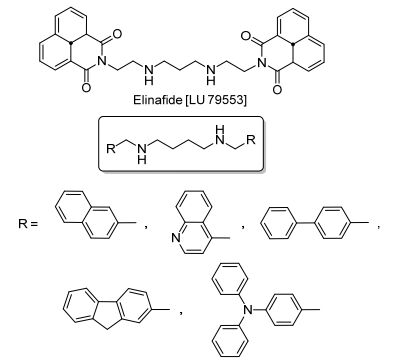
 Figure 1.
Structure of Elinafide and symmetric-substituted diamines
Figure 1.
Structure of Elinafide and symmetric-substituted diamines

双-(1-杂环-β-咔啉)-3-烷氨基衍生物的合成与抗肿瘤活性
English
Synthesis and Antitumor Activities of Novel Bivalent 1-Heterocyclic-β-carbolines Linked by Alkylamino Spacer
-
Key words:
- bivalent β-carboline
- / synthesis
- / antitumor activity
- / structure-activity relationship
-
β-Carboline alkaloids are a class of natural and synthetic products that have a broad spectrum of biochemical effects and pharmacological properties.[1-6] The reported biological applications of β-carboline alkaloids include sedative and anxiolytic, [1] antitumor, [2, 3] antimalarial, [3] antiparasitic, [4] anti-HIV[5] agents, and other pharmacological activities. Recently, the structures of modified β-carboline alkaloids as a new class of antitumor agents have attracted the attention of chemists. It has been reported that β-carboline alkaloids can exhibit antitumor activities through multiple mechanisms, such as DNA binding, [6] inhibition topoisomerases Ⅰ and Ⅱ, [7, 8] cyclin-dependent kinase (CDK), [9] polo-like kinase (PLK1), [10] kinesin-like protein Eg5, [11] and IκB kinases.[12]
Recent reports suggest that bivalent β-carboline alkaloids have much better cytotoxic efficacies than monovalent ones.[13-15]This is most likely because of the dimerization of various intercalating agents by an appropriate spacer, which can increase DNA binding affinity. Thus, there has been tremendous interest in the design of novel polyvalent molecules that possess significant properties different from their monovalent counterparts.[16] For example, Elinafide [LU 79553][17](Figure 1), a naphthalimide derivative, has antitumor activities as a DNA intercalator and topoisomerase Ⅱ inhibitor. Burns et al.[18] synthesized a series of aromatic-substituted diamines (Figure 1) and evaluated their cytotoxic profiles against human breast and prostate tumor cell lines. The results showed that some aryl-substituted diamine compounds demonstrated a nearly 300-fold improvement over the monovalent compounds with respect to cytotoxic properties.
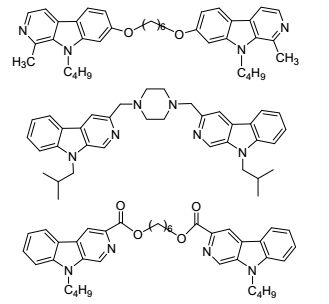
For more than a decade, our group[19~23] has focused on incorporating substituents into positions-1, 2, 3, 7 and 9 of the β-carboline nucleus as an antitumor agent. Our group has also investigated several novel bivalent β-carbolines with an alkyl spacer in positon-1, 3 and 7 of β-carboline nucleus[24~26](Figure 2), and these have exhibited more potent antitumor efficacies than monomers. In the present work, we designed and synthesized a series of 1-heterocyclic substituted bivalent β-carbolines with a spacer of four or five methylene units between the two 3-methylamino groups and to find congeners more active as potential antitumor agents and to study in depth the influence of the substituent in positions-1 and 3 of the β-carboline nucleus. The compounds for this study were synthesized from the starting material ethyl 1-heterocyclic substituted-β-carboline-3-carboxylate via the alkylation, oxidation, condensation, and reduction (Scheme 1). The antitumor activities of these compounds were studied in vitro. The details of the synthesis and biological activities of these compounds are presented herein.
1 Results and discussion
1.1 Synthesis
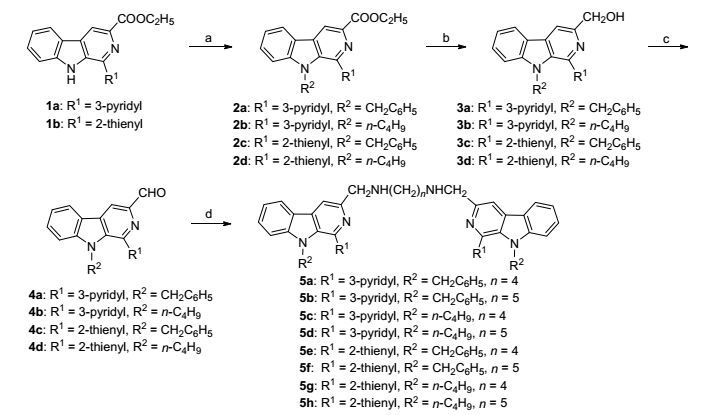
The synthetic route for the 1-heterocyclic substituted bivalent β-carbolines is outlined in Scheme 1. The starting material ethyl 1-heterocyclic substituted-β-carboline-3-car-boxylate (1a~1b). The N9 of 1 was alkylated or arylated by the action of sodium hydride in anhydrous DMF followed by the addition of 1-iodobutane or benzyl bromide to obtain intermediates 2. The ester group in position-3 of 2 was reduced to its corresponding alcohols by LiBH4 in dry THF to provide compounds 3, and further oxidized by MnO2 in CH3CN to give 3-carboxaldehydes 4. The reaction of compounds 4 with the corresponding diamines to form schiff bases took place readily at room temperature in good yield. The crude Schiff bases without further purification were directly reduced with NaBH3CN in anhydrous methanol to give the target 1-heterocyclic substituted bivalent β-carbolines 5 in 40%~64% yield.
1.2 In vitro cell cytotoxicity assay
All the synthesized compounds were subjected to in vitro anticancer evaluation using methyl thiazolyl tetrazolium (MTT)[26] assay in ten human cancer cell lines and compared with the reference drug cisplatin (DDP). In order to enhance the solubility in aqueous solution, all compounds were prepared in the form of hydrochloride salt before use. IC50 (μmol•L−1) are presented in Table 1.
Comp. IC50/(μmol•L-1) 22RV1 SK-OV-3 MCF-7 LLC Eca-109 BGC-823 HT-29 HepG2 769-P A375 3a > 100 > 100 72.4 70.7 > 100 > 100 67.5 53.6 41.6 > 100 3b 90.5 > 100 > 100 42.4 > 100 > 100 > 100 > 100 66.1 > 100 3c > 100 > 100 76.7 56.8 > 100 76.7 74.9 54.3 32.7 90.1 3d > 100 > 100 97.8 > 100 > 100 > 100 89.2 98.5 79.5 93.2 4a > 100 90.1 > 100 > 100 > 100 > 100 > 100 48.9 18.2 58.8 4b > 100 89.2 > 100 > 100 > 100 > 100 > 100 19.9 66.6 > 100 4c > 100 69.1 77.5 91.1 > 100 83.1 69.1 55.9 27.5 83.2 4d 72.2 65.8 54.8 67.4 36.2 58.7 17.7 56.2 19.9 51.1 5a 3.7 2.8 5.7 5.7 6.0 6.0 6.2 7.6 4.1 6.2 5b 1.9 1.4 7.5 1.3 5.5 5.2 1.6 9.9 1.0 5.5 5c 2.3 1.6 6.9 1.7 7.2 4.0 7.4 7.9 1.8 6.6 5d 1.6 5.5 3.8 1.3 2.1 1.6 2.0 4.0 0.8 1.6 5e 3.9 2.7 2.7 1.4 5.3 7.5 5.8 7.0 2.6 4.7 5f 1.3 2.6 6.3 1.4 5.4 7.4 4.7 5.9 1.6 3.4 5g 3.4 2.2 4.6 1.9 1.8 1.5 5.2 4.4 2.1 2.3 5h 0.6 2.2 2.1 1.9 1.8 1.9 5.4 2.6 1.5 1.8 DDP 5.2 5.6 12.4 7.6 8.9 11.6 26.8 14.8 19.2 9.4 As expected, all the 1-heterocyclic substituted bivalent β-carbolines exhibited excellent cytotoxic activities (IC50 values lower than 10 μmol•L−1) against all human tumor cell lines tested. In particular, these compounds were more potent than the positive control drug cisplatin. Meanwhile, the monovalent intermediates 3a~3d and 4a~4d showed weaker cytotoxic activities, all bivalent β-carbolines had significant cytotoxic efficacies in comparable to the mon-ovalent β-carbolines. The results indicate that C3-sub-stituted dimers markedly improve cytotoxic activities.
We also examined the influence of the spacer length of 1-heterocyclic substituted bivalent β-carbolines and the effect of the substituent in position-9 of the β-carboline ring on the cytotoxic activities. The data show that compounds 5b, 5d, 5f and 5h (each with a spacer of five methylene units) had the highest cytotoxicity. Moreover, the 22RV1 and 769-P cell lines were more sensitive to these compounds than other cell lines. Compounds 5d and 5h, both of which had n-butyl in position-9 of the β-carboline ring, had the most potent inhibitory activity against 769-P and 22RV1. The IC50values for compounds 5d and 5h were 0.8 and 0.6 μmol•L-1, respectively.
2 Conclusions
A new series of 1-heterocyclic substituted bivalent β-carbolines with a spacer of four or five methylene units between the 3-methylamino group have been designed and synthesized. Their cytotoxic potential against human tumor cell lines in culture were investigated. The results demonstrated that all bivalent β-carbolines had significant cytotoxic efficacies in comparable to the reference drug cisplatin and monovalent β-carbolines, and compound 5d showed significant inhibitory activity against 769-P with IC50values of 0.8 μmol•L-1 and compound 5h showed significant inhibitory activity against 22RV1 with IC50values of 0.6 μmol•L-1. Preliminary SARs analysis indicated that 2-thienyl into position-1 of the β-carboline nucleus and the spacer of five methylene units between the 3-methylamino group provided compounds with greatly enhanced cytotoxic potencies. Further investigations to confirm the antitumor efficacy in animal models and elucidate the pharmacological mechanisms of this class of compounds are underway in our laboratory.
3 Experimental
3.1 Materials and characterization
MS spectra were obtained from a Micromass ZQ4000 spectrometer; HRMS were obtained from a Bruker ultrafleXtreme MALDI-TOF/TOF spectrometer. 1H NMR and 13C NMR spectra were recorded on a Varian inova-400 spectrometer at 400 MHz and 100 MHz, respectively, using TMS as internal standard and CDCl3 or DMSO-d6 as solvent. Melting points were determined in capillary tubes on an electrothermal WRS-3 apparatus and without correction. Elemental analyses (C, H and N) were carried out on an Elementar Vario ELⅢ CHNS Elemental Analyzer.
All solvents and reagents were obtained from commercial sources without further purification. Silica GF254 used in the analytical thin-layer chromatography (TLC) and silica gel (200-300 mesh) for column chromatography were produced by the Qingdao Haiyang Chemical Co., Ltd.
3.2 Chemistry
3.2.1 General procedure for the preparation of 2a~2d
A mixture of 1a (3.17g, 10 mmol) and anhydrous DMF (60 mL) was stirred at room temperature for 0.5 h, and then 95% NaH (0.37 g, 15 mmol) and benzyl bromide (1.3 mL, 12 mmol) were added. The mixture was stirred at room temperature for 0.5~2 h. After completion of the reaction as indicated by TLC, the solution was poured into H2O (150 mL) and extracted with ethyl acetate. The organic phase was washed with water and brine, then dried over anhy-drous sodium sulfate, filtered and evaporated. The resulting oil was crystallized from ethyl ether or ethyl ether-petro-leum ether.
Ethyl 9-benzyl-1-(3-pyridyl)-β-carboline-3-carboxylate (2a): White solid, yield 89%. m.p. 148.4~149.7 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.98 (s, 1H), 8.70 (d, J=2.4 Hz, 1H), 8.66 (dd, J=4.8, 1.6 Hz, 1H), 8.32 (d, J=7.2 Hz, 1H), 7.61~7.68 (m, 2H), 7.42~7.46 (m, 2H), 7.21~7.24 (m, 1H), 7.15~7.18 (m, 1H), 7.08~7.12 (m, 2H), 6.45 (d, J=7.6 Hz, 2H), 5.29 (s, 2H), 4.53 (q, J=7.2 Hz, 2H), 1.47 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 165.87, 149.88, 149.36, 143.06, 138.10, 137.06, 136.05, 135.80, 131.04, 129.47, 129.44, 128.70, 128.61, 127.56, 125.29, 122.91, 121.93, 121.56, 121.44, 117.11, 110.78, 61.72, 48.35, 14.50; ESI-MS m/z: 409 [M+H]+. Anal. calcd for C26H21N3O2: C 76.64, H 5.19, N 10.31; found C 76.39, H 5.37, N 10.38.
Ethyl 9-n-butyl-1-(3-pyridyl)-β-carboline-3-carboxylate (2b): White solid, yield 91%. m.p. 156.1~156.9 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.93 (s, 1H), 8.92 (s, 1H), 8.78 (d, J=5.6 Hz, 1H), 8.27 (d, J=8.0 Hz, 1H), 8.06 (d, J=7.2 Hz, 1H), 7.64~7.68 (m, 1H), 7.49~7.54 (m, 2H), 7.38~7.42 (m, 1H), 4.53 (q, J=7.2 Hz, 2H), 4.01 (t, J=8.0 Hz, 2H), 1.48 (t, J=7.2 Hz, 3H), 1.33~1.40 (m, 2H), 0.84~0.96 (m, 4H), 0.66 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 165.91, 149.91, 149.33, 142.49, 140.14, 137.67, 137.43, 135.87, 130.87, 129.12, 123.33, 121.89, 121.04, 117.13, 110.61, 61.67, 44.67, 30.92, 19.77, 14.50, 13.45; ESI-MS m/z: 374 [M+H]+. Anal. calcd for C23H23N3O2: C 73.97, H 6.21, N 11.25; found C 73.85, H 6.07, N 11.36.
Ethyl 9-benzyl-1-(2-thienyl)-β-carboline-3-carboxylate (2c):White solid, yield 94%. m.p. 171.2~172.6 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.91 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 7.55~7.59 (m, 1H), 7.36~7.43 (m, 3H), 7.12~7.19 (m, 3H), 7.03 (dd, J=3.6, 1.2 Hz, 1H), 6.95 (dd, J=5.2, 3.6 Hz, 1H), 6.68~6.70 (m, 2H), 5.40 (s, 2H), 4.52 (q, J=7.2 Hz, 2H), 1.47 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 165.94, 143.05, 139.98, 137.82, 137.39, 136.64, 136.62, 131.02, 129.23, 128.71, 128.59, 127.59, 127.33, 126.68, 125.65, 121.81, 121.71, 121.30, 116.96, 111.14, 61.66, 48.10, 14.48; ESI-MS m/z: 414 [M+H]+. Anal. calcd for C25H20N2O2S: C 72.79, H 4.89, N 6.79, S 7.77; found C 72.52, H 4.63, N 6.52, S 7.49.
Ethyl 9-n-butyl-1-(2-thienyl)-β-carboline-3-carboxylate (2d): White solid, yield 86%. m.p. 100.3~100.9 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.88 (s, 1H), 8.25 (d, J=7.6 Hz, 1H), 7.62~7.66 (m, 1H), 7.49~7.53 (m, 2H), 7.36~7.40 (m, 1H), 7.30 (dd, J=3.6, 1.2 Hz, 1H), 7.17 (dd, J=5.2, 3.6 Hz, 1H), 4.52 (q, J=7.2 Hz, 2H), 4.13 (t, J=8.0 Hz, 2H), 1.43~1.52 (m, 5H), 0.93~1.04 (m, 2H), 0.73 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 165.94, 142.58, 140.12, 137.25, 136.97, 136.41, 130.78, 128.96, 128.89, 127.39, 126.73, 121.85, 121.63, 120.89, 117.02, 110.68, 61.64, 44.49, 31.36, 19.96, 14.50, 13.56; ESI-MS m/z: 379 [M+H]+. Anal. calcd for C22H22N2O2S: C 69.81, H 5.86, N 7.40, S 8.47; found C 69.47, H 6.05, N 7.12, S 8.24.
3.2.2 General procedure for the preparation of 3a~3d
To a solution of compound 2a (4.07g, 10 mmol) in THF (200 mL) was added LiBH4(30 mmol). The mixture was then stirred at room temperature until the reaction is completed. Then the reaction was quenched with cool water (200 mL), and adjusted the pH of the aqueous phase to 3~4 by the addition of aqueous HCl, and stirred for 4 h. The reaction mixture was neutralized with aqueous NaOH solution and extracted with ethyl acetate. The organic layer was washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated. The residue obtained was purified by silica column chromatography with ethyl acetate as the eluent. Upon recrystallization, compound 3a was obtained. Products 3b~3d were prepared according to the same method of 3a.
9-Benzyl-1-(3-pyridyl)-3-hydroxymethyl-β-carboline (3a): White solid, yield 72.3%. m.p. 162.3~163.8 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.60~8.71 (m, 2H), 8.23 (d, J=8.0 Hz, 1H), 8.06 (s, 1H), 7.53~7.63 (m, 2H), 7.32~7.40 (m, 2H), 7.05~7.21 (m, 4H), 6.46 (d, J=7.2 Hz, 2H), 5.24 (s, 2H), 4.97 (s, 2H); 13C NMR (CDCl3, 100 MHz) δ: 149.90, 149.36, 148.57, 143.37, 139.62, 136.60, 136.21, 135.20, 134.04, 132.17, 129.24, 128.61, 127.39, 125.36, 122.75, 121.81, 121.15, 120.56, 111.02, 110.43, 64.71, 48.21; ESI-MS m/z: 366 [M+H]+. Anal. calcd for C24H19N3O: C 78.88, H 5.24, N 11.50; found C 78.69, H 5.36, N 11.73.
9-n-Butyl-1-(3-pyridyl)-3-hydroxymethyl-β-carboline (3b): Light yellow solid, yield 78.2%. m.p. > 270 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.18 (d, J=1.6 Hz, 1H), 9.02 (dd, J=5.2, 1.2 Hz, 1H), 8.87 (s, 1H), 8.62 (d, J=8.0 Hz, 1H), 8.53~8.56 (m, 1H), 7.89~7.93 (m, 2H), 7.82~7.86 (m, 1H), 7.47~7.51 (m, 1H), 4.98 (s, 2H), 3.99 (t, J=7.2 Hz, 2H), 1.26~1.34 (m, 2H), 0.80~0.89 (m, 2H), 0.60 (t, J=7.2 Hz, 3H); 13C NMR (DMSO-d6, 100 MHz) δ: 147.87, 146.92, 146.00, 145.17, 143.46, 135.67, 132.81, 132.55, 129.17, 125.64, 124.02, 122.25, 119.68, 115.20, 111.87, 60.11, 44.59, 30.83, 19.60, 13.71; ESI-MS m/z: 332 [M+H]+. Anal. calcd for C21H21N3O: C 76.11, H 6.39, N 12.68; found C 76.27, H 6.15, N 12.56.
9-Benzyl-1-(2-thienyl)-3-hydroxymethyl-β-carboline (3c): Light yellow solid, yield 73.5%. m.p. 111.4~112.8 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.17~8.20 (m, 1H), 7.98 (s, 1H), 7.52~7.56 (m, 1H), 7.42 (dd, J=5.2, 1.2 Hz, 1H), 7.30~7.34 (m, 2H), 7.12~7.18 (m, 3H), 7.02 (dd, J=3.6, 1.2 Hz, 1H), 6.96 (dd, J=5.2, 3.6 Hz, 1H), 6.70~6.72 (m, 2H), 5.37 (s, 2H), 4.95 (s, 2H); 13C NMR (CDCl3, 100 MHz) δ: 148.39, 143.41, 140.45, 137.02, 136.10, 134.55, 132.21, 129.06, 128.55, 128.23, 127.37, 127.20, 126.71, 125.73, 121.71, 121.32, 120.47, 110.98, 110.81, 64.68, 47.98; ESI-MS m/z: 371 [M+H]+. Anal. calcd for C23H18N2OS: C 74.57, H 4.90, N 7.56, S 8.65; found C 74.84, H 4.99, N 7.41, S 8.78.
9-n-Butyl-1-(2-thienyl)-3-hydroxymethyl-β-carboline (3d): Light yellow solid, yield 66.4%. m.p. 101.8~103.1 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.16 (d, J=8.0 Hz, 1H), 7.95 (s, 1H), 7.58~7.62 (m, 1H), 7.52 (dd, J=5.2, 0.8 Hz, 1H), 7.46 (d, J=8.0 Hz, 1H), 7.28~7.32 (m, 2H), 7.19 (dd, J=5.2, 3.6 Hz, 1H), 4.96 (s, 2H), 4.12 (t, J=8.0 Hz, 2H), 1.38~1.46 (m, 2H), 0.93~1.03 (m, 2H), 0.72 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 147.95, 142.91, 140.75, 135.75, 134.35, 132.02, 128.73, 128.38, 127.15, 126.72, 121.73, 121.22, 119.97, 111.01, 110.36, 64.72, 44.27, 31.14, 19.97, 13.59; ESI-MS m/z: 337 [M+H]+. Anal. calcd for C20H20N2OS: C 71.40, H 5.99, N 8.33, S 9.53; found C 71.03, H 6.10, N 8.20, S 9.74.
3.2.3 General procedure for the preparation of 4a~4d
The mixture of compound 3a (2.20 g, 6 mmol), activated MnO2 (30 mmol) in CH3CN (150 mL), was stirred under reflux for 2 h. After the completion of the reaction (monitored by TLC), then cooled to room temperature and filtered through Celite. The filtrate was passed through silica gel and washed with dichloromethane, and the solvent was removed under reduced pressure. The residue was crystallized from acetone or acetone-petroleum ether to give the corresponding compound 4a.
9-Benzyl-1-(3-pyridyl)-β-carboline-3-carbaldehyde (4a): White solid, yield 86.7%. m.p. 127.6~128.9 ℃; 1H NMR (CDCl3, 400 MHz) δ: 10.23 (s, 1H), 8.84 (s, 1H), 8.68~8.71 (m, 2H), 8.31~8.34 (m, 1H), 7.61~7.66 (m, 2H), 7.43~7.48 (m, 2H), 7.22~7.26 (m, 2H), 7.16~7.20 (m, 1H), 7.10~7.15 (m, 2H), 6.47~6.49 (m, 2H), 5.32 (s, 2H); 13C NMR (CDCl3, 100 MHz) δ: 193.36, 148.95, 143.45, 142.74, 140.12, 137.88, 137.76, 136.42, 131.13, 128.57, 129.47, 128.33, 127.33, 126.91, 125.41, 122.11, 121.85, 121.62, 120.87, 113.40, 111.24, 49.23; ESI-MS m/z: 364 [M+H]+. Anal. calcd for C24H17N3O: C 79.32, H 4.72, N 11.56; found C 79.29, H 4.83, N 11.33.
9-n-Butyl-1-(3-pyridyl)-β-carboline-3-carbaldehyde (4b): Yellow solid, yield 94.2%. m.p. 146.1~148.7 ℃; 1H NMR (CDCl3, 400 MHz) δ: 10.23 (s, 1H), 8.93 (dd, J=2.0, 0.8 Hz, 1H), 8.82 (dd, J=5.2, 1.6 Hz, 1H), 8.79 (s, 1H), 8.25~8.28 (m, 1H), 8.02~8.05 (m, 1H), 7.65~7.69 (m, 1H), 7.53~7.56 (m, 1H), 7.51 (d, J=8.4 Hz, 1H), 7.39~7.43 (m, 1H), 4.02 (t, J=8.0 Hz, 2H), 1.36~1.44 (m, 2H), 0.86~0.94 (m, 2H), 0.68 (t, J=7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ: 193.00, 149.96, 149.94, 143.49, 142.52, 140.49, 136.78, 136.61, 135.38, 130.88, 129.42, 123.30, 121.99, 121.79, 121.38, 113.79, 110.71, 44.72, 30.95, 19.78, 13.44; ESI-MS m/z: 330 [M+H]+. Anal. calcd for C21H19N3O: C 76.57, H 5.81, N 12.76; found C 76.92, H 5.85, N 12.66.
9-Benzyl-1-(2-thienyl)-β-carboline-3-carbaldehyde (4c): White solid, yield 87.3%. m.p. 117.7~119.5℃; 1H NMR (CDCl3, 400 MHz) δ: 10.27 (s, 1H), 8.79 (s, 1H), 8.29 (d, J=8.0 Hz, 1H), 7.58~7.62 (m, 1H), 7.47 (dd, J=5.2, 1.2 Hz, 1H), 7.37~7.44 (m, 2H), 7.16~7.20 (m, 3H), 7.04 (dd, J=3.6, 1.2 Hz, 1H), 6.99 (dd, J=5.2, 3.6 Hz, 1H), 6.70~6.73 (m, 2H), 5.41 (s, 2H); 13C NMR (CDCl3, 100 MHz) δ: 193.21, 143.64, 143.11, 139.47, 137.49, 137.46, 136.36, 131.07, 129.59, 128.68, 128.65, 127.78, 127.46, 126.93, 125.59, 121.94, 121.84, 121.67, 113.49, 111.26, 48.18; ESI-MS m/z: 369 [M+H]+. Anal. calcd for C23H16N2OS: C 74.98, H 4.38, N 7.60, S 8.70; found C 74.69, H 4.50, N 7.48, S 8.80.
9-n-Butyl-1-(2-thienyl)-β-carboline-3-carbaldehyde (4d): White solid, yield 86%. m.p. 105.2~107.2 ℃; 1H NMR (CDCl3, 400 MHz) δ: 10.25 (s, 1H), 8.75 (s, 1H), 8.25 (d, J =8.0 Hz, 1H), 7.64~7.68 (m, 1H), 7.58 (dd, J=5.2, 1.2 Hz, 1H), 7.51 (d, J=8.4 Hz, 1H), 7.40 (t, J=7.6 Hz, 1H), 7.34 (dd, J=3.6, 1.2 Hz, 1H), 7.22 (dd, J=5.2, 3.6 Hz, 1H), 4.13 (t, J=8.0 Hz, 2H), 1.46~1.54 (m, 2H), 0.98~1.07 (m, 2H), 0.75 (t, J=7.2 Hz, 4H); 13C NMR (CDCl3, 100 MHz) δ: 193.21, 143.21, 142.57, 139.80, 137.22, 137.03, 130.78, 129.29, 128.76, 127.56, 126.98, 121.94, 121.79, 121.26, 113.56, 110.76, 44.57, 31.39, 20.00, 13.57; ESI-MS m/z: 335 [M+H]+. Anal. calcd for C20H18N2OS: C 71.83, H 5.43, N 8.38, S 9.59; found C 71.50, H 5.53, N 8.26, S 9.58.
3.2.4 General procedure for the preparation of the target compounds 5a~5h
To a solution of compound 4a (0.73g, 2 mmol) in anhydrous methanol (30 mL) and anhydrous CH2Cl2(10 mL) was stirred at room temperature for 10 min, and the corresponding diamine (1.0 mmol) was added. The mixture was refluxed for 2~5 h, The solvent was evaporated under vacuum to give the crude schiff base, which was used directly in the next step without further purification. Then the crude Schiff base in anhydrous CH3OH (30 mL) at 0 ℃, NaBH3CN (5 mmol) was added. The mixture was stirred at room temperature for 4~6 h. After the completion of the reaction (monitored by TLC), the pH of the aqueous phase was adjusted to 2~3 by the addition of aqueous HCl, and stirred for 1 h. The reaction mixture was neutralized with aqueous NaOH and the reaction mixture was concentrated under vacuum. The residue was dissolved in CH2Cl2 (150 mL) and washed with aqueous Na2CO3 (pH 10, 50 mL). The organic layer was separated, dried over anhydrous Na2SO4, filtered, and concentrated under vacuum. The residue was purified by flash chromatography on silica gel [V(CH2-Cl2):V(CH3OH):V(NH4OH)=100:1:0.8] to provide target products.
N, N-Bis[((9-benzyl-1-(3-pyridyl)-β-carboline)-3-yl)me-tyl]butane-1, 4-diamine (5a): Yellow solid, yield 47.4%. m.p. 159.0~161.2 ℃; 1H NMR (CDCl3, 400 MHz) δ: 8.59 (m, 4H), 8.21 (d, J=7.6 Hz, 2H), 8.08 (s, 2H), 7.50~7.57 (m, 4H), 7.30~7.35 (m, 4H), 7.05~7.14 (m, 8H), 6.45 (d, J=7.6 Hz, 4H), 5.19 (s, 4H), 4.08 (s, 4H), 2.79 (t, J=6.4 Hz, 4H), 1.67~1.70 (m, 4H); 13C NMR (CDCl3, 100 MHz) δ: 149.89, 149.23, 143.06, 140.17, 136.52, 136.29, 135.48, 133.73, 131.71, 128.94, 128.53, 127.28, 125.33, 122.69, 121.82, 121.24, 120.41, 112.87, 110.27, 54.89, 49.40, 48.10, 27.99. HRMS calcd for C52H47N8 [M+H]+ 783.3918, found 783.3918.
N, N-Bis[((9-benzyl-1-(3-pyridyl)-β-carboline)-3-yl)met-yl]pentane-1, 5-diamine (5b): Yellow solid, yield 40.1%. m.p. 97.8~99.9 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.82~8.85 (m, 4H), 8.40 (d, J=7.6 Hz, 2H), 8.27 (d, J=8.0 Hz, 2H), 7.82 (d, J=8.4 Hz, 2H), 7.67~7.76 (m, 4H), 7.41~7.45 (m, 4H), 7.04~7.13 (m, 6H), 6.35 (d, J=7.2 Hz, 4H), 5.44 (s, 4H), 4.45 (t, J=5.2 Hz, 4H), 2.91 (t, J=8.0 Hz, 4H), 1.68~1.75 (m, 4H), 1.33~1.40 (m, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 143.78, 143.38, 143.10, 142.81, 140.61, 136.93, 136.28, 133.33, 131.68, 129.74, 128.45, 127.09, 125.26, 124.96, 121.89, 120.93, 120.08, 115.52, 111.11, 50.16, 47.65, 46.34, 24.60, 23.03. HRMS calcd for C53H49N8[M+H]+ 797.4080, found 797.4077.
N, N-Bis[((9-n-butyl-1-(3-pyridyl)-β-carboline)-3-yl)me-tyl]butane-1, 4-diamine (5c): Yellow solid, yield 57.7%. m.p. 225.7~227.2 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.32 (s, 2H), 9.00 (dd, J=5.2, 1.2 Hz, 2H), 8.71 (d, J=8.0 Hz, 2H), 8.62 (s, 2H), 8.35 (d, J=8.0 Hz, 2H), 8.03~8.06 (m, 2H), 7.84 (d, J=8.4 Hz, 2H), 7.70~7.74 (m, 2H), 7.41 (t, J=8.0 Hz, 2H), 4.50 (s, 4H), 4.08 (t, J=8.0 Hz, 4H), 3.03~3.10 (m, 4H), 1.80~1.89 (m, 4H), 1.21~1.29 (m, 4H), 0.77~0.87 (m, 4H), 0.57 (t, J=7.2 Hz, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 145.19, 144.96, 142.56, 139.82, 136.90, 135.97, 133.25, 131.50, 129.59, 125.18, 121.81, 120.66, 120.02, 115.52, 111.17, 50.02, 45.90, 43.91, 30.10, 22.50, 18.96, 13.12. HRMS calcd for C46H51N8 [M+H]+ 715.4231, found 715.4231.
N, N-Bis[((9-n-butyl-1-(3-pyridyl)-β-carboline)-3-yl)me-tyl]pentane-1, 5-diamine (5d): Yellow solid, yield 64.5%. m.p. 100.4~102.8 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.40 (s, 2H), 9.05 (d, J=4.4 Hz, 2H), 8.82 (d, J=6.0 Hz, 2H), 8.67 (s, 2H), 8.35 (d, J=8.0 Hz, 2H), 8.11~8.14 (m, 2H), 7.85 (d, J=8.4 Hz, 2H), 7.73 (t, J=7.6 Hz, 2H), 7.41 (t, J=8.0 Hz, 2H), 4.50 (s, 4H), 4.08 (t, J=8.0 Hz, 4H), 3.01~3.04 (m, 4H), 1.73~1.81 (m, 4H), 1.39~1.45 (m, 2H), 1.11~1.29 (m, 4H), 0.77~0.87 (m, 4H), 0.57 (t, J=7.2 Hz, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 145.01, 144.76, 143.25, 140.25, 136.87, 136.62, 133.81, 132.28, 130.31, 126.16, 122.45, 121.33, 120.57, 116.40, 111.80, 50.41, 46.83, 44.56, 31.18, 30.65, 25.24, 23.61, 19.54, 13.70. HRMS calcd for C47H53N8 [M+H]+ 729.4388, found 729.4348.
N, N-Bis[((9-benzyl-1-(2-thienyl)-β-carboline)-3-yl)me-tyl]butane-1, 4-diamine (5e): Yellow solid, yield 45.6%. m.p. 161.5~163.7 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.52 (s, 2H), 8.34 (d, J=8.0 Hz, 2H), 7.63~7.75 (m, 6H), 7.39 (t, J=7.6 Hz, 2H), 7.30 (d, J=2.4 Hz, 2H), 7.08~7.17 (m, 8H), 6.54~6.56 (m, 4H), 5.55 (s, 4H), 4.43 (s, 4H), 3.08 (t, J=7.6 Hz, 4H), 1.76~1.84 (m, 4H); 13C NMR (DMSO-d6, 100 MHz) δ: 142.97, 140.15, 138.81, 137.01, 136.01, 133.64, 131.56, 129.58, 129.27, 128.57, 128.31, 127.00, 126.81, 125.51, 121.79, 120.88, 120.29, 114.92, 111.51, 50.16, 47.02, 46.12, 22.53. HRMS calcd for C50H45N6S2 [M+H]+ 793.3142, found 793.3142.
N, N-Bis[((9-benzyl-1-(2-thienyl)-β-carboline)-3-yl)metyl]pentane-1, 5-diamine (5f): Yellow solid, yield 49.4%. m.p. 242.6~244.4 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.47 (s, 2H), 8.33 (d, J=8.0 Hz, 2H), 7.71~7.75 (m, 4H), 7.62~7.66 (m, 2H), 7.39 (t, J=7.6 Hz, 2H), 7.30 (dd, J=3.6, 1.2 Hz, 2H), 7.24 (s, 2H), 7.08~7.16 (m, 10H), 5.55 (s, 4H), 4.42 (s, 4H), 3.03 (t, J=7.6 Hz, 4H), 1.68~1.76 (m, 4H), 1.35~1.43 (m, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 142.80, 140.66, 139.85, 137.11, 136.48, 133.63, 131.31, 129.31, 128.87, 128.26, 126.97, 126.77, 125.51, 121.64, 120.72, 120.36, 114.33, 111.47, 50.50, 46.95, 46.51, 24.74, 23.02. HRMS calcd for C51H47N6S2 [M+H]+ 807.3298, found 807.3304.
N, N-Bis[((9-n-butyl-1-(2-thienyl)-β-carboline)-3-yl)me-tyl]butane-1, 4-diamine (5g): Yellow solid, yield 43.1%. m.p. 195.9~197.7 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.69 (s, 2H), 8.33 (d, J=8.0 Hz, 2H), 7.91 (dd, J=5.2, 0.8 Hz, 2H), 7.83 (d, J=8.4 Hz, 2H), 7.68~7.75 (m, 2H), 7.54 (dd, J=3.6, 1.2 Hz, 2H), 7.41 (t, J=7.6 Hz, 2H), 7.30 (dd, J=5.2, 3.6 Hz, 2H), 4.50 (t, J=5.2 Hz, 4H), 4.21 (t, J=8.0 Hz, 4H), 3.06~3.13 (m, 4H), 1.80~1.91 (m, 4H), 1.28~1.36 (m, 4H), 0.86~0.96 (m, 4H), 0.65 (t, J=7.6 Hz, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 142.84, 138.81, 135.10, 133.46, 131.65, 130.08, 129.81, 128.82, 127.02, 121.90, 120.77, 119.92, 115.65, 111.25, 49.53, 46.03, 43.68, 30.72, 22.54, 19.16, 13.23. HRMS calcd for C44H49N6S2 [M+H]+ 725.3455, found 725.3447.
N, N-Bis[((9-n-butyl-1-(2-thienyl)-β-carboline)-3-yl)me-tyl]pentane-1, 5-diamine (5h): Yellow solid, yield 50%. m.p. 262.7~264.6 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.63 (s, 2H), 8.32 (d, J=8.0 Hz, 2H), 7.89 (dd, J=5.2, 0.8 Hz, 2H), 7.82 (d, J=8.4 Hz, 2H), 7.69~7.74 (m, 2H), 7.52 (dd, J=3.6, 1.2 Hz, 2H), 7.40 (t, J=7.6 Hz, 2H), 7.28~7.30 (m, 2H), 4.48 (t, J=5.2 Hz, 4H), 4.21 (t, J=8.0 Hz, 4H), 3.00~3.09 (m, 4H), 1.73~1.80 (m, 4H), 1.41~1.46 (m, 2H), 1.27~1.35 (m, 4H), 0.85~0.95 (m, 4H), 0.64 (t, J=7.6 Hz, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 142.75, 139.07, 135.29, 133.46, 131.52, 129.90, 129.68, 128.67, 127.00, 121.84, 120.69, 119.95, 115.42, 111.23, 49.71, 46.40, 43.64, 30.71, 24.72, 23.04, 19.16, 13.23; HRMS calcd for C45H51N6S2 [M+H]+ 739.3611, found 739.3611.
3.2.5 Biology cell viability assay
Cytotoxicity assays in vitro were carried out using 96-well plate cultures and MTT staining according to the procedures described by Chen et al.[27] Briefly, the cancer cells were grown in RPMI-1640 medium, supplemented with 10% (V/V) fetal calf serum, 100 μg•mL-1 penicillin and 100 μg•mL-1 streptomycin. Cultured cells were propagated at 37 ℃ in a humidified atmosphere containing 5% CO2. In the experiments, cells were allowed to acclimate for 24 h before any treatments. Cell lines were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science. DMSO was used as the solution for drugs. The human tumor cell lines were inoculated into fresh media containing fivefold diluted target compounds. Final concentration of DMSO in the growth medium was 2% (V/V) or lower, concentration without effect on cell replication. After incubation for 48 h, MTT assay was carried out in triplication and automatic microplate reader was used to determine absorbance. Cisplatin was the positive control. The optical density (OD) was read at 490 nm. In all of these experiments, three replicate wells were used to determine each point. IC50 values were calculated by the Logit method.
Supporting Information 1H NMR, 13C NMR, HRMS data of all the target compounds. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn/.
-
-
[1]
Michael, C.; Robert, W. W.; Fil, G.; James, M. C.; Steven, A. B.; Kenner, C. R.; Jacqueline, N. C.; Steven, M. P.; Phil, S. J. Med. Chem. 1982, 25, 1081. doi: 10.1021/jm00351a015
-
[2]
Rashid, M. A.; Gustafson, K. R.; Boyd, M. R. J. Nat. Prod. 2001, 64, 1454. doi: 10.1021/np010214+
-
[3]
Kuo, P.-C.; Shi, L.-S.; Damu, A. G.; Su, C.-R.; Huang, C.-H.; Ke, C.-H.; Wu, J.-B.; Lin, A.-J.; Bastow, K. F.; Lee, K.-H.; Wu, T.-S. J. Nat. Prod. 2003, 66, 1324. doi: 10.1021/np030277n
-
[4]
Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. Bioorg. Med. Chem. 1999, 7, 1223. doi: 10.1016/S0968-0896(99)00050-4
-
[5]
Wang, Y.-H.; Tang, J.-G.; Wang, R.-R.; Yang, L.-M.; Dong, Z.-J.; Du, L.; Shen, X.; Liu, J.-K.; Zheng, Y.-T. Biochem. Biophys. Res. Commun. 2007, 355, 1091. doi: 10.1016/j.bbrc.2007.02.081
-
[6]
Shankaraiah, N.; Siraj, K. P.; Nekkanti, S.; Srinivasulu, V.; Sharma, P.; Senwar, K. R.; Sathish, M.; Vishnuvardhan, M. V. P. S.; Ramakrishna, S.; Jadala, C.; Nagesh, N.; Kamal, A. Bioorg. Chem. 2015, 59, 130. doi: 10.1016/j.bioorg.2015.02.007
-
[7]
Kamal, A.; Sathish, M.; Nayak, V. L.; Srinivasulu, V.; Kavitha, B.; Tangella, Y.; Thummuri, D.; Bagul, C.; Shankaraiah, N.; Nagesh, N. Bioorg. Med. Chem. 2015, 23, 5511. doi: 10.1016/j.bmc.2015.07.037
-
[8]
Figueiredo, P. O.; Perdomo, R. T.; Garcez, F. R.; Matos, M. F. C.; Carvalho, J. E.; Garcez, W. S. Bioorg. Med. Chem. Lett. 2014, 24, 1358. doi: 10.1016/j.bmcl.2014.01.039
-
[9]
Li, Y.; Liang, F.-S.; Jiang, W.; Yu, F.-S.; Cao, R.-H.; Ma, Q.-H.; Dai, X.-Y.; Jiang, J.-D.; Wang, Y.-C.; Si, S.-Y. Cancer Biol. Ther. 2007, 6, 1193.
-
[10]
Zhang, J.; Li, Y.; Guo, L.; Cao, R.-H.; Zhao, P.; Jiang, W.; Ma, Q.; Yi, H.; Li, Z.-R.; Jiang, J.-D.; Wu, J.-L.; Wang, Y.-C.; Si, S.-Y. Cancer Biol. Ther. 2009, 8, 2374. doi: 10.4161/cbt.8.24.10182
-
[11]
Barsanti, P. A.; Wang, W.; Ni, Z.; Duhl, D.; Brammeier, N.; Martin, E.; Bussiere, D.; Walter, A. O. Bioorg. Med. Chem. Lett. 2010, 20, 157. doi: 10.1016/j.bmcl.2009.11.012
-
[12]
Castro, A. C.; Dang, L. C.; Soucy, F.; Grenier, L.; Mazdiyasni, H.; Hottelet, M.; Parent, L.; Pien, C.; Palombella, V.; Adams, J. Bioorg. Med. Chem. Lett. 2003, 13, 2419. doi: 10.1016/S0960-894X(03)00408-6
-
[13]
Gaugain, B.; Barbet, J.; Capelle, N.; Roques, B. P.; Le Pecp, J. B.; Le Bret, M. Biochemisry 1978, 17, 5078. doi: 10.1021/bi00617a002
-
[14]
Capelle, N.; Barbet, J.; Dessen, P.; Blanquet, S.; Roques, B. P.; Le Pecq, J. B. Biochemisry 1979, 18, 3354. doi: 10.1021/bi00582a023
-
[15]
Wang, K.-B.; Di, Y.-T.; Bao, Y.; Yuan, C.-M.; Chen, G.; D. Li, H.; Bai, J.; He, H.-P.; Hao, X.-J.; Pei, Y.-H.; Jing, Y.-K.; Li, Z.-L.; Hua, H.-M. Org. Lett. 2014, 16, 4028. doi: 10.1021/ol501856v
-
[16]
Joshi, A.; Vance, D.; Rai, P.; Thiyagarajan, A.; Kane, R. S. Chem. Eur. J. 2008, 14, 7738. doi: 10.1002/chem.v14:26
-
[17]
Brana, M. F.; Castellano, J. M.; Moran, M.; Perez de Vega, M. J.; Perron, D.; Conlon, D.; Bousquet, P. F.; Romerdahl, C. A.; Robinson, S. P. Anticancer Drug Des. 1996, 11, 297
-
[18]
Burns, M. R.; Turner, S. L.; Ziemer, J.; Vean, M. M.; Devens, B.; Carlson, C. L.; Graminski, G. F.; Vanderwerf, S. M.; Weeks, R. S.; Carreon, J. Bioorg. Med. Chem. Lett. 2002, 12, 1263. doi: 10.1016/S0960-894X(02)00156-7
-
[19]
郭亮, 孙洁, 范文玺, 马芹, 中国现代应用药学, 2012, 29, 385.Guo, L.; Sun, J.; Fan, W.-X.; Ma, Q. Chin. J. Modern Appl. Pharm. 2012, 29, 385(in Chinese).
-
[20]
郭亮, 曹日晖, 范文玺, 马芹, 高等学校化学学报, 2014, 35, 518. doi: 10.7503/cjcu20130683Guo, L.; Cao, R.-H.; Fan, W.-X.; Ma, Q. Chem. J. Chin. Univ. 2014, 35, 518(in Chinese). doi: 10.7503/cjcu20130683
-
[21]
郭亮, 范文玺, 陈雪梅, 马芹, 曹日晖, 有机化学, 2013, 33, 332.Guo, L.; Fan, W.-X.; Chen, X.-M.; Ma, Q.; Cao, R.-H. Chin. J. Org. Chem. 2013, 33, 332(in Chinese).
-
[22]
Guo, L.; Fan, W.-X.; Chen, W.; Ma, Q.; Cao, R.-H. J. Chin. Pharm. Sci. 2015, 24, 801.
-
[23]
Zhang, G.-X.; Cao, R.-H.; Guo, L.; Ma, Q.; Fan, W.-X.; Chen, X.-M., Li, J.-R., Shao, G.; Qiu, L.-Q.; Ren, Z.-H. Eur. J. Med. Chem. 2013, 65, 21. doi: 10.1016/j.ejmech.2013.04.031
-
[24]
Shi, B.-X.; Cao, R.-H.; Fan, W.-X.; Guo, L.; Ma, Q.; Chen, X.-M.; Zhang, G.-X.; Qiu, L.-Q.; Song, H.-C. Eur. J. Med. Chem. 2013, 60, 10. doi: 10.1016/j.ejmech.2012.11.033
-
[25]
Wu, Q.-F.; Bai, Z.-S.; Ma, Q.; Fan, W.-X.; Guo, L.; Zhang, G.-X.; Qiu, L.-Q.; Yu, H.; Shao, G.; Cao, R.-H. Med. Chem. Commun. 2014, 5, 953. doi: 10.1039/C4MD00098F
-
[26]
郭亮, 曹日晖, 范文玺, 甘紫云, 马芹, 有机化学, 2016, 37, 1093. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345396.shtmlGuo, L.; Cao, R.-H.; Fan, W.-X.; Gan, Z.-Y.; Ma, Q. Chem. J. Chin. Univ. 2016, 37, 1093(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345396.shtml
-
[27]
Chen, J.; Zhang, Y.-K.; Zhan, X.-P.; Liu, Z.-L.; Mao, Z.-M. Chin. J. Org. Chem. 2016, 36, 572(in Chinese). doi: 10.6023/cjoc201510016
-
[1]
-
Table 1. Cytotoxicity of compounds 3a~3d, 4a~4d and 5a~5h against human tumour cells
Comp. IC50/(μmol•L-1) 22RV1 SK-OV-3 MCF-7 LLC Eca-109 BGC-823 HT-29 HepG2 769-P A375 3a > 100 > 100 72.4 70.7 > 100 > 100 67.5 53.6 41.6 > 100 3b 90.5 > 100 > 100 42.4 > 100 > 100 > 100 > 100 66.1 > 100 3c > 100 > 100 76.7 56.8 > 100 76.7 74.9 54.3 32.7 90.1 3d > 100 > 100 97.8 > 100 > 100 > 100 89.2 98.5 79.5 93.2 4a > 100 90.1 > 100 > 100 > 100 > 100 > 100 48.9 18.2 58.8 4b > 100 89.2 > 100 > 100 > 100 > 100 > 100 19.9 66.6 > 100 4c > 100 69.1 77.5 91.1 > 100 83.1 69.1 55.9 27.5 83.2 4d 72.2 65.8 54.8 67.4 36.2 58.7 17.7 56.2 19.9 51.1 5a 3.7 2.8 5.7 5.7 6.0 6.0 6.2 7.6 4.1 6.2 5b 1.9 1.4 7.5 1.3 5.5 5.2 1.6 9.9 1.0 5.5 5c 2.3 1.6 6.9 1.7 7.2 4.0 7.4 7.9 1.8 6.6 5d 1.6 5.5 3.8 1.3 2.1 1.6 2.0 4.0 0.8 1.6 5e 3.9 2.7 2.7 1.4 5.3 7.5 5.8 7.0 2.6 4.7 5f 1.3 2.6 6.3 1.4 5.4 7.4 4.7 5.9 1.6 3.4 5g 3.4 2.2 4.6 1.9 1.8 1.5 5.2 4.4 2.1 2.3 5h 0.6 2.2 2.1 1.9 1.8 1.9 5.4 2.6 1.5 1.8 DDP 5.2 5.6 12.4 7.6 8.9 11.6 26.8 14.8 19.2 9.4 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 1515
- HTML全文浏览量: 104


 下载:
下载:


 下载:
下载:

