
Citation: Yang Quanli, Song Yuye, Yu Ping, Wang Long, Liu Mingguo, Huang Nianyu. 1, 8-Diazabicyclo[5.4.0]undec-7-ene (DBU)-Promoted Stere-oselective Synthesis of Oxazolidin-2-(thi)one Derivatives[J]. Chinese Journal of Organic Chemistry, 2017, 37(12): 3177-3185. doi: 10.6023/cjoc201704036

1, 8-二氮杂二环十一碳-7-烯(DBU)立体选择性催化合成噁唑啉-2-(硫)酮衍生物
-
关键词:
- 噁唑啉-2-(硫)酮
- / 1, 8-二氮杂二环十一碳-7-烯
- / 立体选择性
English
1, 8-Diazabicyclo[5.4.0]undec-7-ene (DBU)-Promoted Stere-oselective Synthesis of Oxazolidin-2-(thi)one Derivatives
-
噁唑啉酮作为一类非常重要的含氮杂环化合物, 因其独特的作用机制、口服生物利用度以及良好的构效关系(SAR)[1]而在农药、医药等精细化学品[2]和聚合物材料[3]中广泛应用.其中, 噁唑啉-2-酮衍生物代表了一类重要的合成试剂[4]和活性药效团[5], 例如商品化的Evans手性助剂因其良好的配位和手性诱导性能, 在形成新的碳-碳键、碳-杂原子键及光学活性的天然产物中广泛使用[6]; 此外, 在抗生素类药物Linezolid[7]和Tedizolid[8]中均含有该结构单元, 可选择性作用于真菌蛋白合成酶而发挥抗菌作用, 并与其它抗菌药无交叉耐药现象.
噁唑啉酮的合成方法研究是有机合成领域的研究热点之一, 目前有多种合成该母核的方法[9].例如, 金、钯或银等过渡金属[10]可催化炔丙基胺或醇和CO2发生分子间环化得到噁唑啉酮; 碳酸酯与氨基醇的反应[11]也可以用于构建噁唑啉酮; 卤化酮、醋酸银等过渡金属盐[12]亦可催化异氰酸酯和炔丙醇环化生成噁唑啉酮.为进一步拓展杂环化合物的合成方法[13], 本文报道了在无金属催化条件, 1, 8-二氮杂二环十一碳-7-烯(DBU)催化促进炔丙醇与异(硫)氰酸酯发生环化反应, 高收率立体选择性地合成了一系列(Z)-4-芳亚甲基取代噁唑啉-2-(硫)酮衍生物, 丰富了噁唑啉酮骨架的制备方法.
1 结果与讨论
为筛选噁唑啉-2-(硫)酮衍生物的最佳合成条件, 本工作首先通过苯乙炔(1a)和3-戊酮(2a)制备得了炔丙醇中间体3a, 将其作为模型反应底物与4-甲基苯基异氰酸酯(4a)进行条件实验(Eq. 1), 以筛选合适的催化剂、溶剂和反应温度及时间(表 1).
Entry Catalysta Solvent Temp./ ℃ Time/ h Yieldb/% Carbamate ester 5a 1 AgNO3 Toluene 30 24 0 0 2 PdCl2 CH3CN 30 24 0 0 3 CuI CH3CN 30 24 0 0 4 I2 Toluene 30 24 0 0 5 DMAP DMF 50 24 80 0 6 DBU CH2Cl2 30 12 70 30 7 DBU CH2Cl2 30 24 5 85 8 DBU Toluene 30 24 0 0 9 DBU CHCl3 30 24 90 0 10 DBU Et2O 30 24 0 0 11 DBU EtOAc 30 24 0 0 12 DBU CH3OH 30 24 0 0 13 DBU CH3CN 30 12 0 90 14 K2CO3 CH3CN 30 24 0 40 15 Morpholine CH3CN 30 24 0 0 16 Et3N CH3CN 30 24 0 30 17 NaOH CH3CN 30 24 0 0 18 DBUc CH3CN 30 24 0 60 19 DBUd CH3CN 30 12 0 90 20 DBUe CH3CN 30 12 0 80 21 DBU CH3CN 40 8 0 90 22 DBU CH3CN 50 8 0 60 23 DBU CH3CN 20 24 40 60 aGeneral conditions: catalyst (20 mol%) was added into the mixture of 3a (95 mg, 0.5 mmol) and 4a (67 mg, 0.5 mmol) in corresponding solvent (2.0 mL). b Isolated yield. c10 mol% of DBU was used. d30 mol% of DBU was used. e40 mol% of DBU was used. 首先考察了文献报道的催化剂如AgNO3[12b]、PdCl2[14]、CuI[15]等的催化效果, 但经24 h反应后没有观察到氨基甲酸酯中间体或产物(表 1, Entries 1~4).当使用4-二甲基氨基吡啶(DMAP)时[12b], 将温度加热至50 ℃发现氨基甲酸酯中间体是唯一的产物(Entry 5).有文献[12c]报道DBU可以促进炔丙醇和苯基异氰酸酯在二氯甲烷回流条件下的反应, 经证实该反应在30 ℃下可进行, 但反应12 h后仅得到30%的环化产物5a (Entry 6);当延长时间至24 h后环化产物产率可以达到85% (Entry 7);采用甲苯、氯仿、乙醚、乙酸乙酯或甲醇为溶剂时, 该反应却不能进行(Entries 8~12);而当溶剂为乙腈时, 12 h后环化产率可达到90% (Entry 13).以乙腈为溶剂时, K2CO3和Et3N的催化效果较差, 其它有机或无机碱[28]对该反应没有催化作用(Entries 14~17).鉴于此, DBU被确定为最合适的催化剂, 接下来我们对其用量进行了筛选.减少DBU的用量到10 mol%后, 反应24 h后5a的收率仅为60% (Entry 18); 30 mol% DBU的催化效果与20 mol%用量时相同(Entries 13, 19);增加到40 mol%用量时产物的收率降到80% (Entry 20);因此20 mol% DBU被确定为最佳用量.通过对反应温度的筛选发现, 当升高温度至40 ℃时产物收率与30 ℃时相当, 但反应时间可由12 h缩短至8 h (Entries 13, 21);进一步升高反应温度至50 ℃时产物收率明显降低(Entry 20), 可能为高温下反应过于剧烈所致; 反应温度为20 ℃时, 24 h后仍有较多中间体存在, 反应不完全(Entry 23).综上所述, 该模型反应的最佳条件确定为:催化剂DBU的用量为20 mol%, 使用乙腈作溶剂, 在40 ℃下反应8 h.
鉴于此, 我们根据模型反应的最佳条件制备了25个(Z)-4-芳亚甲基取代的噁唑啉-2-(硫)酮衍生物(Scheme 1), 并且该反应表现出良好的立体选择性和官能团兼容性(表 2).所合成的目标化合物都通过核磁共振(NMR)、红外光谱(IR)和高分辨质谱(HR-ESI-MS)表征.在 1H NMR光谱中, 5i和5j的噁唑啉-2-酮骨架的环外双键的烯烃质子信号分别位于δ 4.36和4.24, 而其余化合物的环外烯基质子在δ 5.53~5.69处出现单峰.在13C NMR中, 化合物噁唑啉-2-酮5a~5q的羰基碳化学位移在δ 153.9~157.2处, 而噁唑啉-2-硫酮5r~5t的羰基碳在δ 187.6~188.9处出现共振信号, 其中化合物5s还通过了单晶X射线衍射分析进行了进一步确证(CCDC号码1545013), 其晶体结构见图 1.在红外光谱中, 目标化合物中羰基的强红外吸收峰位于1745~1777 cm-1处.在HRMS谱中, 所有化合物都可以观察到(M+H)+或(M+Na)+的离子加合物离子峰.
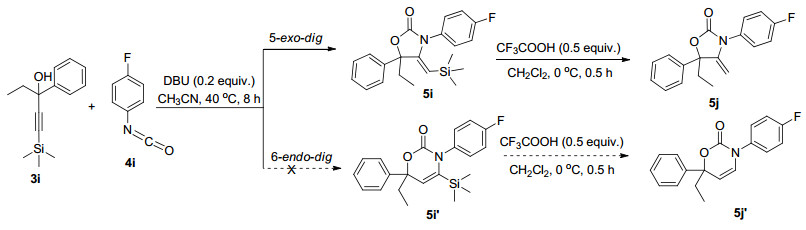
Entry Product Substitution Isolated yield/% R1 R2 R3 R4 X 1 5a C6H5 Me Et p-MeC6H4 O 91 2 5b C6H5 Et Et p-MeC6H4 O 90 3 5c C6H5 Me i-Pr p-MeC6H4 O 86 4 5d C6H5 Me i-Bu p-MeC6H4 O 85 5 5e C6H5 Me Cyclopropyl p-MeC6H4 O 80 6 5f p-MeC6H4 Cyclopentyl p-MeC6H4 O 78 7 5g C6H5 Me i-Bu p-FC6H4 O 87 8 5h C6H5 Me Cyclopropyl p-FC6H4 O 86 9 5i TMS Et C6H5 p-FC6H4 O 75 10 5j H Et C6H5 p-FC6H4 O 91 11 5k C6H5 Et Et Naphthyl O 85 12 5l C6H5 Et Et i-Pr O 87 13 5m C6H5 Me i-Bu i-Pr O 86 14 5n C6H5 Me Cyclopropyl i-Pr O 87 15 5o C6H5 Cyclopentyl i-Pr O 88 16 5p C6H5 Et Et Et O 86 17 5q C6H5 Me Cyclopropyl Et O 84 18 5r C6H5 Et Et C6H5 S 83 19 5s C6H5 Et Et Et S 87 20 5t C6H5 Me i-Bu C6H5 S 80 21 5u p-FC6H4 Me Et p-MeC6H4 O 90 22 5v p-MeOC6H4 Me Et p-MeC6H4 O 80 23 5w p-CNC6H4 Et Et p-MeC6H4 O 91 24 5x n-Bu Et Et p-MeC6H4 O 65 25 5y Cyclopropyl Et Et p-MeC6H4 O 62 为进一步研究化合物的光谱性质和环化机理, 使用三甲基甲硅烷基保护的炔丙醇(3i)在DBU的催化作用下与4-氟苯基异氰酸酯(4i)反应(Scheme 2), 然后脱去三甲硅烷基保护基后分离反应产物, 经鉴定产物为五元环噁唑啉-2-酮骨架, 从其13C NMR谱中没有观察到经6-endo-dig机制得到的六元环产物(5j')的特征信号峰, 而在化合物5j的DEPT-135光谱得到末端烯键的特征信号δ 84.0, HSQC谱分析表明该碳分别与δ 4.25 (d, J=3.0 Hz, 1H)和4.23 (d, J=3.0 Hz, 1H)处的质子相关.因此可以证明化合物5i和5j的噁唑啉-2-酮的骨架均通过5-exo-dig环化机制[16]得到.
经文献调研[17], DBU促进亲核加成/环化反应的可能机理如Scheme 3所示.首先, 将炔丙醇中间体3经DBU去质子化得到烷氧基阴离子A, 再亲核进攻异氰酸酯4得到氨基甲酸酯中间体B; 然后氨基甲酸酯B中的N作为亲核中心与DBU活化的炔键加成, 经5-exo-dig环化机制得到DBU复合物C; 最后, DBU离去得到相应的目标化合物噁唑啉-2-(硫)酮衍生物5.
2 结论
在无金属催化剂的条件下, 通过DBU促进炔醇与异氰酸酯亲核加成/5-exo-dig环化合成了一系列(Z)-4-芳亚甲基取代的噁唑啉-2-(硫)酮衍生物, 该反应具有高度立体选择性、反应条件温和、后处理简单及产率高等优点, 适合于快速构建化合物库并用于生物活性筛选.由于其无金属性质, 该反应亦可满足生物和药物化学的特定纯度要求, 其在活性天然产物结构修饰中的应用已在本实验室展开.
3 实验部分
3.1 仪器与试剂
核磁共振谱(NMR)用Bruker AVANCE Ⅲ 400 MHz Plus NMR光谱仪测试, 使用TMS为内标, CDCl3为溶剂.质谱(MS)使用MALDI TOF/TOF 5800测量.红外辐射光谱(IR)在PE-983红外光谱仪上记录, 吸收值为cm-1.熔点由X-4数字显示显微熔点测定仪测定, 温度计未校正.减压蒸发溶剂使用EYELA SB-1100旋转蒸发仪在50 ℃下进行.
所有试剂均为分析纯, 乙腈、二氯甲烷和乙酸乙酯用CaCl2干燥并蒸馏后使用, 四氢呋喃用钠丝干燥并在氮气氛下蒸馏后使用.
3.2 实验方法
3.2.1 炔丙醇3的合成
在-78 ℃及氩气氛的条件下, 将正丁基锂(2.0 mol•L-1, n-C6H14, 3.0 mL)缓慢滴加到炔(5.0 mmol)的无水四氢呋喃(THF) (20.0 mL)溶液中. 30 min后, 加入醛或酮2 (5.0 mmol), 将混合物温热至室温, 搅拌2 h.反应完成后, 加入饱和NaHCO3水溶液淬灭反应, 并用乙酸乙酯萃取有机相.将合并的有机萃取物用盐水洗涤, 用无水Na2SO4干燥并真空浓缩, 通过柱色谱纯化, 得到炔丙醇3.
1-(苯基乙炔基)环戊烷基-1-醇(3a)[18a]:无色油状, 产率90%. 1H NMR (CDCl3, 400 MHz) δ: 7.43~7.41 (m, 2H), 7.31~7.28 (m, 3H), 2.08~2.02 (m, 4H), 1.95 (s, 1H), 1.89~1.80 (m, 2H), 1.79~1.63 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ: 131.6, 128.3, 128.2, 122.9, 92.9, 83.1, 74.9, 42.5, 23.5.
3-乙基-1-苯基-1-炔-3-戊醇(3b)[18b]:无色油状, 产率92%. 1H NMR (CDCl3, 400 MHz) δ: 7.44~7.41 (m, 2H), 7.31~7.29 (m, 3H), 2.04 (s, 1H), 1.82~1.71 (m, 4H), 1.16 (t, J=7.4 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 131.7, 128.3, 128.2, 122.9, 91.7, 84.5, 72.6, 34.5, 8.7.
2-环丙基-4-苯基-3-炔-2-丁醇(3c)[18c]:无色油状, 产率91%. 1H NMR (CDCl3, 400 MHz) δ: 7.41~7.37 (m, 2H), 7.30~7.28 (m, 3H), 2.23 (s, 1H), 1.67 (s, 3H), 1.24~1.21 (m, 1H), 0.56~0.53 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 131.7, 128.3, 128.2, 122.5, 89.9, 83.8, 70.5, 29.8, 22.1, 2.6, 1.7.
3, 4-二甲基-1-苯基-1-炔-3-戊醇(3d):白色固体, 产率90%. m.p. 42~43 ℃(文献值[18d]: 42~43 ℃); 1H NMR (CDCl3, 400 MHz) δ: 7.43~7.41 (m, 2H), 7.30~7.29 (m, 3H), 2.05 (s, 1H), 1.93~1.87 (m, 1H), 1.54 (s, 3H), 1.11~1.06 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 131.7, 128.3, 128.2, 122.9, 92.0, 84.0, 72.1, 39.1, 27.2, 18.0, 17.5.
3-甲基-1-苯基-1-炔-3-戊醇(3e):无色油状物, 产率94%. b.p. 151~153 ℃(文献值[18d]: 151~154 ℃); 1H NMR (CDCl3, 400 MHz) δ: 7.43~7.41 (m, 2H), 7.30~7.29 (m, 3H), 2.07 (s, 1H), 1.82~1.76 (m, 2H), 1.57 (s, 3H), 1.11 (t, J=7.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 131.6, 128.3, 128.2, 122.8, 92.6, 83.4, 69.1, 36.6, 29.3, 9.1.
3, 5-二甲基-1-苯基-1-炔-3-己醇(3f)[18c]:无色油状, 产率93%. 1H NMR (CDCl3, 400 MHz) δ: 7.42~7.40 (m, 2H), 7.31~7.29 (m, 3H), 2.05~1.97 (m, 2H), 1.70 (s, 1H), 1.68 (s, 1H), 1.58 (s, 3H), 1.07~1.05 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 131.5, 128.3, 128.2, 122.9, 93.3, 83.5, 68.5, 51.9, 30.9, 25.2, 24.3, 24.1.
3-苯基-1-三甲基硅烷-1-炔-3-戊醇(3g)[18e]:无色油状, 产率84%. 1H NMR (CDCl3, 400 MHz) δ: 7.62~7.60 (m, 2H), 7.37~7.34 (m, 2H), 7.30~7.28 (m, 1H), 2.37 (s, 1H), 1.97~1.88 (m, 2H), 0.94 (t, J=7.4 Hz, 3H), 0.22 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 144.2, 128.1, 127.6, 125.5, 107.6, 90.7, 74.2, 38.3, 9.1, 0.0.
1-(4-甲基苯基乙炔基)环戊烷基-1-醇(3h):白色固体, 产率95%. m.p. 55~57 ℃(文献值[18f]: 55~57 ℃); 1H NMR (CDCl3, 400 MHz) δ: 7.32~7.30 (m, 2H), 7.11~7.09 (m, 2H), 2.34 (s, 3H), 2.07~2.01 (m, 4H), 1.92 (s, 1H), 1.89~1.76 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 138.2, 131.5, 129.0, 119.8, 92.1, 83.2, 74.9, 42.5, 23.5, 21.4.
1-(4-氟苯基)-3-甲基-1-炔-3-戊醇(3i):无色油状, 产率90%. 1H NMR (CDCl3, 400 MHz) δ: 7.41~7.37 (m, 2H), 7.01~6.97 (m, 2H), 2.15 (s, 1H), 1.79~1.77 (m, 2H), 1.56 (s, 3H), 1.10 (t, J=7.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 163.7, 161.2, 133.6, 133.5, 118.9, 118.8, 115.6, 155.4, 92.3, 82.3, 69.1, 36.6, 29.3, 9.1; IR (KBr) ν: 3406, 2973, 2366, 1603, 1509, 1463, 1369, 1232, 1152, 832 cm-1; HRMS (ESI) calcd for C12H13FONa [M+Na]+ 215.0848, found 215.0849.
3-乙基-1-(4-氰基苯基)-1-炔-3-戊醇(3j):无色油状, 产率90%. 1H NMR (CDCl3, 400 MHz) δ: 7.61~7.59 (m, 2H), 7.51~7.49 (m, 2H), 2.01 (s, 1H), 1.80~1.75 (m, 4H), 1.10 (t, J=7.4 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 132.2, 132.0, 127.8, 118.4, 111.6, 96.3, 82.9, 72.6, 34.3, 8.6; IR (KBr) ν: 3446, 2986, 2373, 2280, 1606, 1496, 1456, 1409, 1279, 1142, 836 cm-1; HRMS (ESI) calcd for C14H15NONa [M+Na]+ 236.1051, found 236.1044.
4-(4-甲氧基苯基)-2-甲基-3-炔-2-丁醇(3k):白色固体, 产率94%. m.p. 175~176 ℃(文献值:[18d] 175~176 ℃); 1H NMR (CDCl3, 400 MHz) δ: 7.36~7.33 (m, 2H), 6.84~6.81(m, 2H), 3.79 (s, 3H), 2.23 (s, 1H), 1.79~1.76 (m, 2H), 1.55 (s, 3H), 1.10 (t, J=7.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 159.5, 133.1, 114.9, 113.8, 91.2, 83.2, 69.2, 55.3, 36.7, 29.4, 9.1.
3-乙基-4-炔-3-壬醇(3l)[18g]:无色油状, 产率87%. 1H NMR (CDCl3, 400 MHz) δ: 2.22~2.19 (m, 2H), 1.68~1.60 (m, 4H), 1.58~1.38 (m, 4H), 1.01 (t, J=6.8 Hz, 6H), 0.91 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 84.8, 82.5, 72.2, 34.6, 30.9, 21.9, 18.3, 13.6, 8.6.
1-环丙基-3-乙基-1-炔-3-戊醇(3m):无色油状, 产率86%. 1H NMR (CDCl3, 400 MHz) δ: 1.81 (s, 1H), 1.65~1.59 (m, 4H), 1.26~1.23 (m, 1H), 1.01 (t, J=7.4 Hz, 6H), 0.98~0.74 (m, 2H), 0.67~0.64 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ: 88.0, 77.7, 72.1, 34.5, 8.5, 8.3, 0.6; IR (KBr) ν: 3453, 2920, 1739, 1549, 1509, 1463, 1082, 809, 669 cm-1; HRMS (ESI) calcd for C10H17O [M+H]+ 153.1279, found 153.1272.
3.2.2 噁唑啉酮衍生物5的合成
在氮气氛的条件下, 将异氰酸酯或异硫氰酸酯4 (1.0 mmol)加入到炔丙醇3 (1.0 mmol)和无水乙腈(5.0 mL)混合液中, 然后滴加DBU (30.5 μL, 0.2 mmol), 并在30 ℃下搅拌24 h.然后将反应用水(15.0 mL)淬灭, EtOAc (10.0 mL×3)萃取, 将合并的有机层用盐水洗涤, 用Na2SO4干燥.除去溶剂后, 残余物通过1H NMR分析E/Z选择性.通过柱色谱进一步纯化(洗脱液:己烷/ EtOAc, V:V=50:1), 得到相应的噁唑啉酮衍生物5.
(Z)-4-芳亚甲基-5-乙基-5-甲基-3-(对甲苯基)噁唑啉-2-酮(5a):白色固体, 产率91%. m.p. 101~103 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.90~6.82 (m, 7H), 6.67~6.65 (m, 2H), 5.53 (s, 1H), 2.19 (s, 3H), 2.07~1.98 (m, 1H), 1.95~1.85 (m, 1H), 1.68 (s, 3H), 1.08 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.7, 141.2, 136.9, 133.4, 132.4, 128.7, 128.4, 126.9, 125.7, 125.5, 99.4, 85.4, 34.2, 26.9, 20.8, 7.5; IR (KBr) ν: 3413, 2927, 1777, 1661, 1511, 1319, 1229, 1130, 809, 701 cm-1; HRMS (ESI) calcd for C20H21NO2Na [M+Na]+ 330.1473, found 330.1470.
(Z)-4-芳亚甲基-5, 5-二乙基-3-(对甲苯基)噁唑啉-2-酮(5b):白色固体, 产率90%. m.p. 71~73 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.92~6.81 (m, 7H, ), 6.68~6.65 (m, 2H), 5.50 (s, 1H), 2.19 (s, 3H), 2.09~2.00 (m, 2H), 1.88~1.80 (m, 2H), 1.09 (t, J=7.2 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 156.2, 139.4, 137.0, 133.5, 132.4, 128.7, 128.4, 126.9, 125.8, 125.4, 99.3, 88.3, 33.1, 20.8, 7.2; IR (KBr) ν: 3436, 2965, 1754, 1665, 1344, 1232, 1123, 950, 701, 624 cm-1; HRMS (ESI) calcd for C21H24NO2 [M+ H]+ 322.1807, found 322.1810.
(Z)-4-芳亚甲基-5-异丙基-5-甲基-3-(对甲苯基)噁唑啉-2-酮(5c):白色固体, 产率86%. m.p. 93~95 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.90~6.81 (m, 7H), 6.67~6.64 (m, 2H), 5.55 (s, 1H), 2.18 (s, 3H), 2.10~2.03 (m, 1H), 1.85~1.79 (m, 1H), 1.68 (s, 3H), 1.16 (d, J=6.8 Hz, 3H), 1.12 (d, J=6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 156.0, 141.6, 136.9, 133.5, 132.3, 128.7, 128.4, 126.9, 125.6, 125.4, 99.5, 87.3, 38.0, 25.2, 20.8, 16.5, 16.4; IR (KBr) ν: 3445, 2971, 1761, 1665, 1514, 1399, 1251, 1136, 1030, 816, 704 cm-1; HRMS (ESI) calcd for C21H23NO2Na [M+Na]+ 344.1626, found 344.1623.
(Z)-4-芳亚甲基-5-异丁基-5-甲基-3-(对甲苯基)噁唑啉-2-酮(5d):白色固体, 产率85%. m.p. 97~99 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.90~6.82 (m, 7H), 6.66~6.64 (m, 2H), 5.53 (s, 1H), 2.19 (s, 3H), 2.04~1.90 (m, 2H), 1.85~1.81 (m, 1H), 1.68 (s, 3H), 1.06 (d, J=6.4 Hz, 3H), 1.04 (d, J=6.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 155.6, 141.9, 136.9, 133.4, 132.3, 128.7, 128.3, 126.9, 125.7, 125.4, 99.5, 85.4, 49.2, 28.1, 24.4, 24.1, 20.9; IR (KBr) ν: 3439, 2962, 1754, 1677, 1514, 1412, 1309, 1219, 1130, 819, 707 cm-1; HRMS (ESI) calcd for C22H25NO2Na [M+Na]+ 358.1783, found 358.1781.
(Z)-4-芳亚甲基-5-环丙基-5-甲基-3-(对甲苯基)噁唑啉-2-酮(5e):白色固体, 产率80%. m.p. 133~135 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.92~6.82 (m, 7H), 6.69~6.66 (m, 2H), 5.63 (s, 1H), 2.18 (s, 3H), 1.59 (s, 3H), 1.39~1.32 (m, 1H), 0.66~0.56 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 155.6, 141.4, 136.8, 133.3, 132.3, 128.7, 128.4, 126.9, 125.5, 125.5, 100.4, 84.0, 25.7, 20.8, 20.6, 1.4, 0.7; IR (KBr) ν: 3449, 3019, 1767, 1671, 1508, 1399, 1255, 1123, 816, 707 cm-1; HRMS (ESI) calcd for C21H22NO2 [M+H]+ 320.1651, found 320.1647.
(Z)-4-(4-甲基芳亚甲基)-3-(对甲苯基)-1-氧杂-3-氮杂螺[4.4]壬-2-酮(5f):白色固体, 产率78%. m.p. 112~114 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.89~6.83 (m, 4H), 6.67~6.64 (m, 2H), 6.54~6.51 (m, 2H), 5.58 (s, 1H), 2.35~2.30 (m, 2H), 2.20 (s, 3H), 2.15 (s, 3H), 2.08~1.91 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ: 155.7, 140.5, 136.7, 135.2, 132.4, 130.4, 128.6, 128.2, 127.6, 125.8, 99.3, 93.1, 41.4, 24.4, 20.9, 20.8; IR (KBr) ν: 3413, 2965, 1764, 1665, 1514, 1344, 1255, 1101, 982, 835 cm-1; HRMS (ESI) calcd for C22H23NO2Na [M+Na]+ 356.1626, found 356.1627.
(Z)-4-芳亚甲基-3-(4-氟苯基)-5-异丁基-5-甲基噁唑啉-2-酮(5g):白色固体, 产率87%. m.p. 59~61 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.97~6.87 (m, 5H), 6.75~6.69 (m, 2H), 6.67~6.64 (m, 2H), 5.58 (s, 1H), 2.05~1.91 (m, 2H), 1.86~1.79 (m, 1H), 1.69 (s, 3H), 1.06 (d, J=6.4 Hz, 3H), 1.04 (d, J=6.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 162.3, 159.9, 155.4, 141.8, 133.2, 131.7, 130.9, 130.9, 128.3, 128.0, 127.7, 127.6, 127.2, 125.9, 115.1, 114.9, 99.8, 85.6, 49.2, 28.1, 24.4, 24.1; IR (KBr) ν: 3429, 2955, 1777, 1668, 1501, 1309, 1232, 1130, 829, 707 cm-1; HRMS (ESI) calcd for C21H22FNO2Na [M+Na]+ 362.1532, found 362.1533.
(Z)-4-芳亚甲基-5-环丙基-3-(4-氟苯基)-5-甲基噁唑啉-2-酮(5h):白色固体, 产率86%. m.p. 80~82 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.98~6.88 (m, 5H), 6.75~6.67 (m, 4H), 5.68 (s, 1H), 1.71 (s, 3H), 1.40~1.32 (m, 1H), 0.66~0.60 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 162.3, 169.9, 155.3, 141.5, 133.2, 131.0, 128.4, 127.6, 127.5, 127.2, 126.0, 115.1, 114.9, 100.6, 84.2, 25.7, 20.7, 1.4, 0.8; IR (KBr) ν: 3442, 3019, 1770, 1668, 1511, 1399, 1258, 1130, 1034, 835, 701 cm-1; HRMS (ESI) calcd for C20H18FNO2Na [M+Na]+ 346.1219, found 346.1220.
(Z)-5-乙基-3-(4-氟苯基)-5-苯基-4-((三甲基甲硅烷基)亚甲基)噁唑啉-2-酮(5i):无色油状, 产率86%. 1H NMR (CDCl3, 400 MHz) δ: 7.54~7.51 (m, 2H), 7.42~7.40 (m, 3H), 7.16~7.14 (m, 2H), 7.13~7.11 (m, 2H), 4.35 (s, 1H), 2.36~2.31 (m, 1H), 2.23~2.17 (m, 1H), 1.06 (t, J=8.0 Hz, 3H), -0.30 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 163.5, 161.1, 155.8, 153.0, 140.8, 130.0, 128.7, 128.3, 124.9, 116.7, 116.5, 96.4, 89.3, 33.1, 7.8, 0.3; IR (KBr) ν: 3442, 2968, 1777, 1636, 1514, 1514, 1226, 1136, 835, 752, 697 cm-1; HRMS (ESI) calcd for C21H25FNO2Si [M+H]+ 370.1639, found 370.1781.
(Z)-5-乙基-3-(4-氟苯基)-4-亚甲基-5-苯基噁唑啉-2-酮(5j):无色油状, 产率91%. 1H NMR (CDCl3, 400 MHz) δ: 7.57~7.54 (m, 2H), 7.44~7.37 (m, 3H), 7.31~7.27 (m, 2H), 7.18~7.13 (m, 2H), 4.25 (d, J=3.0 Hz, 1H), 4.23 (d, J=3.0 Hz, 1H), 2.38~2.29 (m, 1H), 2.25~2.16 (m, 1H), 1.07 (t, J=7.3 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 163.3, 160.9, 154.7, 149.2, 140.6, 129.8, 129.2, 129.1, 128.7, 128.4, 124.7, 116.7, 116.5, 87.8, 84.0, 33.6, 7.7; IR (KBr) ν: 3436, 2978, 1681, 1463, 1383, 1277, 1136, 1079, 1027, 749, 701 cm-1; HRMS (ESI) calcd for C18H17FNO2 [M+H]+ 298.1243, found 298.1241.
(Z)-4-芳亚甲基-5, 5-二乙基-3-(萘-1-基)噁唑啉-2-酮(5k):白色固体, 产率85%. m.p. 123~125 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.80 (d, J=8.4 Hz, 1H), 7.72 (d, J=8.1 Hz, 1H), 7.58~7.51 (m, 2H), 7.47~7.43 (m, 1H), 7.08~7.01 (m, 2H), 6.64 (t, J=7.4 Hz, 1H), 6.47 (t, J=7.7 Hz, 2H), 6.39 (d, J=7.4 Hz, 2H), 5.52 (s, 1H), 2.19~2.10 (m, 2H), 1.98~1.89 (m, 2H), 1.29 (t, J=7.3 Hz, 3H), 1.22 (t, J=7.3 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 156.1, 134.0, 133.0, 128.8, 128.1, 127.9, 126.9, 126.4, 126.1, 125.7, 125.5, 124.7, 122.4, 99.4, 88.8, 33.4, 33.3, 7.7, 7.3; IR (KBr) ν: 3420, 2959, 1761, 1684, 1565, 1405, 1239, 1143, 777, 954 cm-1; HRMS (ESI) calcd for C24H23NO2Na [M+Na]+ 380.1626, found 380.1631.
(Z)-4-芳亚甲基-5, 5-二乙基-3-异丙基噁唑啉-2-酮(5l):白色固体, 产率87%. m.p. 69~71 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.35~7.30 (m, 2H), 7.27~7.18 (m, 3H), 5.32 (s, 1H), 3.74~3.68 (m, 1H), 1.92~1.87 (m, 2H), 1.70~1.62 (m, 2H), 1.25 (d, J=6.8 Hz, 6H), 0.98 (t, J=7.2 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 156.1, 140.7, 135.4, 128.8, 128.1, 126.7, 97.0, 87.3, 47.1, 33.1, 18.8, 7.1. IR (KBr) ν: 3436, 2968, 1758, 1671, 1456, 1408, 1328, 1280, 1030, 758 cm-1; HRMS (ESI) calcd for C17H23NO2Na [M+Na]+ 296.1626, found 296.1625.
(Z)-4-芳亚甲基-5-异丁基-3-异丙基-5-甲基噁唑啉-2-酮(5m):无色油状, 产率87%. 1H NMR (CDCl3, 400 MHz) δ: 7.45~7.42 (m, 2H), 7.29~7.28 (m, 3H), 4.45 (s, 1H), 3.81 (d, J=6.1 Hz, 1H), 2.05~2.00 (m, 2H), 1.80 (s, 3H), 1.67 (s, 1H), 1.15 (d, J=6.5 Hz, 6H, ), 1.04 (d, J=6.3 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 153.98, 131.75, 128.14, 128.10, 122.93, 90.58, 84.53, 77.20, 75.54, 49.67, 42.72, 27.87, 24.99, 24.16, 23.89, 23.03; IR (KBr) ν: 3327, 2962, 1687, 1527, 1463, 1248, 1139, 1066, 765 cm-1; HRMS (ESI) calcd for C18H25NO2Na [M+Na]+ 310.1783, found 310.1777.
(Z)-4-芳亚甲基-5-环丙基-3-异丙基-5-甲基噁唑啉-2-酮(5n):白色固体, 产率87%. m.p. 95~97 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.35~7.30 (m, 2H), 7.26~7.19 (m, 3H), 5.47 (s, 1H), 3.69~3.61 (m, 1H), 1.54 (s, 3H), 1.27~1.15 (m, 7H, CH), 0.56~0.40 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 155.7, 143.5, 135.3, 128.8, 128.1, 126.7, 98.2, 82.8, 47.2, 25.8, 20.6, 18.8, 18.6, 1.2, 0.5; IR (KBr) ν: 3442, 2975, 1745, 1668, 1450, 1376, 1293, 1024, 694 cm-1; HRMS (ESI) calcd for C17H21NO2Na [M+Na]+ 294.1470, found 294.1464.
(Z)-4-芳亚甲基-3-异丙基-1-氧杂-3-氮杂螺[4.4]壬-2-酮(5o):白色固体, 产率88%. m.p. 119~121 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.34~7.30 (m, 2H), 7.26~7.18 (m, 3H), 5.44 (s, 1H), 3.74~3.67 (m, 1H), 2.18~2.17 (m, 2H), 1.92~1.84 (m, 6H), 1.27 (d, J=6.8 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 155.7, 143.0, 135.2, 128.8, 128.1, 126.7, 97.3, 92.2, 47.3, 41.4, 24.4, 18.8. IR (KBr) ν: 3423, 2971, 1751, 1674, 1408, 1290, 1107, 1030, 701, 646 cm-1; HRMS (ESI) calcd for C17H21NO2Na [M+Na]+ 294.1470, found 294.1472.
(Z)-4-芳亚甲基-3, 5, 5-三乙基噁唑啉-2-酮(5p):白色固体, 产率86%. m.p. 76~78 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.34~7.31 (m, 2H), 7.27~7.23 (m, 1H), 7.21~7.19 (m, 2H), 5.42 (s, 1H), 3.39 (q, J=7.1 Hz, 2H), 1.98~1.88 (m, 2H), 1.77~1.66 (m, 2H), 0.91 (t, J=7.4 Hz, 6H), 0.74 (t, J=7.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 157.2, 139.4, 134.9, 131.6, 129.5, 128.2, 128.1, 127.9, 126.8, 97.7, 87.6, 38.0, 33.0, 12.3, 7.1; IR (KBr) ν: 3481, 2975, 1748, 1674, 1456, 1360, 1264, 1197, 1050, 950, 713 cm-1; HRMS (ESI) calcd for C16H22NO2 [M+H]+ 260.1651, found 260.1649.
(Z)-4-芳亚甲基-5-环丙基-3-乙基-5-甲基噁唑啉-2-酮(5q):白色固体, 产率84%. m.p. 74~76 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.34~7.30 (m, 3H), 7.25~7.19 (m, 2H), 5.56 (s, 1H), 3.42~3.29 (m, 2H), 1.57 (s, 3H), 1.32~1.00 (m, 2H), 0.74 (t, J=7.1 Hz, 3H), 0.63~0.48 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 156.71, 142.05, 134.89, 130.10, 129.47, 128.07, 127.95, 126.90, 99.65, 83.28, 38.03, 25.94, 20.61, 12.38, 1.37, 0.64; IR (KBr) ν: 3439, 2975, 1745, 1674, 1674, 1364, 1059, 720, 633 cm-1; HRMS (ESI) calcd for C16H19NO2Na [M+Na]+ 280.1313, found 280.1314.
(Z)-4-芳亚甲基-5, 5-二乙基-3-苯基噁唑啉-2-硫酮(5r):白色固体, 产率83%. m.p. 127~129 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.09~7.00 (m, 5H), 6.90~6.84 (m, 3H), 6.82~6.63 (m, 2H), 5.56 (s, 1H), 2.18~2.09 (m, 2H), 1.89~1.82 (m, 2H), 1.13 (t, J=7.3 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 188.9, 140.2, 136.7, 132.6, 128.4, 128.3, 128.1, 127.1, 125.8, 101.1, 94.3, 33.5, 7.4; IR (KBr) ν: 3455, 2971, 2360, 1658, 1591, 1463, 1341, 1117, 970, 688 cm-1; HRMS (ESI) calcd for C20H22NOS [M+H]+ 324.1422, found 324.1072.
(Z)-4-芳亚甲基-3, 5, 5-三乙基噁唑啉-2-硫酮(5s):白色固体, 产率87%. m.p. 109~111 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.37~7.27 (m, 3H), 7.23~7.20 (m, 2H), 5.53 (s, 1H), 3.77 (q, J=7.0 Hz, 2H), 2.04~1.96 (m, 2H), 1.77~1.69 (m, 2H), 0.97 (t, J=7.3 Hz, 6H), 0.80 (t, J=7.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 188.4, 139.2, 134.1, 129.4, 128.0, 127.3, 100.3, 93.0, 77.6, 41.6, 33.4, 11.4, 7.2; IR (KBr) ν: 3445, 2971, 1674, 1456, 1389, 1277, 1136, 915, 701 cm-1; HRMS (ESI) calcd for C16H22NOS [M+H]+ 276.1422, found 276.0996.
(Z)-4-芳亚甲基-3-乙基-5-异丁基-5-甲基噁唑啉-2-硫酮(5t):无色油状, 80%.1H NMR (CDCl3, 400 MHz) δ: 7.37~7.28 (m, 3H), 7.21~7.18 (m, 2H), 5.55 (s, 1H, ), 3.76 (q, J=7.0 Hz, 2H), 1.91~1.84 (m, 2H), 1.72~1.69 (m, 1H), 1.58 (s, 3H), 1.00 (t, J=6.7 Hz, 6H), 0.81 (t, J=7.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 187.6, 141.9, 134.0, 129.3, 128.0, 127.3, 100.4, 89.7, 49.3, 41.5, 28.3, 24.3, 24.2, 23.8, 11.3; IR (KBr) ν: 3442, 2955, 1677, 1463, 1389, 1277, 1223, 1136, 745, 701 cm-1; HRMS (ESI) calcd for C17H24NOS [M+H]+ 290.1579, found 290.1571.
(Z)-5-乙基-4-(4-氟苯亚甲基)-5-甲基-3-(对甲苯基)噁唑啉-2-酮(5u):白色固体, 产率90%. m.p. 81~83 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.86~6.85 (m, 4H), 6.62~6.53 (m, 4H), 5.48 (s, 1H), 2.22 (s, 3H), 2.05~1.86 (m, 2H), 1.68 (s, 3H), 1.08 (t, J=7.6 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 162.1, 159.7, 155.7, 141.5, 137.3, 132.2, 130.0, 129.9, 128.9, 125.9, 113.9, 113.7, 98.2, 85.5, 34.2, 27.0, 20.9, 7.6; IR (KBr) ν: 2960, 1756, 1676, 1513, 1406, 1326, 1232, 1139, 1066, 812, 626 cm-1; HRMS (ESI) calcd for C20H21FNO2 [M+H]+ 326.1556, found 326.1554.
(Z)-5-乙基-4-(4-甲氧基苯亚甲基)-5-甲基-3-(对甲苯基)噁唑啉-2-酮(5v):白色固体, 产率80%. m.p. 93~95 ℃; 1H NMR (CDCl3, 400 MHz) δ: 6.87~6.86 (m, 4H), 6.58~6.56 (m, 2H), 6.41~6.39 (m, 2H), 5.48 (s, 1H), 3.66 (s, 3H), 2.21 (s, 3H), 2.02~1.87 (m, 2H), 1.67 (s, 3H), 1.08 (t, J=7.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 157.6, 155.9, 140.1, 136.8, 132.5, 129.5, 128.7, 125.9, 125.7, 112.5, 99.2, 85.5, 55.2, 34.3, 27.0, 20.9, 7.6; IR (KBr) ν: 2973, 1773, 1663, 1509, 1329, 1239, 1126, 1029, 812, 616 cm-1; HRMS (ESI) calcd for C21H24NO3 [M+H]+ 338.1756, found 338.1752.
(Z)-5-乙基-4-(4-氰基苯亚甲基)-5-甲基-3-(对甲苯基)噁唑啉-2-酮(5w):无色油状, 产率91%. 1H NMR (CDCl3, 400 MHz) δ: 7.58~7.56 (m, 2H), 7.35~7.32 (m, 2H), 7.23~7.20 (m, 4H), 5.66 (s, 1H), 2.41 (s, 3H), 1.88~1.83 (dq, J=14.6, 7.3 Hz, 2H), 1.60~1.53 (m, 2H), 1.07 (t, J=7.3 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 155.1, 144.2, 140.2, 139.2, 131.9, 130.9, 130.6, 129.5, 127.7, 118.6, 110.6, 99.8, 89.2, 31.9, 21.2, 7.3; IR (KBr) ν: 2966, 2360, 1759, 1669, 1409, 1356, 1166, 1062, 826, 669 cm-1; HRMS (ESI) calcd for C22H23N2O2 [M+H]+ 347.1760, found 347.1753.
(Z)-5, 5-二乙基-4-戊基-3-(对甲苯基)噁唑啉-2-酮(5x):白色固体, 产率65%. m.p. 77~79 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.22 (d, J=8.1 Hz, 2H), 7.16 (d, J=8.2 Hz, 2H), 4.16 (t, J=7.6 Hz, 1H), 2.38 (s, 3H), 1.90 (dd, J=14.4, 7.2 Hz, 2H), 1.67 (dd, J=14.4, 7.3 Hz, 2H), 1.43 (dd, J=14.4, 7.2 Hz, 2H), 1.12~1.05 (m, 4H), 1.02 (t, J=7.3 Hz, 6H), 0.71 (t, J=7.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 156.5, 138.4, 138.3, 133.9, 129.8, 127.6, 99.6, 88.0, 32.9, 32.3, 24.4, 21.9, 21.1, 13.6, 7.2, 0. IR (KBr) ν: 2973, 1706, 1606, 1546, 1466, 1406, 1323, 1246, 1052, 816 cm-1; HRMS (ESI) calcd for C19H28NO2 [M+H]+ 302.2120, found 302.2113.
(Z)-4-(环丙基)-5, 5-二乙基-3-(对甲苯基)噁唑啉-2-酮(5y):白色固体, 产率62%. m.p. 79~82 ℃; 1H NMR (CDCl3, 400 MHz) δ: 7.26~7.20 (m, 4H), 3.72 (d, J=9.0 Hz, 1H), 2.37 (s, 3H), 1.89~1.85 (dq, J=14.5, 7.3 Hz, 2H), 1.65~1.59 (m, 2H), 1.01 (t, J=7.3 Hz, 6H), 0.61~0.57 (m, 1H), 0.41~0.40 (m, 2H), 0.12 (m, 2H); 13C NMR (CDCl3, 100 MHz) δ: 156.3, 138.3, 138.1, 133.6, 129.5, 127.3, 103.4, 87.8, 32.9, 21.1, 7.8, 7.5, 7.2. IR (KBr) ν: 3293, 2980, 1706, 1596, 1539, 1316, 1239, 1052, 912, 816 cm-1; HRMS (ESI) calcd for C18H23NO2Na [M+Na]+ 308.1626, found 308.1620.
辅助材料(Supporting Information)所有化合物的 1H NMR和13C NMR谱图.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
Zappia, G.; Menendez, P.; Monache, G. D.; Misiti, D.; Nevola, L.; Botta, B. Mini-Rev. Med. Chem. 2007, 7, 389. doi: 10.2174/138955707780363783
-
[2]
(a) Phillips, O. A.; Sharaf, L. H. Expert Opin. Ther. Pat. 2016, 26, 591.
(b) Jadhavar, P. S.; Vaja, M. D.; Dhameliya, T. M.; Chakraborti, A. K. Curr. Med. Chem. 2015, 22, 4379. -
[3]
(a) Fujita, T.; Yamago, S. Chem.-Eur. J. 2015, 21, 18547.
(b) Green, R.; Peed, J.; Taylor, J. E.; Blackburn, R. A.; Bull, S. D. Nat. Protoc. 2013, 8, 1890. -
[4]
Ferreira, J.; Rees-Jones, S. C.; Ramaotsoa, V.; Msutu, A.; Hunter, R. Org. Biomol. Chem. 2016, 14, 1545. doi: 10.1039/C5OB02560E
-
[5]
(a) Guo, B.; Fan, H.; Xin, Q.; Chu, W.; Wang, H.; Huang, Y.; Chen, X.; Yang, Y. J. Med. Chem. 2013, 56, 2642.
(b) Friggeri, L.; Ballante, F.; Ragno, R.; Musmuca, I.; De Vita, D.; Manetti, F.; Biava, M.; Scipione, L.; Di Santo, R.; Costi, R.; Feroci, M.; Tortorella, S. J. Chem. Inf. Model. 2013, 53, 1463. -
[6]
(a) Njiojob, C. N.; Bozell, J. J.; Long, B. K.; Elder, T.; Key, R. E.; Hartwig, W. T. Chem.-Eur. J. 2016, 22, 12506.
(b) Mydock-McGrane, L.; Rath, N. P.; Covey, D. F. J. Org. Chem. 2014, 79, 5636. -
[7]
Mendes, R. E.; Deshpande, L. M.; Jones, R. N. Drug Resist. Updates 2014, 17, 1. doi: 10.1016/j.drup.2014.04.002
-
[8]
Chahine, E. B.; Sucher, A. J.; Knutsen, S. D. Consult. Pharm. 2015, 30, 386. doi: 10.4140/TCP.n.2015.386
-
[9]
(a) Xu, J. X.; Zhao, J. W.; Jia, Z. B. Chin. Chem. Lett. 2011, 22, 1063.
(b) Li, Y. W.; Liu, Y.; Jia, Y. C.; Yuan, J. Y. Chin. Chem. Lett. 2013, 24, 230. -
[10]
(a) Sadeghzadeh, S. M. Appl. Organomet. Chem. 2016, 30, 835.
(b) Bacchi, A.; Chiusoli, G. P.; Costa, M.; Gabriele, B.; Righi, C.; Salerno, G. Chem. Commun. 1997, 1209.
(c) Song, Q. W.; Zhou, Z. H.; Wang, M. Y.; Zhang, K.; Liu, P.; Xun, J. Y.; He, L. N. ChemSusChem 2016, 9, 2054.
(d) Hu, J.; Ma, J.; Zhu, Q.; Zhang, Z.; Wu, C.; Han, B. Angew. Chem., Int. Ed. 2015, 54, 5399.
(e) Hase, S.; Kayaki, Y.; Ikariya, T. ACS Catal. 2015, 5, 5135. -
[11]
(a) Fujisaki, F.; Abe, N.; Sumoto, K. Heterocycles 2008, 75, 1681.
(b) Steiner, B.; Langer, V.; Koóš, M. Carbohydr. Res. 2009, 344, 2079. -
[12]
a) Li, S. Q.; Xiong, P.; Zhu, L.; Qian, X. Y.; Xu, H. C. Eur. J. Org. Chem. 2016, 2016, 3449.
(b) Sekine, K.; Mawatari, T.; Yamada, T. Synlett 2015, 26, 2447.
(c) Kim, W. S.; Yoon, E.; Jo, K. A.; Kang, E. J. Bull. Korean Chem. Soc. 2011, 32, 3158. -
[13]
(a) Lu, X. F.; Yang, Z.; Huang, N. Y.; He, H. B.; Deng, W. Q.; Zou, K. Bioorg. Med. Chem. Lett. 2015, 25, 3726.
(b) Wang, L.; Xie, Y. B.; Huang, N. Y.; Yan, J. Y.; Hu, W. M.; Liu, M. G.; Ding, M. W. ACS Catal. 2016, 6, 4010.
(c) Li, R. K.; Yang, Q. L.; Liu, Y.; Li, D. W.; Huang, N. Y.; Liu, M. G. Chin. Chem. Lett. 2016, 27, 345. -
[14]
Li, S.; Ye, J.; Yuan, W.; Ma, S. Tetrahedron 2013, 69, 10450. doi: 10.1016/j.tet.2013.09.087
-
[15]
Zhao, J.; Huang, H.; Qi, C.; Jiang, H. Eur. J. Org. Chem. 2012, 29, 5665.
-
[16]
(a) Ritter, S.; Horino, Y.; Lex, J.; Schmalz, H. G. Synlett 2006, 3309.
(b) Ramesh, R.; Chandrasekaran, Y.; Megha, R.; Chandrasekaran, S. Tetrahedron 2007, 63, 9153.
(c) Hu, M.; Song, R. J.; Li, J. H. Angew. Chem., Int. Ed. 2015, 54, 608. -
[17]
(a) Doherty, S.; Knight, J. G.; Perry, D. O.; Ward, N. A.; Bittner, D. M.; McFarlane, W.; Probert, M. R. Organometallics 2016, 35, 1265.
(b) Wang, F.; Wang, Y.; Cai, L.; Miao, Z.; Chen, R. Adv. Synth. Catal. 2008, 350, 2733.
(c) Hu, Y.; Xin, X.; Wan, B. Tetrahedron Lett. 2014, 55, 32. -
[18]
(a) Hashmi, A.; Stephen K.; Wang, T.; Shi, S.; Rudolph, M. J. Org. Chem. 2012, 77, 7761.
(b) Engel, D. A.; Dudley, G. B. Org. Lett. 2006, 8, 4027.
(c) Jagtap, S. R.; Bhanage, B. M. J. Chem. Res. 2007, 6, 370.
(d) Smissman, E. E.; Johnsen, R. H.; Carlson, A. W.; Aycock, B. F. J. Org. Chem. 1956, 78, 3395.
(e) Cooper, M. A.; Lucas, M. A.; Taylor, J. M.; Ward, A. D.; Williamson, N. M. Synthesis 2001, 621.
(f) Moran, W. J.; Rodríguez, A. Org. Biomol. Chem. 2012, 10, 8590.
(g) Zhang, H.; Tanimoto, H.; Morimoto, T.; Nishiyama, Y.; Kakiuchi, K. Org. Lett. 2013, 20, 5222.
-
[1]
-
表 1 模型反应的条件优化
Table 1. Optimization of reaction conditions for the model reaction
Entry Catalysta Solvent Temp./ ℃ Time/ h Yieldb/% Carbamate ester 5a 1 AgNO3 Toluene 30 24 0 0 2 PdCl2 CH3CN 30 24 0 0 3 CuI CH3CN 30 24 0 0 4 I2 Toluene 30 24 0 0 5 DMAP DMF 50 24 80 0 6 DBU CH2Cl2 30 12 70 30 7 DBU CH2Cl2 30 24 5 85 8 DBU Toluene 30 24 0 0 9 DBU CHCl3 30 24 90 0 10 DBU Et2O 30 24 0 0 11 DBU EtOAc 30 24 0 0 12 DBU CH3OH 30 24 0 0 13 DBU CH3CN 30 12 0 90 14 K2CO3 CH3CN 30 24 0 40 15 Morpholine CH3CN 30 24 0 0 16 Et3N CH3CN 30 24 0 30 17 NaOH CH3CN 30 24 0 0 18 DBUc CH3CN 30 24 0 60 19 DBUd CH3CN 30 12 0 90 20 DBUe CH3CN 30 12 0 80 21 DBU CH3CN 40 8 0 90 22 DBU CH3CN 50 8 0 60 23 DBU CH3CN 20 24 40 60 aGeneral conditions: catalyst (20 mol%) was added into the mixture of 3a (95 mg, 0.5 mmol) and 4a (67 mg, 0.5 mmol) in corresponding solvent (2.0 mL). b Isolated yield. c10 mol% of DBU was used. d30 mol% of DBU was used. e40 mol% of DBU was used. 表 2 噁唑啉-2-(硫)酮衍生物5衍生物
Table 2. Oxazolidin-2-(thi)one derivatives 5
Entry Product Substitution Isolated yield/% R1 R2 R3 R4 X 1 5a C6H5 Me Et p-MeC6H4 O 91 2 5b C6H5 Et Et p-MeC6H4 O 90 3 5c C6H5 Me i-Pr p-MeC6H4 O 86 4 5d C6H5 Me i-Bu p-MeC6H4 O 85 5 5e C6H5 Me Cyclopropyl p-MeC6H4 O 80 6 5f p-MeC6H4 Cyclopentyl p-MeC6H4 O 78 7 5g C6H5 Me i-Bu p-FC6H4 O 87 8 5h C6H5 Me Cyclopropyl p-FC6H4 O 86 9 5i TMS Et C6H5 p-FC6H4 O 75 10 5j H Et C6H5 p-FC6H4 O 91 11 5k C6H5 Et Et Naphthyl O 85 12 5l C6H5 Et Et i-Pr O 87 13 5m C6H5 Me i-Bu i-Pr O 86 14 5n C6H5 Me Cyclopropyl i-Pr O 87 15 5o C6H5 Cyclopentyl i-Pr O 88 16 5p C6H5 Et Et Et O 86 17 5q C6H5 Me Cyclopropyl Et O 84 18 5r C6H5 Et Et C6H5 S 83 19 5s C6H5 Et Et Et S 87 20 5t C6H5 Me i-Bu C6H5 S 80 21 5u p-FC6H4 Me Et p-MeC6H4 O 90 22 5v p-MeOC6H4 Me Et p-MeC6H4 O 80 23 5w p-CNC6H4 Et Et p-MeC6H4 O 91 24 5x n-Bu Et Et p-MeC6H4 O 65 25 5y Cyclopropyl Et Et p-MeC6H4 O 62 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 1
- 文章访问数: 1020
- HTML全文浏览量: 158




 下载:
下载:


 下载:
下载:

