction were investigated. Either higher or lower temperature from 100 ℃ to 130 ℃ can improve the yield (Table 1, Entries 2 and 16~18). Therefore, the optimized condition was to use a combination of NiBr2 (20 mol%) as catalyst and O2 as oxidant in MeCN at 120 ℃ (Table 1, Entry 2).
With this optimum condition in hand, we proceeded to investigate the reaction of NBS with different 8-amido-quinoline derivatives. Generally, the dibromination showed excellent functional group tolerance toward diversely substituted 8-amidoquinolines (Table 2). Both electron-withdrawing and electron-donating aromatic substitutions of carboxamides were turned into the corresponding halogenation products in good yields (3b~3k). From the results, it can be concluded that the reactants with electron-donating substituents afforded the desired products in higher yields compare to those with electron-withdrawing groups (3d 90% yield and 3k 91% yield). In addition, naphthalene substituent was also a suitable substrate for this reaction, affording the product 3l in 82% yield. Moreover, the reaction of NBS with thiophene-fused carboxamides also took place efficiently, providing N-(5, 7-dibromoquinolin-8-yl)-thiophene-2-carboxamide (3m) in 77% yield. The carboxamides with alkyl substitutions were also went smoothly in high yields. For instance, N-(quinolin-8-yl)acetamide and N-(quinolin-8-yl)cyclo-hexanecarboxamide could act as good reaction substrates under the optimized conditions. The desired products 3n and 3o were obtained in yields of 80% and 81%, respectively. In the further study, it is found that chlorination could be occurred as well as bromination at the C(5) and C(7) position of quinolines, and deliver the desire dichlorination products 3p and 3q in good yield. On the other hand, substitution at the quinoline moiety was also explored. The electron-donating methyl-substituents (1r) and electron-withdrawing fluorine-substituents (1s) delivered the desired products 3r and 3s in moderate yields.
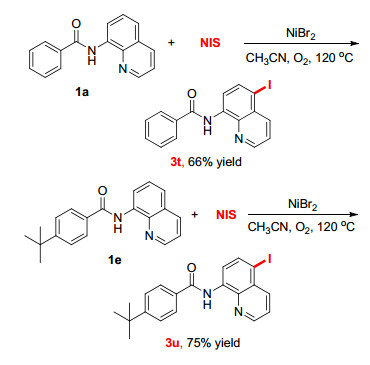
Next, we also explored the iodination under the standard condition. To our surprise, we only got the mono-iodi-nation product at the C(5) position of quinoline (Scheme 3).
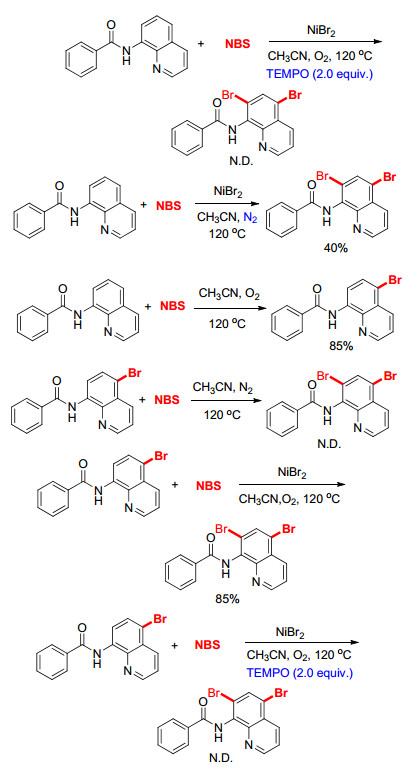
In order to insight into the reaction mechanism more clearly, a series of control experiments were carried out. The reaction was suppressed completely by using the radical scavenger 2, 2, 6, 6-tetramethyl-1-piperidinyl-oxy (TEM-PO) probably suggesting that the reaction may follow a radical pathway. Further experiments about reaction atmosphere replaced with nitrogen gas were also carried out, the yields of products is 40%. This phenomenon indicates that NBS has a dual role in the bromination, not only producing the bromine radical also oxidizing the intermediate instead of O2 in the process of bromination. Only mono-bromination product at the C(5) position of quinoline was obtained in the absence of nickel catalyst and mono-bromination product at the C(5) position of quinoline could not further turn into the C(5) and C(7) di-bromination quinoline without nickel as the catalyst. These two phenomena show that the nickel is the key in formation the bromination product at the C(7) position of quinoline. Treating 2a with NBS under the standard condition gave 3a in a yield of 85%, indicating that 2a is most likely an intermediate for the formation of 3a from 1a. The reaction was also completely suppressed by treating 2a with NBS and NiBr2 in the presence of 2 equiv. of TEMPO, indicating that the C(7) halogenation of 2a may mainly proceed via a radical pathway.
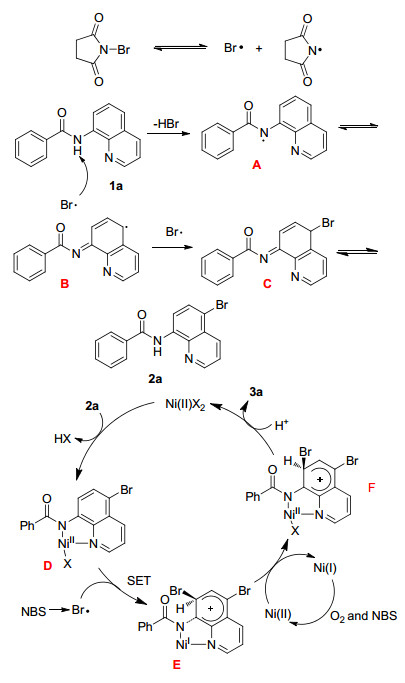
On the basis of the above results and previous reports, [13] a plausible mechanism for the formation of 3a is proposed in Scheme 4. First, a radical Br• was generated from NBS under the high temperature, which then reacted with 8-aminoquinoline amides (1a) to generate the intermediate A. Subsequently, intermediate A transferred to a radical intermediate B through electron delocalization. Then, Intermediate B was trapped by Br• in the system to form the intermediate C, which underwent isomerization to give the C(5) halogenation product 2a. Second, 2a coordinates with NiBr2 to give intermediate D. Then intermediate D was attacked by Br• and the intermediate turned into complex E through a single electron transfer process (SET). Soon afterwards, the intermediate E transformed into F through oxidation. After a proton transfer process, reductive elimination occurs with F to afford terminal product 3a.
2 Conclusion
In conclusion, we have described an efficient route for the synthesis of C(5) and C(7) di-halogenated quinoline derivatives via a nickel-catalyzed C—H halogenation reaction of 8-aminoquinolines. The reaction proceeds smoothly under mild conditions, leading to the diverse products in good to excellent yields. More transformations incorporating C—H halogenation reaction for the synthesis of biologically interesting moleculars are ongoing in our laboratory.
3 Experimental section
3.1 General procedure for the synthesis of product 3
A 50 mL of schlenk tube was equipped with a magnetic stir bar and charged with the mixture of 1 (0.25 mmol), NXS (2.0 equiv.), NiBr2 (10.9 mg, 20 mol%), in MeCN (2.0 mL) under O2. The mixture was stirred at 120 ℃ for overnight. After completion, the mixture was cooled to room temperature and diluted with water (10 mL), extracted with EtOAc (10 mL×3). The combined organic phase was dried over Na2SO4. After filtering, the acquired solution was collected and the solvent was removed at reduced pressure. The residue was subjected to silica gel column chromatography to give pure products 3 by using mixed petroleum ether and ethyl acetate [V(petroleum ether):V(ethyl acetate)=5:1].
N-(5, 7-Dibromoquinolin-8-yl)benzamide (3a): Yield 87%, white solid. m.p. 156.0~157.0 ℃ (lit.[6b] 156~157 ℃); 1H NMR (400 MHz, CDCl3) δ: 9.15 (s, 1H), 8.84 (dd, J=4.2, 1.6 Hz, 1H), 8.50 (dd, J=8.5, 1.4 Hz, 1H), 8.11 (s, 1H), 8.08 (d, J=4.0 Hz, 2H), 7.64~7.47 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 165.4, 150.6, 143.8, 136.2, 134.5, 134.1, 134.0, 132.3, 128.7, 128.1, 126.9, 122.8, 120.2, 118.9.
N-(5, 7-Dibromoquinolin-8-yl)-4-fluorobenzamide (3b): Yield 83%, white solid. m.p. 178.6~179.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.16 (s, 1H), 8.84 (d, J=4.0 Hz, 1H), 8.51 (d, J=8.3 Hz, 1H), 8.13~8.05 (m, 3H), 7.57 (dd, J=8.7, 3.7 Hz, 1H), 7.19 (t, J=8.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 165.3 (d, 1JCF=252 Hz), 164.4, 150.6, 143.7, 136.3, 134.5, 133.9, 130.5 (d, 3JCF=9 Hz), 130.1, 127.0, 122.9, 120.5, 119.1, 115.8 (d, 2JCF=22 Hz); HRMS (ESI+) calcd for C16H10Br2FN2O [M+H]+ 422.9138, found 422.9139.
N-(5, 7-Dibromoquinolin-8-yl)-4-(trifluoromethyl)benza-mide (3c): Yield 85%, white solid. m.p. 205.4~206.6 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.23 (s, 1H), 8.85 (dd, J=4.3, 1.6 Hz, 1H), 8.54 (dd, J=8.5, 1.6 Hz, 1H), 8.18 (d, J=8.1 Hz, 2H), 8.13 (s, 1H), 7.77 (d, J=8.1 Hz, 2H), 7.59 (q, J=8.5, 4.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 164.2, 150.7, 143.8, 137.1, 136.3, 134.4, 134.0, 133.7, 133.6, 128.5, 127.0, 125.8 (q, J=4 Hz), 125.0, 123.0, 122.3, 120.8, 119.5; HRMS (ESI+) calcd for C17H10Br2-F3N2O [M+H]+ 472.9106, found 472.9107.
N-(5, 7-Dibromoquinolin-8-yl)-4-methylbenzamide (3d): Yield 90%, white solid. m.p. 166.0~167.0 ℃(lit.[6b] 166~167 ℃); 1H NMR (400 MHz, CDCl3) δ: 9.12 (s, 1H), 8.85 (dd, J=4.3, 1.6 Hz, 1H), 8.52 (dd, J=8.5, 1.5 Hz, 1H), 8.12 (s, 1H), 7.97 (d, J=8.0 Hz, 2H), 7.56 (q, J=4.2 Hz, 1H), 7.32 (d, J=8.0 Hz, 2H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.3, 150.5, 143.8, 142.9, 136.0, 134.4, 134.3, 131.1, 129.4, 128.1, 126.9, 122.8, 120.1, 118.7, 21.6.
4-(tert-Butyl)-N-(5, 7-dibromoquinolin-8-yl)benzamide (3e): Yield 94%, white solid. m.p. 219.2~221.6 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.51 (s, 1H), 8.79 (dd, J=4.3, 1.6 Hz, 1H), 8.44 (dd, J=8.6, 1.5 Hz, 1H), 8.07 (s, 1H), 8.00 (d, J=8.4 Hz, 2H), 7.50~7.44 (m, 3H), 1.35 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 165.3, 155.8, 150.4, 143.9, 136.1, 134.4, 134.3, 131.0, 127.9, 127.9, 126.9, 125.6, 122.8, 120.6, 118.8, 35.0, 31.2; HRMS (ESI+) calcd for C20H19Br2N2O [M+H]+ 460.9859, found 460.9857.
N-(5, 7-Dibromoquinolin-8-yl)-2-fluorobenzamide (3f): Yield 79%, white solid. m.p. 148.1~149.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.27 (d, J=13.1 Hz, 1H), 8.91 (dd, J=4.2, 1.6 Hz, 1H), 8.51 (dd, J=8.5, 1.6 Hz, 1H), 8.23 (td, J=7.8, 1.9 Hz, 1H), 8.12 (s, 1 H), 7.59~7.54 (m, 2H), 7.32 (td, J=7.6, 1.1 Hz, 1H), 7.26~7.21 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 161.3, 161.1(d, 1JCF=248 Hz), 159.9, 151.1, 144.3, 136.0, 134.2, 134.0 (d, 3JCF=9 Hz), , 133.8, 132.6, 127.1, 124.9, 122.8, 121.3, 119.8, 116.4 (d, 2JCF=24 Hz); HRMS (ESI+) calcd for C16H10Br2FN2O [M+H]+ 422.9138429, found 422.9139.
2-Chloro-N-(5, 7-dibromoquinolin-8-yl)benzamide (3g): Yield 75%, white solid. m.p. 178.4~179.7 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.88 (s, 2H), 8.48 (d, J=8.3 Hz, 1H), 8.09 (s, 1H), 7.91 (d, J=7.2 Hz, 1H), 7.55 (q, J=4.0 Hz, 1H), 7.48 (d, J=7.6 Hz, 1H), 7.44~7.36 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 164.2, 150.9, 144.1, 136.0, 134.6, 134.4, 134.3, 133.5, 131.8, 131.7, 130.7, 127.0, 127.0, 122.9, 120.8, 119.6; HRMS (ESI+) calcd for C16H10Br2ClN2O [M+H]+ 438.8843, found 438.8845.
N-(5, 7-Dibromoquinolin-8-yl)-3-fluorobenzamide (3h): Yield 79%, white solid. m.p. 152.1~152.4 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.43 (s, 1H), 8.81 (dd, J=4.3, 1.6 Hz, 1H), 8.46 (dd, J=8.5, 1.6 Hz, 1H), 8.07 (s, 1H), 7.84 (d, J=8.4 Hz, 1H), 7.72 (dt, J=9.3, 2.0 Hz, 1H), 7.53 (q, J=8.5, 4.2 Hz, 1H), 7.44 (td, J=8.0, 5.4 Hz, 1H), 7.27~7.22 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 164.2, 162.8 (d, 1JCF=246 Hz), 150.6, 143.9, 136.2, 134.4, 133.8, 130.4 (d, 3JCF=8 Hz), 127.0, 123.5 (d, 2JCF=29 Hz), 122.9, 120.7, 119.4, 119.1, 115.3, 115.2; HRMS (ESI+) calcd for C16H10Br2FN2O [M+H]+ 422.9138, found 422.9138.
3-Bromo-N-(5, 7-dibromoquinolin-8-yl)benzamide (3i): Yield 81%, white solid. m.p. 175.2~177.5 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.81 (dd, J=4.2, 1.6 Hz, 1H), 8.45 (dd, J=8.6, 1.6 Hz, 1H), 8.16 (s, 1H), 8.05 (s, 1H), 7.97 (d, J=8.0 Hz, 1H), 7.65 (d, J=8.0 Hz, 1H), 7.53 (q, J=4.2 Hz, 1H), 7.31 (t, J=7.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 164.1, 150.7, 143.9, 136.2, 135.8, 135.2, 134.4, 133.7, 131.2, 130.2, 127.0, 126.5, 122.9, 122.9, 120.8, 119.5; HRMS (ESI+) calcd for C16H10Br3N2O [M+H]+ 482.8338, found 482.8338.
N-(5, 7-Dibromoquinolin-8-yl)-3-methylbenzamide (3j): Yield 79%, white solid. m.p. 146.7~148.2 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.15 (s, 1H), 8.85 (d, J=3.2 Hz, 1H), 8.50 (d, J=8.4 Hz, 1H), 8.11 (s, 1H), 7.88~7.86 (m, 2H), 7.56 (q, J=4.0 Hz, 1H), 7.40 (d, J=4.5 Hz, 2H), 2.44 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.6, 150.5, 143.7, 138.6, 136.2, 134.5, 134.1, 133.9, 133.1, 128.7, 128.6, 126.9, 125.1, 122.8, 120.2, 118.8, 21.4; HRMS (ESI+) calcd for C17H13Br2N2O [M+H]+ 418.9389, found 418.9388.
N-(5, 7-Dibromoquinolin-8-yl)-3, 5-dimethylbenzamide (3k): Yield 91%, white solid. m.p. 158.5~160.2 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.11 (s, 1H), 8.84 (d, J=4.0 Hz, 1H), 8.48 (d, J=8.4 Hz, 1H), 8.09 (s, 1H), 7.68 (s, 2H), 7.54 (q, J=4.0 Hz, 1H), 7.21 (s, 1H), 2.40 (s, 6H); 13C NMR (100 MHz, CDCl3) δ: 165.7, 150.6, 143.8, 138.5, 136.1, 134.5, 134.2, 133.9, 133.8, 126.9, 125.8, 122.8, 120.3, 118.8, 21.3; HRMS (ESI+) calcd for C18H15Br2-N2O[M+H]+ 432.9547, found 432.9547.
N-(5, 7-Dibromoquinolin-8-yl)-2-naphthamide (3l): Yield 82%, white solid. m.p. 174.7~176.9 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.39 (s, 1H), 8.83 (d, J=4.2 Hz, 1H), 8.58 (s, 1H), 8.48 (d, J=8.5 Hz, 1H), 8.11~8.06 (m, 2H), 7.93~7.85 (m, 3H), 7.59~7.51 (m, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.6, 150.6, 143.9, 136.1, 135.2, 134.4, 134.2, 132.6, 131.2, 129.2, 128.9, 128.6, 128.0, 127.8, 126.9, 126.8, 124.3, 122.8, 120.3, 119.0; HRMS (ESI+) calcd for C20H13Br2N2O [M+H]+ 454.9389, found 454.9389.
N-(5, 7-Dibromoquinolin-8-yl)thiophene-2-carboxamide (3m): Yield 77%, yellow liquid. 1H NMR (400 MHz, CDCl3) δ: 9.59 (s, 1H), 8.84 (d, J=4.0 Hz, 1H), 8.48 (d, J=8.4 Hz, 1H), 8.08 (s, 1H), 7.87 (d, J=3.7 Hz, 1H), 7.56~7.54 (m, 2H), 7.12 (t, J=4.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 159.9, 150.5, 143.9, 138.5, 136.3, 134.5, 133.6, 131.4, 130.1, 127.9, 127.0, 122.9, 120.8, 119.2; HRMS (ESI+) calcd for C14H9Br2N2OS [M+H]+ 410.8797, found 410.8797.
N-(5, 7-dibromoquinolin-8-yl)acetamide (3n): Yield 80%, white solid. m.p. 87~88 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.86 (dd, J=4.4, 1.6 Hz, 1H), 8.46 (dd, J=8.8, 1.6 Hz, 2H), 8.05 (s, 1H), 7.53 (q, J=4.4 Hz, 1H), 2.32 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 168.7, 150.8, 144.2, 136.1, 134.2, 133.9, 130.9, 128.8, 127.0, 122.7, 23.7; HRMS (ESI+) calcd for C11H9Br2N2O [M+H]+ 342.9076, found 342.9078.
N-(5, 7-Dibromoquinolin-8-yl)cyclohexanecarboxamide (3o): Yield 81%, white solid. m.p. 97~98 ℃ (lit.[6b] 202~203 ℃); 1H NMR (400 MHz, CDCl3) δ: 8.86 (dd, J=4.2, 1.6 Hz, 1H), 8.46 (dd, J=8.5, 1.6 Hz, 1H), 8.28 (s, 1H), 8.03 (s, 1H), 7.54 (dd, J=8.5, 4.2 Hz, 1H), 2.52 (tt, J=11.6, 3.5 Hz, 1H), 2.16~2.10 (m, 2H), 1.91~1.85 (m, 2H), 1.75~1.62 (m, 3H), 1.41~1.28 (m, 3H); 13C NMR (100 MHz, CDCl3) δ: 174.1, 150.6, 144.0, 135.9, 134.3, 133.9, 126.9, 122.7, 120.5, 118.8, 45.9, 29.7, 25.8, 25.7.
N-(5, 7-Dichloroquinolin-8-yl)benzamide (3p): Yield 64%, yellow solid. m.p. 111.3~113.3 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.23 (s, 1H), 8.86 (dd, J=4.3, 1.6 Hz, 1H), 8.52 (dd, J=8.5, 1.6 Hz, 1H), 8.11~8.04 (m, 2H), 7.74 (s, 1H), 7.59~7.46 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 165.4, 150.6, 143.5, 133.9, 133.5, 132.3, 131.5, 129.9, 128.7, 128.6, 128.6, 128.1, 125.2, 122.4; HRMS (ESI+) calcd for C16H11Cl2N2O [M+H]+ 317.0243, found 317.0242.
N-(5, 7-Dichloroquinolin-8-yl)acetamide (3q): Yield 62%, yellow solid. m.p. 180.3~180.5 ℃ (lit.[6a] 179~180 ℃); 1H NMR (400 MHz, CDCl3) δ: 8.87 (dd, J=4.0, 1.6 Hz, 1H), 8.67 (brs, 1H), 8.49 (dd, J=4.0, 1.6 Hz, 1H), 7.68 (s, 1H), 7.51~7.55 (m, 1H), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3) δ: 168.8, 150.7, 143.8, 136.1, 133.5, 131.8, 128.4, 125.3, 122.6, 122.3, 23.6.
N-(5, 7-Dibromo-6-methylquinolin-8-yl)benzamide (3r): Yield 65%, white solid. m.p. 188.2~188.7 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.19 (s, 1H), 8.76 (dd, J=4.4, 1.6 Hz, 1H), 8.58 (dd, J=8.8, 1.2 Hz, 1H), 8.07 (d, J=7.2 Hz, 2H), 7.57 (t, J=7.6 Hz 1H), 7.47~7.52 (m, 3H), 2.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 165.7, 149.8, 142.4, 137.4, 136.6, 134.1, 134.0, 132.2, 128.7, 128.1, 127.2, 125.1, 122.9, 120.8, 25.8; HRMS (ESI+) calcd for C17H12Br2N2NaO [M+Na]+ 440.9209, found 440.9214.
N-(5, 7-Dibromo-6-fluoroquinolin-8-yl)benzamide (3s): Yield 59%, white solid. m.p. 203.3~204.3 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.34 (s, 1H), 8.79 (d, J=3.6 Hz, 1H), 8.51 (d, J=8.0 Hz, 1H), 8.08 (d, J=7.2 Hz, 2H), , 7.56~7.62 (m, 3H), 7.52 (t, J=7.6 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 165.2, 154.3 (d, 1JCF=245 Hz), 149.6 (d, 4JCF=3 Hz), 140.3, 136.1 (d, 4JCF=3 Hz), 135.4, (d, 3JCF=7 Hz), 133.8, 132.5, 128.8, 128.1 (d, 2JCF=29 Hz), 126.9 (d, 4JCF=3 Hz), 110.8 (d, 2JCF=27 Hz), 102.9, (d, 2JCF=24 Hz), 100.0; HRMS (ESI+) calcd for C16H9Br2FN2NaO [M+Na]+ 444.8958, found 44.8948.
N-(5-Iodoquinolin-8-yl)benzamide (3t): Yield 66%, white solid. m.p. 154~157 ℃ (lit.[6c] 154~157 ℃); 1H NMR (400 MHz, CDCl3) δ: 10.75 (s, 1H), 8.82 (dd, J=4.2, 1.6 Hz, 1H), 8.71 (d, J=8.3 Hz, 1H), 8.39 (dd, J=8.5, 1.6 Hz, 1H), 8.13 (d, J=8.3 Hz, 1H), 8.07 (dd, J=6.5, 1.7 Hz, 2H), 7.59~7.54 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 165.4, 148.9, 140.8, 139.4, 138.3, 135.5, 134.9, 132.1, 129.7, 128.9, 127.3, 123.2, 117.9, 89.5.
4-(tert-Butyl)-N-(5-iodoquinolin-8-yl)benzamide (3u): Yield 75%. yellow liquid. 1H NMR (400 MHz, CDCl3) δ: 10.52 (s, 1H), 8.59 (dd, J=4.2, 1.5 Hz, 1H), 8.52 (d, J=8.3 Hz, 1H), 8.14 (dd, J=8.5, 1.5 Hz, 1H), 7.92~7.86 (m, 3H), 7.43 (d, J=8.4 Hz, 2H), 7.32 (dd, J=8.5, 4.2 Hz, 1H), 1.25 (s, 9H); 13C NMR (101 MHz, CDCl3) δ: 165.3, 155.6, 148.8, 140.7, 139.3, 138.3, 135.6, 132.0, 129.6, 127.2, 125.8, 123.2, 117.8, 89.4, 35.1, 31.2; HRMS (ESI+) calcd for C20H20IN2O[M+H]+ 431.0615, found 431.0614.
Supporting Information The 1H NMR and 13C NMR spectra for compound 3 and the single crystal data of compound 3a. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn.
-
-
[1]
(a) Kouznetsov, V. V. ; Mendez, L. Y. ; Gomez, C. M. Curr. Org. Chem. 2005, 9, 141.
(b) Michael, J. P. Nat. Prod. Rep. 2008, 25, 166.
(c) Tokoro, Y. ; Nagai, A. ; Kokado, K. ; Chujo, Y. Macromolecules 2009, 42, 2988.
(d) Solomon, V. R. ; Lee, H. Curr. Med. Chem. 2011, 18, 1488.
(e) Liu, G. ; Yi, M. ; Liu, L. ; Wang, J. ; Wang, J. Chem. Commun. 2015, 51, 2911. -
[2]
(a) Madrid, P. B. ; Sherrill, J. ; Liou, A. P. ; Weisman, J. L. ; Derisi, J. L. ; Guy, R. K. Bioorg. Med. Chem. Lett. 2005, 15, 1015.
(b) Tardito, S. ; Barilli, A. ; Bassanetti, I. ; Tegoni, M. ; Bussolati, O. ; Franchi-Gazzola, R. ; Mucchino, C. ; Marchio, L. J. Med. Chem. 2012, 55, 10448.
(c) Abouelhassan, Y. ; Garrison, V. G. ; Burch, M. ; Wong, W. ; Norwood, V. M. ; Huigens, R. W. Bioorg. Med. Chem. Lett. 2014, 24, 5076.
(d) Vippagunta, S. R. ; Dorn, A. ; Matile, H. ; Bhattacharjee, A. K. ; Karle, J. M. ; Ellis, W. Y. ; Ridley, R. G. ; Vennerstrom, J. L. J. Med. Chem. 1999, 42, 4630.
(e) Bhat, S. ; Shim, J. S. ; Zhang, F. ; Chong, C. R. ; Liu, J. O. Org. Biomol. Chem. 2012, 10, 2979.
(f) Borchardt, R. T. J. Med. Chem. 1973, 16, 382.
(g) Liu, Y. -C. ; Wei, J. -H. ; Chen, Z. -F. ; Liu, M. ; Gu, Y. -Q. ; Huang, K. -B. ; Li, Z. -Q. ; Liang, H. Eur. J. Med. Chem. 2013, 69, 554. -
[3]
(a) Jiang, H. ; Taggart, H. ; Zhang, X. ; Benbrook, D. M. ; Lind, S. E. ; Ding, W. -Q. Cancer Lett. 2011, 312, 11.
(b) Heidary, D. K. ; Howerton, B. S. ; Glazer, E. C. J. Med. Chem. 2014, 57, 8936. -
[4]
(a) Sun, K. ; Lv, Y. ; Wang, J. ; Sun, J. ; Liu, L. ; Jia, M. ; Liu, X. ; Z. Li, Z. ; Wang, X. ; Org. Lett. 2015, 17, 4408.
(b) Wasa, M. ; Worrell, B. T. ; Yu, J. Q. Angew. Chem. , Int. Ed. 2010, 49, 1275.
(c) Tobisu, M. ; Hyodo, I. ; Chatani, N. J. Am. Chem. Soc. 2009, 131, 12070.
(d) Berman, A. M. ; Lewis, J. C. ; Bergman, R. G. ; Ellman, J. A. J. Am. Chem. Soc. 2008, 130, 14926.
(e) Zhao, D. ; Wang, W. ; Yang, F. ; Lan, J. ; Yang, L. ; Gao, G. ; You, J. Angew. Chem. , Int. Ed. 2009, 48, 3296. -
[5]
(a) Boudet, N. ; Lachs, J. R. ; Knochel, P. Org. Lett. 2007, 9, 5525.
(b) Kwak, J. ; Kim, M. ; Chang, S. J. Am. Chem. Soc. 2011, 133, 3780.
(c) Chen, Q. ; du Jourdin, X. M. ; Knochel, P. J. Am. Chem. Soc. 2013, 135, 4958.
(d) Tsai, C. -C. ; Shih, W. -C. ; Fang, C. -H. ; Li, C. -Y. ; Ong, T. -G. ; Yap, G. P. J. Am. Chem. Soc. 2010, 132, 11887. -
[6]
(a) Guo, H. ; Chen, M. ; Jiang, P. ; Chen, J. ; Pan, L. ; Wang, M. ; Xie, C. ; Zhang, Y. Tetrahedron 2015, 71, 70.
(b) Xu, J. ; Zhu, X. ; Zhou, G. ; Ying, B. ; Ye, P. ; Su, L. ; Shen, C. ; Zhang, P. Org. Biomol. Chem. 2016, 14, 3016.
(c) Liu, X. X. ; Wu, Z, Y. ; Luo, X. L. ; He, Y, Q. ; Zhou, X, Q. ; Fan, Y. X. ; Huang. G. S. RSCAdv. 2016, 6, 71485. -
[7]
(a) Gu, L.; Lu, T.; Zhang, M.; Tou, L.; Zhang, Y. Adv. Synth. Catal. 2013, 355, 1077.
(b) Nishina, Y.; Takami, K. Green Chem. 2012, 14, 2380.
(c) Nishina, Y.; Hashimoto, H.; Kimura, N.; Miyata, N.; Fujii, T.; Ohtanid, B.; Takada, J. RSC Adv. 2012, 2, 6420.
(d) Janin, Y. L. Chem. Rev. 2012, 112, 3924.
(e) Podgorsek, A.; Zupan, M.; Iskra, J. Angew. Chem., Int. Ed. 2009, 48, 8424.
(f) Zhang, J.; Hao, X.; Wang, Z.; Ren, C.; Niu, J.; Song, M. Chin. J. Org. Chem. 2017, 37, 1237(in Chinese).
(张家恒, 郝新奇, 王正龙, 任常久, 牛俊龙, 宋毛平, 有机化学, 2017, 37, 1237.)
(g) Luo, F.; Long, Y.; Li, Z.; Zhou, X. Acta Chim. Sinica 2016, 74, 805(in Chinese).
(罗飞华, 龙洋, 李正凯, 周向葛, 化学学报, 2016, 74, 805.) -
[8]
For selected example, (a) Zhang, J. ; Chen, T. ; Yang, J. ; Han, L. Chem. Commun. 2015, 51, 7540.
(b) Li, X. ; Feng, Z. ; Jiang, Z. ; Zhang, X. Org. Lett. 2015, 17, 5570.
(c) Li, K. ; Wu, Q. ; Lan, J. ; You, J. Nat. Commun. 2015, 6, 8404.
(d) Jin, L. ; Wan, L. ; Feng, J. ; Cai, C. Org. Lett. 2015, 17, 4726. -
[9]
(a) Cho, C. S. ; Lee, J. W. ; Lee, D. Y. ; Shim, S. C. ; Kim, T. J. Chem. Commun. 1996, 2115.
(b) Nogi, K. ; Fujihara, T. ; Terao, J. ; Tsuji, Y. J. Org. Chem. 2015, 80, 11618.
(c) Tjutrins, J. ; Shao, J. ; Yempally, V. ; Bengali, A. A. ; Arndtsen, B. A. Organometallics 2015, 34, 1802.
(d) Lo, W. ; Hu, C. ; Berenson, T. ; Tracer, N. ; Shlian, D. ; Khaloo, M. ; Benhaim, A. ; Jiang, J. Chem. Commun. 2015, 51, 9432.
(e) Hoshimoto, Y. ; Ohata, T. ; Sasaoka, Y. ; Ohashi, M. ; Ogoshi, S. J. Am. Chem. Soc. 2014, 136, 15877. -
[10]
(a) Ohashi, M. ; Kishizaki, O. ; Ikeda, H. ; Ogoshi, S. J. Am. Chem. Soc. 2009, 131, 9160.
(b) Sato, Y. ; Saito, N. ; Mori, M. J. Am. Chem. Soc. 2000, 122, 2371.
(c) Yeh, C. H. ; Korivi, R. P. ; Cheng, C. H. Angew. Chem. , Int. Ed. 2008, 47, 4892.
(d) Stolley, R. M. ; Duong, H. A. ; Thomas, D. R. ; Louie, J. J. Am. Chem. Soc. 2012, 134, 15154.
(e) Thakur, A. ; Facer, M. E. ; Louie, J. Angew. Chem. , Int. Ed. 2013, 52, 12161. -
[11]
Zhan, B.-B.; Liu, Y.-H.; Hu, F.; Shi, B.-F. Chem. Commun. 2016, 52, 4934. doi: 10.1039/C6CC00822D
-
[12]
(a) Hao, W. Y. ; Tian, J. ; Li, W. ; Shi, R. ; Huang, Z. ; Lei. A. W. Chem. Asian J. 2016, 11, 1664.
(b) Wan, J. -P. ; Li, Y. ; Liu, Y. Org. Chem. Front. 2016, 3, 768.
(c) Liu, Y. ; Huang, M. ; Wei, L. Asian J. Org. Chem. 2017, 6, 41. -
[13]
He, Y.; Zhao, N.; Qiu, L.; Zhang, X.; Fan, X. Org. Lett. 2016, 18, 6054. doi: 10.1021/acs.orglett.6b02998
-
[1]
-
Table 1. Screening of reaction conditions for dibromination of quinolinesa

Entry Catalyst Oxidant Base Solvent Yield/% of 3a/2a 1 NiCl2 O2 — MeCN 55/30 2 NiBr2 O2 — MeCN 87/— 3 Ni(acac)2 O2 — MeCN 60/20 4 Ni(NO3)2 O2 — MeCN 57/33 5 — O2 — MeCN —/85 6 NiBr2 BQ — MeCN 55/27 7 NiBr2 TBHP — MeCN 65/25 8 NiBr2 Ag2O — MeCN 60/30 9 NiBr2 O2 Cs2CO3 MeCN 30/54 10 NiBr2 O2 Na2CO3 MeCN 28/60 11 NiBr2 O2 NaHCO3 MeCN 33/59 12 NiBr2 O2 — Toluene 55/35 13 NiBr2 O2 — PET —/25 14 NiBr2 O2 — DCM —/82 15 NiBr2 O2 — DCE 15/45 16 b NiBr2 O2 — MeCN 84/15 17 c NiBr2 O2 — MeCN 87/12 18 d NiBr2 O2 — MeCN 85/14 a Reaction conditions: 1a (0.25 mmol), NiBr2 (20 mol%), NBS (2.0 equiv.), MeCN (2.0 mL), stirred at 120 ℃, under O2, overnight, isolated yields. b The reaction temperature is 100 ℃. c The reaction temperature is 110 ℃. d The reaction temperature is 130 ℃. Table 2. Substrate scope of the dibromination and dichlorination reactiona

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 1649
- HTML全文浏览量: 200


 下载:
下载:




 下载:
下载:

