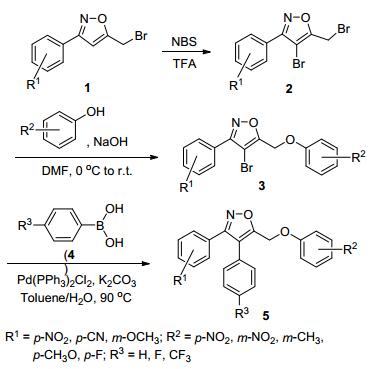
 图式1
3, 4-二芳基-5-芳氧甲基异噁唑的合成路线
Scheme1.
Synthetic route for preparation of 3, 4-diaryl-5-ar-yloxy-methyl-isoxazoles
图式1
3, 4-二芳基-5-芳氧甲基异噁唑的合成路线
Scheme1.
Synthetic route for preparation of 3, 4-diaryl-5-ar-yloxy-methyl-isoxazoles

Citation: Jiang Haifang, Zhang Min, Zhang Li, Chen Yali, Zhu Ning, Song Liping, Deng Hongmei. Synthesis of 3, 4-Diaryl-5-aryloxymethyl Isoxazole Derivatives[J]. Chinese Journal of Organic Chemistry, 2017, 37(9): 2399-2408. doi: 10.6023/cjoc201702026

3, 4-二芳基-5-芳氧甲基异噁唑的合成
-
关键词:
- 异噁唑衍生物
- / 溴代
- / 醚化
- / Suzuki偶联反应
English
Synthesis of 3, 4-Diaryl-5-aryloxymethyl Isoxazole Derivatives
-
Key words:
- isoxazole derivatives
- / bromination
- / etherification
- / suzuki coupling reaction
-
异噁唑类化合物具有非常良好的生物活性, 在农药、医药等领域具有广阔的应用价值[1~4].异噁唑类化合物不仅被广泛应用于农化产品的研究[5~8], 而且在药物化学[9]和材料化学[10]上的应用也相当广泛[11], 可显示多种药物活性, 如抗菌、抗炎、平喘、降血压、抗肿瘤等.因此, 其合成方法也引起了许多药物化学家的注意, 很多异噁唑衍生物已被开发成医药产品[12~19].研究结果同时发现, 异噁唑衍生物在治疗帕金森氏病和老年痴呆症方面也有一定的作用[20, 21]. 3, 4-二芳基异噁唑作为药物骨架被广泛应用于生物制药, 例如非甾体抗炎药物、蛋白激酶抑制剂等[22].目前, 在异噁唑4-位上引入芳基的方法主要可通过Suzuki或Stille交叉偶联反应来制备[22~31], 但3, 4-二取代芳基-5-芳氧甲基异噁唑化合物少见报道[32], 为了进一步研究多取代异噁唑化合物的构效关系, 本文在前期研究的基础上[33, 34], 以3-芳基-5-溴甲基异噁唑(1)为原料, 经溴代、醚化、Suzuki偶联反应合成了系列3, 4-二取代芳基-5-芳氧甲基异噁唑化合物.
1 结果与讨论
目标产物3, 4-二芳基-5-芳氧甲基异噁唑衍生物的合成路线如Scheme 1所示.首先, 我们初步尝试了合成化合物2的溴化条件, 考虑到如果用液溴或H2O2/HBr体系进行溴化时反应不如用N-溴代丁二酰亚胺(NBS)溴化的反应条件温和、容易操作[35~37], 所以我们选择了较为温和的NBS作为溴化试剂, 以3-(4-氰基苯基)-5-溴甲基异噁唑反应为例, 筛选了不同的溴化反应条件.当选择以醋酸为反应溶剂时, 无论是在室温下还是提高反应温度, 反应均无法进行(表 1, Entry 2);当以醋酸/三氟醋酸(V:V=10:1) 为反应溶剂, 反应在25 ℃时亦不能进行, 只有在60 ℃时才能得到少量目标产物(表 1, Entries 3, 4);而当以三氟醋酸为反应溶剂时, 该溴化反应能够顺利进行, 并且在60 ℃下反应6 h的收率达到75% (表 1, Entry 6).随后我们以此为较优的溴代反应条件, 进一步考察了以3-(4-硝基苯基)-5-溴甲基异噁唑、3-(4-甲氧基苯基)-5-溴甲基异噁唑为底物的溴代反应, 分别以83%和86%的产率得到相应的化合物2.化合物2在碱性条件下与取代苯酚发生醚化反应得到化合物3.具体反应条件参见文献[35]方法, 反应产率见表 2.化合物3通过与苯硼酸4在Pd(PPh3)2Cl2催化下的Suzuki反应得到一系列的多芳基取代的异噁唑衍生物5.
 图式1
3, 4-二芳基-5-芳氧甲基异噁唑的合成路线
Scheme1.
Synthetic route for preparation of 3, 4-diaryl-5-ar-yloxy-methyl-isoxazoles
图式1
3, 4-二芳基-5-芳氧甲基异噁唑的合成路线
Scheme1.
Synthetic route for preparation of 3, 4-diaryl-5-ar-yloxy-methyl-isoxazoles
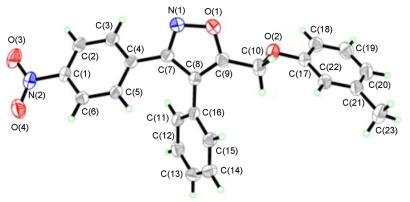
Entry Solvent Temp/℃ T/h Yieldb/% 1 DMF 25 12 —c 2 AcOH 60 12 —c 3 AcOH/TFA (V/V=10/1) 25 12 —c 4 AcOH/TFA (V/V=10/1) 60 12 25 5 TFA 25 12 37 6 TFA 60 6 75 a Reaction conditions: 1:NBS=1:2 (molar ratio). bIsolated yield. cNo product was detected. Entry R1 R2 Product Yieldb/% 1 4-CN 3-NO2 3-1 87 2 4-CN 4-CH3O 3-2 85 3 4-CN 4-F 3-3 84 4 4-NO, 4-CH3O 3-4 88 5 4-NO2 3-NO2 3-5 69 6 4-NO2 3-Me 3-6 85 7 4-CH3O 4-CH3O 3-7 90 8 4-CH3O 3-NO2 3-8 75 9 4-CH3O 4-F 3-9 86 10 4-CH3O 3-Me 3-10 88 aReaction conditions: 25 ℃, 12h; b Isolated yield. 由表 3结果可见, 取代基的电子效应对目标产物产率的影响不是很明显, 该反应的普适性较好.对目标产物的结构鉴定, 不仅通过1H NMR、13C NMR、IR和HRMS进行了表征, 同时还对代表性的化合物5~16进行了单晶培养, 并对所得晶体进行了X-射线晶体结构分析[38].晶体结构见图 1, 进一步确定了化合物的结构.
Entry R1 R2 R3 Product Yieldb/% 1 4-CN 4-NO2 H 5-1 88 2 4-CN 4-NO2 F 5-2 86 3 4-CN 3-NO2 4-CF3 5-3 91 4 4-CN 4-CH3O H 5-4 82 5 4-CN 4-CH3O 4-F 5-5 91 6 4-CN 4-CH3O 4-CF3 5-6 92 7 4-CN 4-F H 5-7 89 8 4-CN 4-F 4-F 5-8 82 9 4-CN 4-F 4-CF3 5-9 85 10 4-NO2 4-CH3O H 5-10 85 11 4-NO2 4-CH3O 4-F 5-11 83 12 4-NO2 4-CH3O 4-CF3 5-12 86 13 4-NO2 3-NO2 H 5-13 84 14 4-NO2 3-NO2 4-F 5-14 81 15 4-NO2 3-NO2 4-CF3 5-15 84 16 4-NO2 3-Me H 5-16 83 17 4-NO2 3-Me 4-F 5-17 83 18 4-NO2 3-Me 4-CF3 5-18 85 19 3-CH3O 4-CH3O H 5-19 89 20 3-CH3O 4-CH3O 4-F 5-20 91 21 3-CH3O 4-CH3O 4-CF3 5-21 88 22 3-CH3O 3-NO2 H 5-22 85 23 3-CH3O 3-NO2 4-F 5-23 85 24 3-CH3O 3-NO2 4-CF3 5-24 82 25 3-CH3O 4-F H 5-25 90 26 3-CH3O 4-F 4-F 5-26 88 27 3-CH3O 4-F 4-CF3 5-27 82 28 3-CH3O 3-Me H 5-28 91 29 3-CH3O 3-Me 4-F 5-29 84 30 3-CH3O 3-Me 4-CF3 5-30 86 a Reaction conditions: 90 ℃, 12 h. b Isolated yield. 化合物5-16的晶体结构属正交晶系, 四个芳环都不在一个平面上, 其中异噁唑环与对硝基苯环的两面角为43.3°, 异噁唑环与苯环的两面角为50.1°, 异噁唑环与3-甲基苯环的两面角为-73.0°.
我们对目标化合物进行了昆虫室内毒力测试, 供试对象是豆蚜Aphis crassivora 成蚜和东方粘虫M. separata 2龄幼虫.发现目标化合物对豆蚜Aphis crassivora成蚜无活性, 对东方粘虫M. separata 2龄幼虫发现测试三天后目标化合物5-19的杀虫率为23.3%, 5-24的杀虫率为26.7%, 其余样品杀虫率较低, 仅为0%~13.3%.
2 结论
以3-芳基-5-溴甲基异噁唑(1)为原料, 经溴代、醚化、Suzuki偶联反应高效地合成了一系列3, 4-二取代芳基-5-芳氧甲基异噁唑化合物5, 并测试了化合物5-16的单晶结构, 为进一步研究多芳基取代的异噁唑化合物的生物活性和构效关系奠定了基础.
3 实验部分
3.1 仪器和试剂
Brucker AV-500核磁共振光谱仪, TMS内标, CDCl3或DMSO-d6作为溶剂; LCMS 2020低分辨质谱仪; Bruker Daltonics APEXIII 7.0 TESALA FTMS高分辨质谱仪; WRS-1A数字熔点仪; 柱层析硅胶为H型(青岛海洋化工厂); 所用试剂均为市售分析, 用前未作处理. 3-芳基-5-溴甲基异噁唑(1)均从APICHEMICAL SHANGHAI CO. LTD公司购买.
3.2 实验方法
3.2.1 化合物2的合成
称取4-氰基苯基-5-溴甲基异噁唑(1) (1.0 g, 3.58 mmol)于50 mL圆底烧瓶中, 加入20 mL三氟醋酸, 室温搅拌至完全溶解后加入NBS (0.96 g, 7.19 mmol), 60℃下反应约6个h, 薄层色谱(TLC)检测至反应完全.混合物冷却后倒入盛有10 mL水的100 mL烧杯中, 用Na2CO3调pH值至中性后用乙酸乙酯萃取, 萃取液用无水MgSO4干燥后浓缩得粗产物, 粗产物用V(乙酸乙酯):V(石油醚)=1:10为淋洗剂, 经柱层析分离得到4-氰基苯基-4-溴-5-溴甲基异噁唑(2-1) 910 mg, 产率为75 %.化合物2-2~2-3按上述方法合成.
3-(4-氰基苯基)-4-溴-5-溴甲基异噁唑(2-1):白色固体, 产率65.2%. m.p. 112.5~113.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.93 (dd, J=6.5, 2.0 Hz, 2H), 7.77 (dd, J=6.5, 2.0 Hz, 2H), 4.54 (s, 2H).
3-(4-硝基苯基)-4-溴-5-溴甲基异噁唑(2-2):白色固体, 产率74.3%. m.p. 121.3~122.9 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.37 (dd, J=9.0, 2.0 Hz, 2H), 8.09 (dd, J=9.0, 2.0 Hz, 2H), 4.72 (s, 0.9H), 4.56 (s, 1.1H).
3-(3-甲氧基苯基)-4-溴-5-溴甲基异噁唑(2-3):白色固体, 产率57.8%. m.p. 104.7~105.9 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.93 (t, J=2.0 Hz, 1H), 7.72 (d, J=9.0 Hz, 1H), 6.96 (d, J=9.0 Hz, 1H), 6.57 (s, 1H), 4.65 (s, 0.7 H), 4.50 (s, 1.4H), 3.95 (s, 3H).
3.2.2 化合物3的合成
称取3-硝基苯酚(0.19 g, 1.39 mmol)于25 mL圆底烧瓶中, 加入10mL N, N-二甲基甲酰胺(DMF)溶解, 室温条件下加入NaOH (0.06 g, 1.52 mmol), 反应1 h后加入4-氰基苯基-4-溴-5-溴甲基异噁唑(2) (0.5 g, 1.39 mmol), 室温下反应12 h, TLC检测反应完全.将反应液倒入30 mL水中, 混合液用乙酸乙酯(30 mL×3) 萃取, 收集有机相, 用无水MgSO4干燥后浓缩得粗产物, 粗产物用V(乙酸乙酯):V(石油醚)=1:8为淋洗剂, 经柱层析分离得3-(4-氰基苯基)-4-溴-5-(3-硝基苯氧甲基)异噁唑(3-1) 480 mg, 产率为87%.化合物3-2~3-10按上述方法合成.
3-(4-氰基苯基)-4-溴-5-(3-硝基苯氧甲基)异噁唑(3-1):白色固体, 产率86.1%. m.p. 149.4~149.8 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.02~8.00 (m, 2H), 7.94~7.92 (m, 1H), 7.89 (t, J=2.5 Hz, 1H), 7.82~7.80 (m, 2H), 7.51 (t, J=8.0 Hz, 1H), 7.37~7.34 (m, 1H), 5.32 (s, 2H); 13C NMR (126 MHz, CDCl3) δ: 165.15, 159.39, 158.04, 149.23, 132.58, 131.55, 130.45, 128.85, 121.96, 118.11, 117.23, 114.34, 109.32, 94.08, 59.91.
3-(4-氰基苯基)-4-溴-5-(4-甲氧基苯氧甲基)异噁唑(3-2):白色固体, 产率83.7%. m.p. 110.8~111.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.02~8.00 (m, 2H), 7.81~7.79 (m, 2H), 6.97~6.95 (m, 2H), 5.17 (s, 2H), 3.78 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 166.58, 159.21, 154.97, 151.77, 132.52, 131.85, 128.85, 118.17, 116.44, 114.81, 114.18, 93.34, 60.69, 55.71.
3-(4-氰基苯基)-4-溴-5-(4-氟苯氧甲基)异噁唑(3-3):白色固体, 产率79.9%. m.p. 106.5~106.7 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.00 (d, J=8.0 Hz, 2H), 7.79 (d, J=8.0 Hz, 2H), 7.10~6.88 (m, 4H), 5.18 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -121.61~-121.64 (m); 13C NMR (126 MHz, CDCl3) δ: 166.17, 159.20, 158.04 (d, 1JC-F=240.8 Hz, ArF), 153.79, 132.53, 131.70, 128.80, 118.18, 116.44, (d, 3JC-F=8.1 Hz, ArF) 116.17 (d, 2JC-F=23.2 Hz, ArF), 114.17, 93.50, 60.44.
3-(4-硝基苯基)-4-溴-5-(4-甲氧基苯氧甲基)异噁唑(3-4):白色固体, 产率85.2%. m.p. 104.1~104.7 ℃; 1H NMR (500 MHz, CDCl3) δ: 9.09~8.19 (m, 2H), 8.18~7.98 (m, 2H), 7.07~6.92 (m, 2H), 6.94~6.80 (m, 2H), 5.18 (s, 2H), 3.78 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 166.70, 159.01, 154.98, 151.76, 148.93, 133.59, 129.24, 123.95, 116.44, 114.82, 93.41, 60.69, 55.70.
3-(4-硝基苯基)-4-溴-5-(3-硝基苯氧甲基)异噁唑(3-5):白色固体, 产率93.1%, m.p. 148.7~149.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.38~8.36 (m, 2H), 8.10~8.07 (m, 2H), 7.90 (t, J=2.5 Hz, 1H), 7.51 (t, J=8.0 Hz, 1H), 7.49~7.37 (m, 1H), 5.33 (s, 2H); 13C NMR (126 MHz, CDCl3) δ: 165.27, 159.18, 158.04, 149.23, 149.02, 133.29, 130.46, 129.26, 124.00, 121.96, 117.24, 109.31, 94.14, 59.90.
3-(4-硝基苯基)-4-溴-5-((3-甲基苯氧甲基)异噁唑(3-6):白色固体, 产率89.3%. m.p. 95.8~96.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.36 (d, J=9.0 Hz, 2H), 8.09 (d, J=9.0 Hz, 2H), 7.22 (t, J=8.0 Hz, 1H), 6.87~6.82 (m, 3H), 5.22 (s, 2H), 2.36 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 166.58, 159.03, 157.70, 148.93, 139.94, 133.59, 129.48, 129.25, 123.95, 123.05, 115.80, 111.68, 93.45, 59.55, 21.54.
3-(3-甲氧基苯基)-4-溴-5-(4-甲氧基苯氧甲基)异噁唑(3-7):白色固体, 产率91.6%. m.p. 116.5~117.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.99 (d, J=2.0 Hz, 1H), 7.73 (dd, J=8.5, 2.0 Hz, 1H), 6.95 (d, J=8.5 Hz, 1H), 6.94~6.90 (m, 2H), 6.87~6.83 (m, 2H), 6.56 (s, 1H), 5.13 (s, 2H), 3.94 (s, 3H), 3.77 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 168.95, 161.01, 157.25, 154.69, 151.89, 131.76, 127.18, 122.62, 116.04, 114.80, 112.17, 111.96, 101.00, 62.29, 56.36, 55.70.
3-(3-甲氧基苯基)-4-溴-5-(3-硝基苯氧甲基)异噁唑(3-8):白色固体, 产率82.8%. m.p. 171.4~173.7 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.00 (d, J=2.0 Hz, 1H), 7.91 (d, J=8.0, 1H), 7.85 (t, J=2.0 Hz, 1H), 7.75 (dd, J=8.5, 2.0 Hz, 1H), 7.49 (t, J=8.0 Hz, 1H), 7.32 (dd, J=8.5, 2.0 Hz, 1H), 6.97 (d, J=8.5 Hz, 1H), 6.64 (s, 1H), 5.28 (s, 2H), 3.95 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 165.22, 159.49, 154.80, 151.89, 148.49, 135.00, 129.80, 129.40, 129.15, 128.84, 128.26, 123.80, 119.36, 116.40, 114.75, 60.62, 55.71.
3-(3-甲氧基苯基)-4-溴-5-(4-氟苯氧甲基)异噁唑(3-9):白色固体, 产率88.5%. m.p. 115.5~116.0 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.99 (d, J=2.0 Hz, 1H), 7.74 (dd, J=8.5, 2.0 Hz, 1H), 7.04~6.98 (m, 2H), 6.96 (d, J=8.5 Hz, 1H), 6.95~6.91 (m, 2H), 6.58 (s, 1H), 5.16 (d, J=0.5 Hz, 2H), 3.95 (s, 3H). 19F NMR (471 MHz, CDCl3) δ: -122.11~-122.27 (m); 13C NMR (126 MHz, DMSO-d6) δ: 168.87, 161.01, 157.48 (d, 1JC—F=237.5 Hz, ArF), 157.31, 154.32, 131.37, 127.95, 122.51, 116.70 (d, 3JC—F=8.1 Hz, ArF), 116.42 (d, 2JC—F=23.3 Hz, ArF), 113.40, 111.76, 102.63, 61.54, 56.86.
3-(3-甲氧基苯基)-4-溴-5-(3-甲基苯氧甲基)异噁唑(3-10):白色固体, 产率84.9%. m.p. 107.7~108.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.00 (d, J=2.0 Hz, 1H), 7.74 (dd, J=8.5, 2.0 Hz, 1H), 7.20 (t, J=8.0 Hz, 1H), 6.96 (d, J=8.5 Hz, 1H), 6.85~6.77 (m, 3H), 6.58 (s, 1H), 5.18 (s, 2H), 3.95 (s, 3H), 2.35 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 168.85, 161.02, 157.82, 157.24, 139.87, 131.73, 129.45, 127.21, 122.76, 122.60, 115.66, 112.17, 111.97, 111.56, 100.99, 61.34, 56.35, 21.56.
3.2.3 化合物5的合成
称取3-氰基苯基-4-溴-5-(3-硝基苯氧甲基)异噁唑(0.4 g, 1.00 mmol)、苯硼酸(0.18 g, 1.50 mmol)、K2CO3 (0.20 g, 2.00 mmol)、Pd(PPh3)2Cl2 (0.07 g, 0.10 mmol)于25 mL圆底烧瓶中, 加入10 mL甲苯和1mL水, 在氮气保护下, 90 ℃反应12 h, 停止反应冷却至室温.将反应液倒入水中并用乙酸乙酯萃取(30 mL×3), 收集有机相, 用无水MgSO4干燥后浓缩得粗产物, 粗产物用V(乙酸乙酯):V(石油醚)=1:8为淋洗剂, 经柱层析分离得4-氰基苯基-4-苯基-5-(3-硝基苯氧甲基)异噁唑(5-1) 344 mg, 产率为88 %.化合物5-2~5-30按上述方法合成.
3-(4-氰基苯基)-4-苯基-5-(4-硝基苯氧甲基)异噁唑(5-1):白色固体, 产率88%. m.p. 155.9~157.0 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.21 (dd, J=7.0, 2.0 Hz, 2H), 7.63 (d, J=8.0 Hz, 2H), 7.58 (d, J=8.0 Hz, 2H), 7.45~7.41 (m, 3H), 7.21 (dd, J=7.0, 2.0 Hz, 2H), 7.00 (dd, J=7.5, 2.0 Hz, 2H), 5.21 (s, 2H); 13C NMR (126 MHz, CDCl3) δ: 163.40, 162.42, 159.91, 142.38, 132.77, 132.43, 129.68, 129.33, 129.15, 129.04, 127.92, 125.99, 119.97, 118.20, 114.84, 113.66, 59.87; IR (KBr) ν: 3078, 2930, 2836, 2224, 1592, 1502, 1334, 1249, 1107, 1012, 845 cm-1. HRMS (ESI) calcd for C23H16N3O4[(M+H)+]: 398.1133, found 398.1133.
3-(4-氰基苯基)-4-(4-氟苯基)-5-(4-硝基苯氧甲基)异噁唑(5-2):白色固体, 产率86%. m.p. 164.0~165.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.22 (dd, J=7.0, 2.0 Hz, 2H), 7.65 (d, J=9.0 Hz, 2H), 7.57 (d, J=8.5 Hz, 2H), 7.21 (dd, J=9.0, 5.0 Hz, 2H), 7.13 (t, J=8.5 Hz, 2H), 7.02 (d, J=8.5 Hz, 2H), 5.19 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -111.82. 13C NMR (126 MHz, CDCl3) δ: 163.55, 163.06 (d, 1JC—F=250.7 Hz, ArF), 162.07, 159.91, 142.40, 132.61, 132.51, 131.57(d, 3JC—F=8.3 Hz, ArF), 129.01, 126.01, 123.89, 123.86, 119.01, 118.14, 116.55(d, 2JC—F=21.7 Hz, ArF), 114.83, 113.75, 59.89; IR (KBr) ν: 3084, 2925, 2850, 2228, 1596, 1509, 1340, 1257, 1105, 842 cm-1. HRMS (ESI) calcd for C23H15FN3O4 [(M+H)+]: 416.1044, found 416.1041.
3-(4-氰基苯基)-4-(4-三氟甲基苯基)-5-(3-硝基苯氧甲基)异噁唑(5-3):白色固体, 产率91%. m.p. 131.0~131.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.94 (ddd, J=8.0, 2.0, 1.0 Hz, 1H), 7.79 (t, J=2.0 Hz, 1H), 7.75~7.68 (m, 4H), 7.59 (dd, J=8.0, 2.0 Hz, 2H), 7.51 (t, J=8.0 Hz, 1H), 7.40 (d, J=8.0 Hz, 2H), 7.33 (ddd, J=8.0, 2.0, 1.0 Hz, 1H), 5.22 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -62.81; 13C NMR (126 MHz, CDCl3) δ: 164.30, 159.87, 158.01, 149.15, 132.60, 132.37, 131.88, 131.12 (q, 2JC—F=32.9 Hz, ArCF3), 130.43, 130.17, 129.09, 126.24 (q, 3JC—F=3.6 Hz, ArCF3), 123.74 (q, 1JC—F=273.4 Hz, CF3), 121.86, 118.61, 118.08, 117.09, 113.88, 109.16, 60.00; IR (KBr) ν: 3101, 2925, 2855, 2228, 1622, 1523, 1331, 1241, 1115, 844 cm-1. HRMS (ESI) calcd for C24H15F3N3O4 [(M+H)+]: 466.1004, found 466.1009.
3-(4-氰基苯基)-4-苯基-5-(4-甲氧基苯氧甲基)异噁唑(5-4):白色固体, 产率82%. m.p. 104.1~105.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.64~7.60 (m, 4H), 7.43~7.37 (m, 3H), 7.20 (dt, J=6.0, 2.0 Hz, 2H), 6.88 (dd, J=6.0, 2.0 Hz, 2H), 6.82 (dd, J=6.0, 2.0 Hz, 2H), 5.04 (s, 2H), 3.77 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 165.11, 159.73, 154.78, 151.89, 133.20, 132.37, 129.79, 129.11, 129.08, 128.77, 128.34, 119.26, 118.32, 116.39, 114.74, 113.41, 60.62, 55.71; IR (KBr) ν: 3051, 2932, 2228, 1640, 1505, 1448, 1331, 1228, 1028, 837 cm-1. HRMS (ESI) calcd for C24H19N2O3[(M+H)+]: 383.1351, found 383.1354.
3-(4-氰基苯基)-4-(4-氟苯基)-5-(4-甲氧基苯氧甲基)异噁唑(5-5):白色固体, 产率91%. m.p. 85.1~85.7 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.65 (d, J=2.0 Hz, 2H), 7.57 (dd, J=6.5, 2.0 Hz, 2H), 7.21~7.15 (m, 2H), 7.09 (dd, J=6.5, 2.0 Hz, 2H), 6.88 (dd, J=6.5, 2.5 Hz, 2H), 6.86 (dd, J=6.5, 2.5 Hz, 2H), 5.03 (s, 2H), 3.77 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -111.97~-111.04 (m); 13C NMR (126 MHz, CDCl3) δ: 165.24, 162.89 (d, 1JC—F=249.8 Hz, ArF), 159.72, 154.84, 151.79, 133.00, 132.45, 131.65 (d, 3JC—F=8.7 Hz, ArF), 129.04, 124.32, 118.31, 118.24, 116.38, 116.27 (d, 2JC—F=21.9 Hz, ArF), 114.77, 113.53, 60.64, 55.69; IR (KBr) ν: 3062, 2942, 2826, 2225, 1507, 1331, 1230, 1050, 829 cm-1. HRMS (ESI) calcd for C24H18FN2O3[(M+H)+]: 401.1257, found 401.1259.
3-(4-氰基苯基)-4-(4-三氟甲基苯基)-5-(4-甲氧基苯氧甲)异噁唑(5-6):白色固体, 产率92%. m.p. 104.2~105.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.68~7.63 (m, 4H), 7.55 (dd, J=6.5, 2.5 Hz, 2H), 7.34 (d, J=8.0 Hz, 2H), 6.87~6.90 (m, 4H), 5.05 (s, 2H), 3.77 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -62.75; 13C NMR (126 MHz, CDCl3) δ: 165.75, 159.73, 154.93, 151.67, 132.67, 132.56, 132.24, 130.85 (q, 2JC—F=32.7 Hz, ArCF3), 130.15, 129.11, 126.03(q, 3JC—F=3.6 Hz, ArCF3), 123.81 (q, 1JC—F=273.4 Hz, CF3), 118.13, 118.03, 116.36, 114.80, 113.76, 60.69, 55.68; IR (KBr) ν: 3062, 2938, 2842, 2229, 1506, 1324, 1231, 1127, 839 cm-1. HRMS (ESI) calcd for C25H18F3N2O3[(M+H)+]: 451.1225, found 451.1229.
3-(4-氰基苯基)-4-苯基-5-(4-氟苯氧甲基)异噁唑(5-7):白色固体, 产率89%. m.p. 103.8~104.5 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.64 (d, J=7.5 Hz, 2H), 7.58 (d, J=7.5 Hz, 2H), 7.43~7.38 (m, 3H), 7.20 (ddd, J=7.0, 2.0, 1.0 Hz, 2H), 7.00 (dt, J=7.0, 2.0 Hz, 2H), 6.91~6.83 (m, 2H), 5.06 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -122.02 (s); 13C NMR (126 MHz, CDCl3) δ: 164.67, 159.78, 157.95 (d, 1JC—F=240.52 Hz, ArF), 153.87, 133.08, 132.39, 129.76, 129.17, 129.07, 128.88, 128.23, 119.43, 118.29, 116.35(d, 3JC—F=8.19 Hz, ArF), 116.06 (d, 2JC—F=23.31Hz, ArF), 113.48, 60.42; IR (KBr) ν: 3070, 2917, 2864, 2225, 1500, 1331, 1203, 1032, 825 cm-1. HRMS (ESI) calcd for C23H16FN2O2 [(M+H)+]: 371.1194, found 371.1190.
3-(4-氰基苯基)-4-(4-氟苯基)-5-(4-氟苯氧甲基)异噁唑(5-8):白色固体, 产率82% m.p. 113.7~114.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.64 (d, J=8.0 Hz, 2H), 7.57 (d, J=8.0 Hz, 2H), 7.23~7.15 (m, 2H), 7.10 (m, 2H), 7.02~6.95 (m, 2H), 6.92~6.84 (m, 2H), 5.05 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -111.76~-111.79 (m), -121.85~-121.89 (m); 13C NMR (126 MHz, CDCl3) δ: 164.79, 162.93 (d, 1JC—F=249.7 Hz, ArF), 159.78, 157.95 (d, 1JC—F=240.4 Hz, ArF), 153.80, 132.88, 132.46, 131.63 (d, 3JC—F=8.3 Hz, ArF), 129.03, 124.21, 118.47, 118.22, 116.34 (d, 2JC—F=21.8 Hz, ArF), 116.30, 116.27(d, 2JC—F=21.7 Hz, ArF), 116.01, 113.58, 60.42; IR (KBr) ν: 3076, 2923, 2857, 2227, 1601, 1501, 1205, 1115, 834cm-1. HRMS (ESI) calcd for C23H15F2N2O2[(M+H)+]: 389.1100, found 389.1102.
3-(4-氰基苯基)-4-(4-三氟甲基苯基) -5-(4-氟苯氧甲基)异噁唑(5-9):白色固体, 产率85%. m.p. 114.6~115.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.70~7.61 (m, 4H), 7.59~7.51 (m, 2H), 7.34 (d, J=2.0 Hz, 2H), 6.96 (t, J=8.0 Hz, 2H), 6.90~6.85 (m, 2H), 5.07 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -62.77 (s), -121.63~121.69 (m); 13C NMR (126 MHz, CDCl3) δ: 165.29, 159.79, 158.04 (d, 1JC—F=240.2 Hz, ArF), 153.69, 132.58, 132.14, 130.95 (q, 2J=32.7 Hz, ArCF3), 130.14, 129.10, 126.10 (q, 3J=3.6 Hz, ArCF3), 123.79, (q, 1JC—F=273.4 Hz, CF3), 118.19, 118.11, 116.30(d, 3JC—F=8.2 Hz, ArF), 116.16 (d, 2JC—F=23.3 Hz, ArF), 113.82, 60.46; IR (KBr) ν: 3076, 2923, 2857, 2227, 1601, 1501, 1205, 1115, 834 cm-1. HRMS (ESI) calcd for C24H15F4N2O2[(M+H)+]: 439.1062, found 439.1064.
3-(4-硝基)-4-苯基-5-(4-甲氧基苯氧甲基)异噁唑(5-10):白色固体, 产率85%. m.p. 121.6~122.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.19 (d, J=9.0 Hz, 2H), 7.68 (d, J=9.0 Hz, 2H), 7.43~7.39 (m, 3H), 7.21 (dd, J=8.0, 2.0 Hz, 2H), 6.91 (dd, J=4.0, 2.0 Hz, 2H), 6.86~6.83 (m, 2H), 5.05 (s, 2H), 3.77 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 165.23, 159.49, 154.78, 151.88, 148.48, 135.00, 129.81, 129.41, 129.16, 128.84, 128.27, 123.80, 119.36, 116.38, 114.74, 60.61, 55.70; IR (KBr) ν: 3063, 2933, 2848, 1603, 1511, 1350, 1226, 1027, 846 cm-1. HRMS (ESI) calcd for C23H19N2O5 [(M+H)+]: 403.1249, found 403.1251.
3-(4-硝基苯基)-4-(4-氟苯基)-5-(4-甲氧基苯氧甲基)异噁唑(5-11):白色固体, 产率83%. m.p. 121.8~122.6 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.20 (d, J=9.0 Hz, 2H), 7.63 (d, J=9.0 Hz, 2H), 7.08 (dd, J=9.0, 5.0 Hz, 2H), 7.10 (t, J=9.0 Hz, 2H), 6.82~6.81 (m, 4H), 5.04 (s, 2H), 3.77 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -111.89 (s); 13C NMR (126 MHz, CDCl3) δ: 165.34, 162.93 (d, 1JC—F=249.8 Hz, ArF), 159.48, 154.86, 151.78, 148.55, 134.79, 131.64 (d, 3JC—F=8.25 Hz, ArF), 129.37, 124.22, 123.88, 118.41, 116.33 (d, 2JC—F=21.86 Hz, ArF), 116.38, 114.78, 60.63, 55.71; IR (KBr) ν: 3078, 2945, 2834, 1595, 1509, 1350, 1216, 1031, 846 cm-1. HRMS (ESI) calcd for C23H18FN2O5[(M+H)+]: 421.1191, found 421.1194.
3-(4-硝基苯基)-4-(4-三氟甲基苯基)-5-(4-甲氧基苯氧甲)异噁唑(5-12):白色固体, 产率86%. m.p. 120.8~121.7 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.20 (d, J=9.0 Hz, 2H), 7.63 (d, J=8.5 Hz, 2H), 7.20 (dd, J=9.0, 5.0 Hz, 2H), 7.10(t, J=8.5 Hz, 2H), 6.87 (d, J=9.5 Hz, 2H), 6.83 (d, J=9.5 Hz, 2H), 5.04 (s, 2H), 3.77 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -62.76 (s); 13C NMR (126 MHz, CDCl3) δ: 165.84, 159.48, 154.95, 151.65, 148.67, 134.45, 132.14, 130.96 (q, 2JC—F=33.0 Hz), 130.15, 129.45, 126.08 (q, 3JC—F=3.7 Hz), 124.00, 123.78 (q, 1JC—F=270.9 Hz), 118.13, 116.35, 114.81, 60.67, 55.70; IR (KBr) ν: 3074, 2915, 2843, 1607, 1513, 1333, 1230, 1116, 850 cm-1. HRMS (ESI) calcd for C24H18F3N2O5[(M+H)+]: 471.1123, found 471.1125.
3-(4-硝基苯基)-4-苯基-5-(3-硝基苯氧甲基)异噁唑(5-13):白色固体, 产率84%. m.p. 137.9~138.8 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.18 (d, J=9.0 Hz, 2H), 7.88 (dd, J=8.0, 1.0 Hz, 1H), 7.75 (d, J=2.5 Hz, 1H), 7.66 (d, J=8.5 Hz, 2H), 7.47~7.41 (m, 4H), 7.28 (dd, J=8.0, 2.0 Hz, 1H), 7.24~7.22 (m, 2H), 5.21 (s, 2H); 13C NMR (126 MHz, CDCl3) δ: 163.80, 159.61, 158.16, 149.15, 148.54, 134.68, 130.32, 129.75, 129.39, 129.35, 129.15, 127.86, 123.84, 121.91, 119.95, 116.94, 109.31, 60.01; IR (KBr) ν: 3074, 2915, 2843, 1607, 1513, 1333, 1230, 1116, 850 cm-1. HRMS (ESI) calcd for C22H16N3O6 [(M+H)+]: 418.1005, found 418.1009.
3-(4-硝基苯基)-4-(4-氟苯基)-5-(3-硝基苯氧甲基)异噁唑(5-14):白色固体, 产率81%. m.p. 130.4~131.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.22~8.20 (m, 2H), 7.90~7.88 (m, 1H), 7.76 (t, J=2.5 Hz, 1H), 7.65~7.63 (m, 2H), 7.48 (t, J=8.0 Hz, 1H), 7.30~7.28 (m, 1H), 7.24~7.21 (m, 2H), 7.16~7.12 (m, 2H), 5.19 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -111.15~-111.21 (m); 13C NMR (126 MHz, CDCl3) δ: 163.95, 163.06 (d, 1JC—F=250.6 Hz, ArF), 159.61, 158.10, 149.13, 148.57, 134.51, 131.68 (d, 3JC—F=8.3 Hz, ArF), 130.39, 129.37, 123.89, 121.88, 118.99, 116.97, 116.53 (d, 2JC—F=21.8 Hz, ArF), 109.21, 59.97; IR (KBr) ν: 3095, 2940, 2861, 1612, 1526, 1343, 1242, 1118, 854 cm-1. HRMS (ESI) calcd for C22H15FN3O6 [(M+H)+]: 436.0934, found 436.0939.
3-(4-硝基苯基)-4-(4-三氟甲基苯基)-5-(3-硝基苯氧甲基)异噁唑(5-15):白色固体, 产率84%. m.p. 132.2~133.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.23 (d, J=8.5 Hz, 2H), 7.90 (d, J=7.5 Hz, 1H), 7.76 (d, J=2.0 Hz, 1H), 7.70 (d, J=7.5 Hz, 2H), 7.64~7.62 (m, 2H), 7.49 (t, J=8.0 Hz, 1H), 7.39 (d, J=7.5 Hz, 2H), 7.29 (d, J=8.0 Hz, 1H), 5.22 (s, 2H); 19F NMR (471 MHz, CDCl3) δ: -62.82; 13C NMR (126 MHz, CDCl3) δ: 164.34, 159.61, 157.97, 149.22, 148.76, 134.11, 131.77, 131.34 (q, 2JC—F=33.1 Hz, CF3), 130.44, 130.13, 129.44, 126.33 (q, 3JC—F=3.6 Hz, ArCF3), 124.06, 123.67 (q, 1JC—F=272.5 Hz, ArCF3), 121.87, 118.72, 117.20, 109.10, 59.96; IR (KBr) ν: 2900, 1620, 1527, 1343, 1247, 1164, 855 cm-1. HRMS (ESI) calcd for C23H15F3N3O6[(M+H)+]: 486.0902, found 486.0907.
3-(4-硝基苯基)-4-苯基-5-(3-甲基苯氧甲基)异噁唑(5-16):白色固体, 产率83%. m.p. 99.8~100.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.21 (d, J=9.0 Hz, 2H), 7.66 (d, J=9.0 Hz, 2H), 7.44~7.39 (m, 3H), 7.22 (dt, J=6.0, 2.0 Hz, 2H), 7.15 (dd, J=9.0, 8.0 Hz, 1H), 6.83 (d, J=7.5 Hz, 1H), 6.75~6.74 (m, 2H), 5.09 (s, 2H), 2.31 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 165.09, 159.51, 157.79, 148.50, 139.80, 135.00, 129.83, 129.43, 129.39, 129.20, 128.87, 128.27, 123.82, 122.77, 119.43, 115.69, 111.84, 59.47, 21.52; IR (KBr) ν: 3085, 2918, 2860, 1597, 1520, 1346, 1252, 1166, 1028, 851 cm-1. HRMS (ESI) calcd for C23H19N2O4[(M+H)+]: 387.1331, found 387.1338.
3-(4-硝基苯基)-4-(4-氟苯基)-5-(3-甲基苯氧甲基)异噁唑(5-17):白色固体, 产率83%. m.p. 94.3~95.7 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.22 (dd, J=8.0, 2.0 Hz, 2H), 7.65 (d, J=9.0 Hz, 2H), 7.23~7.16 (m, 3H), 7.12 (t, J=8.5 Hz, 2H), 6.84 (d, J=7.5 Hz, 1H), 6.75~6.74 (m, 2H), 5.08 (s, 2H), 2.32 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -111.84~111.89 (m); 13C NMR (126 MHz, CDCl3) δ: 165.24, 162.96 (d, 1JC—F=250.0 Hz, ArF), 159.51, 157.72, 148.55, 139.86, 134.80, 131.69 (d, 3JC—F=8.2 Hz, ArF), 129.43, 129.40, 124.26, 123.89, 122.88, 118.47, 116.36 (d, 2JC—F=21.4 Hz, ArF), 116.28, 115.70, 111.80, 59.51, 21.50; IR (KBr) ν: 3083, 2924, 2864, 1597, 1514, 1348, 1227, 1164, 1028, 848 cm-1. HRMS (EI) calcd for C23H18FN2O24 [(M+H)+]: 405.1246, found 405.1245.
3-(4-硝基苯基)-4-(4-三氟甲基苯基)-5-((3-甲基苯氧甲基)异噁唑(5-18):白色固体, 产率85%. m.p. 92.5~93.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.23 (dd, J=7.0, 2.0 Hz, 2H), 7.66 (d, J=8.0 Hz, 2H), 7.63 (dd, J=7.0, 2.0 Hz, 2H), 7.36 (d, J=8.0 Hz, 2H), 7.20 (t, J=7.5 Hz, 1H), 6.85 (d, J=8.0 Hz, 1H), 6.74 (t, J=7.5 Hz, 2H), 5.09 (s, 2H), 2.31 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -62.77. 13C NMR (126 MHz, CDCl3) δ: 165.73, 159.52, 157.59, 148.67, 139.93, 134.46, 132.18, 130.95 (q, 2JC—F=32.7 Hz, ArCF3), 130.20, 129.47, 126.10 (q, 3JC—F=3.7 Hz, ArCF3), 124.00, 123.81 (q, 1JC—F=273.4 Hz, CF3), 123.00, 118.18, 115.69, 111.72, 59.58, 21.47; IR (KBr) ν: 3074, 2928, 2863, 1598, 1521, 1333, 1260, 1117, 851 cm-1. HRMS (ESI) calcd for C24H18F3N2O4[(M+H)+]: 455.1217, found 455.1213.
3-(3-甲氧基苯基)-4-苯基-5-(4-甲氧基苯氧甲基)异噁唑(5-19):白色固体, 产率89%. m.p. 104.5~105.4 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.77 (dd, J=8.5, 2.0 Hz, 2H), 7.55 (d, J=8.5 Hz, 2H), 7.42~7.38 (m, 2H), 7.14~7.08 (m, 1H), 7.04 (d, J=8.5 Hz, 1H), 6.92 (dd, J=7.0, 2.0 Hz, 2H), 6.84 (dd, J=7.0, 5.0 Hz, 2H), 6.60 (s, 1H), 5.14 (s, 2H), 3.86 (s, 3H), 3.77 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -110.33~-118.43 (m); 13C NMR (126 MHz, CDCl3) δ: 168.67, 162.10, 158.03, 154.70, 152.03, 137.81, 131.28, 129.62, 129.48, 128.18, 127.42, 127.36, 121.51, 116.10, 114.84, 111.58, 101.01, 62.30, 56.38, 55.70; IR (KBr) ν: 3060, 2931, 2838, 1610, 1511, 1448, 1231, 1030, 815 cm-1. HRMS (ESI) calcd for C24H22NO4[(M+H)+]: 388.1538, found 388.1543.
3-(3-甲氧基苯基)-4-(4-氟苯基)-5-(4-甲氧基苯氧甲基)异噁唑(5-20):白色固体, 产率91%. m.p. 101.3~102.2 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.80 (dd, J=8.5, 2.5 Hz, 1H), 7.74 (d, J=2.0 Hz, 1H), 7.52 (dd, J=9.0, 5.5 Hz, 2H), 7.11 (t, J=9.0 Hz, 2H), 7.04 (d, J=8.5 Hz, 1H), 6.92 (d, J=9.0 Hz, 2H), 6.85 (d, J=9.0 Hz, 2H), 6.60 (s, 1H), 5.14 (s, 2H), 3.86 (s, 3H), 3.77 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: 115.19~115.17 (m); 13C NMR (126 MHz, CDCl3) δ: 168.71, 162.20 (d, 1JC—F=246.9 Hz, ArF), 161.99, 157.87, 154.68, 151.96, 133.63, 131.19 (d, 3JC—F=7.8 Hz, ArF), 130.23, 129.31, 127.40, 121.53, 116.06, 115.02 (d, 2JC—F=21.3 Hz, ArF), 114.80, 111.49, 101.09, 62.36, 55.73, 55.69; IR (KBr) ν: 3071, 2935, 2834, 1605, 1510, 1448, 1231, 1164, 1027, 814 cm-1. HRMS (ESI) calcd for C24H21FNO3[(M+H)+]: 406.1443, found 406.1449.
3-(3-甲氧基苯基)-4-(4-三氟甲基苯基)-5-(4-甲氧基苯氧甲基)异噁唑(5-21):白色固体, 产率88%. m.p. 114.6~114.8 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.81 (dd, J=8.5, 2.5 Hz, 1H), 7.76 (d, J=2.5 Hz, 1H), 7.65 (dd, J=9.0, 2.5 Hz, 4H), 7.07 (d, J=9.0 Hz, 1H), 6.92 (d, J=9.0 Hz, 2H), 6.85 (d, J=9.0 Hz, 2H), 6.61 (s, 1H), 5.15 (s, 2H), 3.88 (s, 3H), 3.78 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -62.46; 13C NMR (126 MHz, CDCl3) δ: 168.82, 161.85, 157.87, 154.69, 151.93, 129.88, 129.80, 129.33 (q, 2JC—F=32.4 Hz, ArCF3), 129.32, 128.09, 125.01 (q, 3JC—F=3.6 Hz, ArCF3), 124.30 (q, 1JC—F=273.4 Hz, ArCF3), 123.22, 121.68, 116.05, 114.80, 111.60, 101.04, 62.37, 55.77, 55.70; IR (KBr) ν: 2946, 2840, 1612, 1511, 1453, 1323, 1234, 1123, 1025, 814 cm-1. HRMS (EI) calcd for C25H21F3NO4[(M+H)+]: 456.1408, found 456.1417.
3-(3-甲氧基苯基)-4-苯基-5-(3-硝基苯氧甲基)异噁唑(5-22):白色固体, 产率85%. m.p. 158.9~159.4℃; 1H NMR (500 MHz, CDCl3) δ: 7.90~7.88 (m, 1H), 7.85 (t, J=2.5 Hz, 1H), 7.79 (dd, J=8.5, 2.5 Hz, 1H), 7.75 (d, J=2.0 Hz, 1H), 7.55~7.53 (m, 2H), 7.48 (t, J=8.0 Hz, 1H), 7.44~7.41 (m, 2H), 7.38~7.34 (m, 1H), 7.33~7.31 (m, 1H), 7.06 (d, J=8.5 Hz, 1H), 6.67 (s, 1H), 5.28 (s, 2H), 3.87 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 166.90, 162.20, 158.25, 158.11, 149.23, 137.67, 131.31, 130.36, 129.54, 129.47, 128.14, 127.41, 127.30, 121.63, 121.14, 116.87, 111.55, 109.43, 101.82, 61.69, 55.75; IR (KBr) ν: 3106, 2993, 2940, 2840, 1609, 1528, 1350, 1242, 1056, 813 cm-1. HRMS (ESI) calcd for C23H19N2O5[(M+H)+]: 403.1291, found 403.1288.
3-(3-甲氧基苯基)-4-(4-氟苯基)-5-(3-硝基苯氧甲基)异噁唑(5-23):白色固体, 产率85%. m.p. 166.2~167.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.90 (ddd, J=8.0, 2.0, 1.0 Hz, 1H), 7.85 (t, J=2.5 Hz, 1H), 7.78 (dd, J=8.5, 2.5 Hz, 1H), 7.75 (d, J=8.5Hz, 1H), 7.55 (m, 3H), 7.35 (dt, J=8.0, 2.0 Hz, 1H), 7.12 (dd, J=8.5, 2.0 Hz, 2H), 7.05 (d, J=8.5 Hz, 1H), 6.67 (s, 1H), 5.28 (s, 2H), 3.87 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -115.03~-115.09 (m); 13C NMR (126 MHz, CDCl3) δ: 166.93, 162.24 (d, 1JC—F=246.9 Hz), 162.12, 158.23, 158.01, 149.26, 133.52, 131.15 (d, 3JC—F=7.6 Hz, ArF), 130.36, 130.34, 129.32, 127.40, 121.65, 121.19, 116.93, 115.03 (d, 2JC—F=21.4 Hz, ArF), 111.52, 109.40, 101.75, 61.72, 55.76; IR (KBr) ν: 3097, 2918, 2851, 1611, 1529, 1355, 1237, 1164, 1055, 825 cm-1. HRMS (ESI) calcd for C23H18FN2O5[(M+H)+]: 421.1194, found 421.1194.
3-(3-甲氧基苯基)-4-(4-三氟甲基苯基)-5-(3-硝基苯氧甲基)异噁唑(5-24):白色固体, 产率82%. m.p. 157.8~158.0 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.91 (dd, J=8.0, 1.0 Hz, 1H), 7.85 (t, J=2.5 Hz, 1H), 7.82 (dd, J=8.0, 2.0 Hz, 1H), 7.67 (d, J=2.0 Hz, 1H), 7.71~7.63 (m, 4H), 7.49 (t, J=8.0 Hz, 1H), 7.32 (dd, J=8.5, 2.0 Hz, 1H), 7.08 (d, J=8.5 Hz, 1H), 6.67 (s, 1H), 5.29 (s, 2H), 3.88 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -62.39; 13C NMR (126 MHz, CDCl3) δ: 167.07, 161.97, 158.22, 158.00, 149.23, 141.33, 130.37, 129.87, 129.85, 129.33 (q, 2JC—F=32.4 Hz, ArCF3), 129.28, 128.12, 124.30 (q, 1JC—F=272.2 Hz, CF3), 125.01 (q, 3JC—F=3.8 Hz, ArCF3), 121.63, 121.36, 116.90, 111.66, 109.38, 101.74, 61.68, 55.78; IR (KBr) ν: 2932, 2849, 1612, 1524, 1330, 1246, 1119, 1068, 825 cm-1. HRMS (ESI) calcd for C24H18F3N2O5 [(M+H)+]: 471.1160, found 471.1162.
3-(3-甲氧基苯基)-4-苯基-5-(4-氟苯氧甲基)异噁唑(5-25):白色固体, 产率90%. m.p. 121.5~122.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.79 (d, J=8.5 Hz, 1H), 7.75 (d, J=2.0 Hz, 1H), 7.55 (d, J=8.5 Hz, 2H), 7.44 (t, J=8.0 Hz, 2H), 7.35 (d, J=8.5 Hz, 1H), 7.05 (d, J=8.5 Hz, 1H), 7.01 (d, J=8.5 Hz, 2H), 6.96~6.91 (m, 2H), 6.61 (s, 1H), 5.16 (s, 2H), 3.87 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -122.28~-122.34 (m); 13C NMR (126 MHz, CDCl3) δ: 168.11, 162.13, 158.03, 157.91 (d, 1JC—F=240.5 Hz, ArF), 153.94, 137.72, 131.30, 129.54, 129.51, 128.13, 127.38, 127.27, 121.34, 116.13 (d, 2JC—F=23.7 Hz, ArF), 116.11 (d, 3JC—F=7.9 Hz, ArF), 111.50, 101.29, 100.00, 62.18, 55.74; IR (KBr) ν: 3063, 2936, 2840, 1608, 1508, 1446, 1365, 1241, 1025, 817 cm-1. HRMS (ESI) calcd for C23H19FNO3[(M+H)+]: 376.1338, found 376.1343.
3-(3-甲氧基苯基)-4-(4-氟苯基)-5-(4-氟苯氧甲基)异噁唑(5-26):白色固体, 产率88%. m.p. 105.8~106.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.77 (dd, J=8.5, 2.5 Hz, 1H), 7.73 (d, J=2.0 Hz, 1H), 7.54~7.48 (m, 2H), 7.11 (dt, J=7.0, 2.0 Hz, 2H), 7.05 (d, J=8.5 Hz, 1H), 7.03~6.98 (m, 2H), 6.95~6.92 (m, 2H), 6.61 (s, 1H), 5.16 (s, 2H), 3.87 (s, 3H); 19F NMR (471 MHz, DMSO) δ: -115.12~-115.15 (m), -122.76~-122.81 (m); 13C NMR (126 MHz, DMSO) δ: 168.63, 161.98 (d, 1JC—F=244.9 Hz, ArF), 161.97, 158.42, 157.48 (d, 1JC—F=237.5 Hz, ArF), 154.36, 134.10, 134.08, 131.77 (d, 3JC—F=8.07 Hz, ArF), 129.80, 129.15, 127.93, 121.40, 116.73 (d, 3JC-F=8.15 Hz, ArF), 116.43 (d, 2JC—F=23.09 Hz, ArF), 115.33 (d, 2JC—F=21.3 Hz, ArF), 112.71, 102.72, 61.56, 56.20; IR (KBr) ν: 3066, 2956, 2843, 1610, 1512, 1450, 1357, 1220, 1069, 823 cm-1. HRMS (ESI) calcd for C23H18F2NO3[(M+H)+]: 394.1243, found 394.1249.
3-(3-甲氧基苯基)-4-(4-三氟甲基苯基)-5-(4-氟苯氧甲基)异噁唑(5-27):白色固体, 产率82%. m.p. 108.0~108.7 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.82 (dd, J=8.0, 2.0 Hz, 1H), 7.76 (d, J=2.0 Hz, 1H), 7.68 (d, J=8.5 Hz, 2H), 7.65 (d, J=8.5 Hz, 2H), 7.08 (d, J=8.5 Hz, 1H), 7.02 (t, J=9.0 Hz, 2H), 6.94 (dd, J=9.0, 4.0 Hz, 2H), 6.62 (s, 1H), 5.17 (s, 2H), 3.88 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -62.46 (s), -122.22~-122.27 (m); 13C NMR (126 MHz, CDCl3) δ: 168.30, 161.90, 157.94, 157.90 (d, 1JC—F=239.7 Hz), 153.95, 129.90, 129.31 (q, 2J=32.3 Hz, ArCF3), 129.28, 128.12, 125.01 (q, 3JC—F=3.7 Hz, ArCF3), 124.35 (q, 1JC—F=272.0 Hz, CF3), 121.58, 116.13 (d, 2JC—F=23.2 Hz, ArF), 116.08 (d, 3JC-F=8.1 Hz), 111.63, 101.23, 62.10, 55.74; IR (KBr) ν: 3058, 2941, 2844, 1613, 1513, 1430, 1357, 1259, 1103, 830 cm-1. HRMS (ESI) calcd for C24H18F4NO3[(M+H)+]: 444.1225, found 444.1217.
3-(3-甲氧基苯基)-4-苯基-5-(3-甲基苯氧甲基)异噁唑(5-28):白色固体, 产率91%. m.p. 102.6~103.9 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.79 (dd, J=8.5, 2.0 Hz, 1H), 7.75 (d, J=2.5 Hz, 1H), 7.55 (dd, J=8.5, 1.0 Hz, 2H), 7.43 (t, J=7.5 Hz, 2H), 7.35 (t, J=7.5 Hz, 1H), 7.20 (t, J=8.0 Hz, 1H), 7.05 (d, J=8.5 Hz, 1H), 6.83 (d, J=8.0 Hz, 3H), 6.85~6.77 (m, 1H), 5.19 (s, 2H), 3.87 (s, 3H), 2.35 (s, 3H); 13C NMR (126 MHz, CDCl3) δ: 168.11, 162.13, 158.85, 158.03, 156.94, 153.94, 137.72, 131.30, 129.54, 129.51, 128.13, 127.38, 127.27, 121.34, 116.22, 116.14, 116.08, 116.04, 111.50, 101.29, 62.18, 55.74; IR (KBr) ν: 3052, 2924, 2849, 1601, 1508, 1448, 1349, 1257, 1174, 776 cm-1. HRMS (ESI) calcd for C24H22NO3[(M+H)+]: 372.1587, found 372.1594.
3-(3-甲氧基苯基)-4-(4-氟苯基)-5-(3-甲基苯氧甲基)异噁唑(5-29):白色固体, 产率84%. m.p. 103.8~104.3 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.78 (dd, J=8.5, 2.5 Hz, 1H), 7.73 (d, J=2.0 Hz, 1H), 7.52 (dd, J=9.0, 5.0 Hz, 2H), 7.20 (t, J=7.5 Hz, 1H), 7.11 (t, J=8.5 Hz, 2H), 7.04 (d, J=8.5 Hz, 1H), 6.85~6.78 (m, 3H), 6.62 (s, 1H), 5.18 (s, 2H), 3.87 (s, 3H), 2.36 (s, 3H); 19F NMR (471 MHz, CDCl3) δ: -115.06~-115.11 (m); 13C NMR (126 MHz, CDCl3) δ: 168.62, 162.22 (d, 1JC—F=246.9 Hz, ArF), 162.01, 157.87, 157.85, 139.86, 133.61, 131.17 (d, 3JC—F=7.6 Hz, ArF), 130.25, 129.41, 129.34, 127.39, 122.72, 121.52, 115.67, 115.01 (d, 2JC—F=21.4 Hz, ArF), 111.57, 111.47, 101.02, 61.48, 55.75, 21.54; IR (KBr) ν: 3023, 2920, 2849, 1605, 1509, 1453, 1259, 1157, 785 cm-1. HRMS (ESI) calcd for C24H21FNO3[(M+H)+]: 390.1494, found 390.1500.
3-(3-甲氧基苯基)-4-(4-三氟甲基苯基) -5-(3-甲基苯氧甲基)异噁唑(5-30):白色固体, 产率86%. m.p. 109.0~109.1 ℃; 1H NMR (500 MHz, CDCl3) δ: 7.82 (dd, J=8.5, 2.0 Hz, 1H), 7.77 (d, J=2.0 Hz, 1H), 7.71~7.64 (m, 4H), 7.20 (t, J=7.5 Hz, 1H), 7.07 (d, J=8.5 Hz, 1H), 6.84~6.78 (m, 3H), 6.62 (s, 1H), 5.19 (s, 2H), 3.88 (s, 3H), 2.35 (s, 3H); 19F NMR (471 MHz, DMSO) δ: -61.12; 13C NMR (126 MHz, DMSO-d6) δ: 168.92, 161.86, 158.11, 158.03, 141.99, 139.67, 130.56, 129.74, 129.27, 129.23, 128.65, 128.25 (q, 2JC—F=31.5 Hz, ArCF3), 125.26 (q, 3JC—F=3.6 Hz, ArCF3), 124.82 (q, 1JC—F=272.2 Hz, CF3), 122.63, 121.63, 115.90, 112.74, 112.09, 102.50, 60.87, 56.15, 21.43; IR (KBr) ν: 3012, 2928, 2849, 1615, 1456, 1326, 1260, 1117, 842 cm-1. HRMS (ESI) calcd for C25H21F3NO3[(M+H)+]: 440.1462, found 440.1468.
辅助材料(Supporting Information) 所合成化合物的1H NMR、13C NMR及含氟化合物的19F NMR谱图.这些材料可以免费从本刊网站( http://sioc-journal.cn/)上下载.
-
-
[1]
Castellano, S.; Kuck, D.; Viviano, M.; Yoo, J.; Lopeź-Vallejo, F.; Conti, P.; Tamborini, L.; Pinto, A.; Medina-Franco, J. L.; Sbardella, G. J. Med. Chem. 2011, 54, 7663. doi: 10.1021/jm2010404
-
[2]
Kumar, K. A.; Jayaroopa, P. Chem. Biol. Sci. 2013, 3, 294.
-
[3]
Dong, K. Y.; Qin, H. T.; Bao, X. X.; Liu, F.; Zhu, C. Org. Lett. 2014, 16, 5266. doi: 10.1021/ol502246t
-
[4]
Pereź, J. M.; Ramon, D. J. ACS Sustainable Chem. Eng. 2015, 3, 2343. doi: 10.1021/acssuschemeng.5b00689
-
[5]
范玉杰, 农药研究与应用, 2010, 14, 1. http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htmFan, Y. J. Agrochem. Res. Appl. 2010, 14, 1(in Chinese http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htm
-
[6]
贺红武, 李美强, 黄刚良, 农药, 2000, 39, 4. doi: 10.3969/j.issn.1006-0413.2000.05.002He, H. W.; Li, M. Q.; Huang, G. L. Agrochemicals 2000, 39, 4(in Chinese doi: 10.3969/j.issn.1006-0413.2000.05.002
-
[7]
Eccles, S. A.; Massey, A.; Raynaud, F. I.; Sharp, S. Y.; Box, G.; Valenti, M.; Patterson, L.; Brandon, A. D. H.; Gowan, S.; Boxall, F.; Aherne, W.; Rowlands, M.; Hayes, A.; Martins, V.; Urban, F.; Boxall, K.; Prodromou, C.; Pearl, L.; James, K.; Matthews, T. P.; Cheung, K. M.; Kalusa, A.; Jones, K.; McDonald, E.; Barril, X.; Brough, P. A.; Cansfield, J. E.; Dymock, B.; Drysdale, M. J.; Finch, H.; Howes, R.; Hubbard, R. E.; Surgenor, A.; Webb, P.; Wood, M.; Wright, L.; Workman, P. Cancer Res. 2008, 68, 2850. doi: 10.1158/0008-5472.CAN-07-5256
-
[8]
Sun, R.; Li, Y.; Xiong, L.; Liu, Y.; Wang, Q. J. Agric. Food Chem. 2011, 59, 4851. doi: 10.1021/jf200395g
-
[9]
Sperry, J.; Wright, D. Curr. Opin. Drug Discovery Dev. 2005, 8, 723.
-
[10]
Lee, Y.; Koyama, Y.; Yonekawa, M.; Tanaka, T. Macromolecules 2009, 42, 7709. doi: 10.1021/ma9014577
-
[11]
Masafumi, U.; Yuki, I.; Aoi, S.; Yuta, I.; Maiko, K.; Hiroko, S.; Tetsuya, M.; Takeaki, N.; Okiko, M. Tetrahedron 2011, 67, 4612. doi: 10.1016/j.tet.2011.04.083
-
[12]
Zhou, Z. W.; Yan, J. F.; Tang, X. M. Chin. J. Org. Chem. 2010, 30, 582.
-
[13]
Changtam, C.; Hongmanee, P.; Suksamrarn, A. Eur. J. Med. Chem. 2010, 45, 4446. doi: 10.1016/j.ejmech.2010.07.003
-
[14]
John, J. T.; David, L. B.; Jeffery, S. C.; Matthew, J. G.; Carol, M. K.; Jaime, L. M.; William, E. P.; Roland, S. R.; Alexander, F. S.; Zhang, Y. Y.; Ben, S. Z.; Karen, S. J. Med. Chem. 2000, 43, 777.
-
[15]
Yermolina, M. V.; Wang, J.; Caffrey, M. J. Med. Chem. 2011, 54, 765. doi: 10.1021/jm1008715
-
[16]
Jensen, A. A.; Plath, N.; Pedersen, M. H. F.; Isberg, V.; Krall., J. J. Med. Chem. 2013, 56, 1211. doi: 10.1021/jm301656h
-
[17]
Vitale, P.; Tacconelli, S.; Perrone, M. G. J. Med. Chem. 2013, 56, 4277. doi: 10.1021/jm301905a
-
[18]
Fournier, P. A.; Arbour, M.; Cauchon, E. Bioorg. Med. Chem. Lett. 2012, 22, 2670. doi: 10.1016/j.bmcl.2012.03.014
-
[19]
Gomha, S. M.; Badrey, M. G.; Abdalla, M. M.; Arafa, R. K. Med. Chem. Commun. 2014, 5, 1685. doi: 10.1039/C4MD00282B
-
[20]
Crocker, C. E.; Khan, S.; Cameron, M. D.; Robertson, H. A.; Robertson, G. S.; Lograsso, P. ACS Chem. Neurosci. 2011, 2, 207. doi: 10.1021/cn1001107
-
[21]
Chambers, J. W.; Pachori, A.; Howard, S.; Ganno, M.; Hansen, D.; Kamenecka, T.; Song, X.; Duckett, D.; Chen, W.; Ling, Y. Y.; Cherry, L.; Cameron, M. D.; Lin, L.; Ruiz, C. H.; Lograsso, P. ACS Chem. Neurosci. 2011, 2, 198. doi: 10.1021/cn100109k
-
[22]
Kumar, D. J. S.; Ho, M. M.; Leung, J. M.; Toyokuni, T. Adv. Synth. Catal. 2002, 344, 1146. doi: 10.1002/(ISSN)1615-4169
-
[23]
Sahoo, A. K.; Oda, T.; Nakao, Y.; Hiyama, T. Adv. Synth. Catal. 2004, 346, 1715. doi: 10.1002/adsc.200404188
-
[24]
Fu, W. C.; Zhou, Z. Y.; Kwong, F. Y. Org. Chem. Front. 2016, 3, 273. doi: 10.1039/C5QO00400D
-
[25]
Velcicky, J.; Soicke, A.; Steiner, R.; Schmalz, H. G. J. Am. Chem. Soc. 2011, 133, 6948. doi: 10.1021/ja201743j
-
[26]
Oberli, M. A.; Buchwald, S. L. Org. Lett. 2012, 14, 4606. doi: 10.1021/ol302063g
-
[27]
Scott, P. J. L.; Clarke, A.; Richardson, J. Org. Lett. 2015, 17, 476. doi: 10.1021/ol503479g
-
[28]
Yang, Y.; Oldenhuis, N. J.; Buchwald, S. L. Angew. Chem. Int. Ed. 2013, 52, 615. doi: 10.1002/anie.201207750
-
[29]
Haag, B. A.; Sämann, C.; Jana, A.; Knochel, P. Angew. Chem. Int. Ed. 2011, 50, 7290. doi: 10.1002/anie.v50.32
-
[30]
Piller, F. M.; Metzger, A.; Schade, M..; Haag, B. A.; Garyushin, A.; Knochel, P. Chem. Eur. J. 2009, 15, 7192.
-
[31]
Bernhardt, S.; Manolikakes, G.; Kunz, T.; Knochel, P. Angew. Chem. Int. Ed. 2011, 50, 9205. doi: 10.1002/anie.201104291
-
[32]
(a) Rogers, R. S.; Talley, J. J.; Brown, D. L.; Nagarajan, S.; Carter, J. S.; Weier, R. M.; Stealey, Michael A.; Collins, Paul W.; Seibert, K. WO 9625405, 1996[Chem. Abstr. 1996, 125, 247800].(b) Rogers, R. S.; Talley, J. J.; Sikorski, J. A.; Devadas, B.; Graneto, M. J.; Carter, J. S.; Norman, B. H.; Lu, H. F; Brown, D. L.; Nagarajan, S. WO 9638442, 1996[Chem. Abstr. 1997, 126, 104081].
-
[33]
Yang, Y. D.; Zhang, M.; Zhu, Y. W.; Zhang, L.; Xie, Q. Q.; Song, L. P.; Deng, H. M. Chin. J. Chem. 2013, 31, 950. doi: 10.1002/cjoc.v31.7
-
[34]
徐姗姗, 张敏, 张丽, 蒋海芳, 朱宁, 宋力平, 邓红梅, 有机化学, 2015, 35, 2595. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345186.shtmlXu, S. S.; Zhang, M.; Zhang, L.; Jiang, H. F.; Zhu, N.; Song, L. P.; Deng, H. M. Chin. J. Org. Chem. 2015, 35, 2595(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345186.shtml
-
[35]
He, C.; He, Q.; Chen, Q.; Shi, L.; Cao, H.; Cheng, J. Tetrahedron Lett. 2010, 51, 1317. doi: 10.1016/j.tetlet.2009.12.136
-
[36]
Hao, Z. S.; Li, M. J.; Lin, H. X.; Gu, Z. B.; Cui, Y. M. Dyes Pigm. 2014, 109, 54. doi: 10.1016/j.dyepig.2014.04.042
-
[37]
Budzik, B. W.; Evans, K. A.; Wisnoski, D. D.; Jin, J.; Rivero, R. A.; Szewczyk, G. R.; Jayawickreme, C.; Moncol, D. L.; Yu, H. S. Bioorg. Med. Chem. Lett. 2010, 20, 1363. doi: 10.1016/j.bmcl.2010.01.003
-
[38]
CCDC 1540659 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data CEntre via www.ccdc.cam.ac.uk/ data_request/cif.
-
[1]
-
表 1 化合物2a的反应条件优化a
Table 1. Optimization of reaction conditions for preparation 2a
Entry Solvent Temp/℃ T/h Yieldb/% 1 DMF 25 12 —c 2 AcOH 60 12 —c 3 AcOH/TFA (V/V=10/1) 25 12 —c 4 AcOH/TFA (V/V=10/1) 60 12 25 5 TFA 25 12 37 6 TFA 60 6 75 a Reaction conditions: 1:NBS=1:2 (molar ratio). bIsolated yield. cNo product was detected. 表 2 异噁唑衍生物3-1~3-10的合成a
Table 2. Synthesis of isoxazole derivatives 3-1~3-10
Entry R1 R2 Product Yieldb/% 1 4-CN 3-NO2 3-1 87 2 4-CN 4-CH3O 3-2 85 3 4-CN 4-F 3-3 84 4 4-NO, 4-CH3O 3-4 88 5 4-NO2 3-NO2 3-5 69 6 4-NO2 3-Me 3-6 85 7 4-CH3O 4-CH3O 3-7 90 8 4-CH3O 3-NO2 3-8 75 9 4-CH3O 4-F 3-9 86 10 4-CH3O 3-Me 3-10 88 aReaction conditions: 25 ℃, 12h; b Isolated yield. 表 3 异噁唑衍生物5-1~5-30的合成a
Table 3. Synthesis of isoxazole derivatives 5-1~5-30
Entry R1 R2 R3 Product Yieldb/% 1 4-CN 4-NO2 H 5-1 88 2 4-CN 4-NO2 F 5-2 86 3 4-CN 3-NO2 4-CF3 5-3 91 4 4-CN 4-CH3O H 5-4 82 5 4-CN 4-CH3O 4-F 5-5 91 6 4-CN 4-CH3O 4-CF3 5-6 92 7 4-CN 4-F H 5-7 89 8 4-CN 4-F 4-F 5-8 82 9 4-CN 4-F 4-CF3 5-9 85 10 4-NO2 4-CH3O H 5-10 85 11 4-NO2 4-CH3O 4-F 5-11 83 12 4-NO2 4-CH3O 4-CF3 5-12 86 13 4-NO2 3-NO2 H 5-13 84 14 4-NO2 3-NO2 4-F 5-14 81 15 4-NO2 3-NO2 4-CF3 5-15 84 16 4-NO2 3-Me H 5-16 83 17 4-NO2 3-Me 4-F 5-17 83 18 4-NO2 3-Me 4-CF3 5-18 85 19 3-CH3O 4-CH3O H 5-19 89 20 3-CH3O 4-CH3O 4-F 5-20 91 21 3-CH3O 4-CH3O 4-CF3 5-21 88 22 3-CH3O 3-NO2 H 5-22 85 23 3-CH3O 3-NO2 4-F 5-23 85 24 3-CH3O 3-NO2 4-CF3 5-24 82 25 3-CH3O 4-F H 5-25 90 26 3-CH3O 4-F 4-F 5-26 88 27 3-CH3O 4-F 4-CF3 5-27 82 28 3-CH3O 3-Me H 5-28 91 29 3-CH3O 3-Me 4-F 5-29 84 30 3-CH3O 3-Me 4-CF3 5-30 86 a Reaction conditions: 90 ℃, 12 h. b Isolated yield. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 9
- 文章访问数: 2100
- HTML全文浏览量: 201


 下载:
下载:

 下载:
下载:

