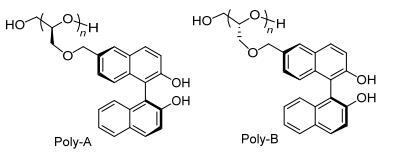
 图 1
手性聚合物配体Poly-A和Poly-B的结构
Figure 1.
Structures of the chiral polymer ligands Poly-A and Poly-B
图 1
手性聚合物配体Poly-A和Poly-B的结构
Figure 1.
Structures of the chiral polymer ligands Poly-A and Poly-B

Citation: Yu Zeng, Liu Feng, Zhang Lingjun, Yang Nianfa. Synthesis of Two 1, 1'-Bi-2-naphthol-Based Chiral Polyethers for the Enantioselective Addition of Phenylacetylene to Aldehydes[J]. Chinese Journal of Organic Chemistry, 2017, 37(8): 2015-2021. doi: 10.6023/cjoc201612059

两种基于联萘酚的手性聚醚诱导苯乙炔对醛的对映选择性加成反应
English
Synthesis of Two 1, 1'-Bi-2-naphthol-Based Chiral Polyethers for the Enantioselective Addition of Phenylacetylene to Aldehydes
-
Key words:
- epichlorohydrin
- / polyether
- / bi-2-naphthol
- / phenylacetylene
-
不对称催化是现代有机合成最具挑战性的领域之一, 一个成功的不对称反应应该具备以下三个特点: (1) 产物应该具有较高的ee值(对映体过量值); (2) 手性配体容易制备和再利用; (3) 能够根据需求制备R和S两种构型的产品.虽然现在已经有很多优异的手性小分子催化剂被合成出来[1~8], 但由于大多数的手性小分子催化剂是非常昂贵并且难以和催化产物分离, 导致催化成本很高.因此, 科学家们对手性催化剂的回收利用越来越感兴趣[9~20].相对于小分子催化剂, 聚合物催化剂在很多的不良溶剂中能够完全沉降, 从而到达很好的分离.因此, 设计和合成聚合物催化剂是解决催化剂回收利用的有效途径之一.我们课题组多年来一直致力于手性聚合物催化剂的研究, 并且在许多的不对称反应中取得了显著的进展[21~28].
众所周知, 手性炔醇是许多手性化合物非常重要的前体[29~35], 而炔基锌对醛的不对称加成是制备手性炔醇的有效途径之一[36~51].虽然已经有大量的手性小分子催化剂能很好地催化制备手性炔醇[36~51], 但是仅仅只有少量可以回收利用的聚合物催化剂被报道[52].因此, 基于我们前期合成的联萘酚衍生的聚醚Poly-A和Poly-B(图 1)在对映选择性硼烷还原潜手性酮的优异表现[26, 27].我们再次尝试将它们应用于诱导苯乙炔对醛的不对称加成中.令人欣慰的是, 我们获得的手性炔丙基醇的ee值最高达95%, 而且这种聚醚还可以被回收利用多次且催化活性没有明显降低.
1 结果与讨论
1.1 带联萘侧基的大分子配体的合成与表征
根据之前的研究[26], 我们合成了(S, S)-6-(2, 3-环氧丙氧基)甲基-2, 2'-二(甲氧基甲氧基)-1, 1'-联萘(8a)和(S, R)-6-(2, 3-环氧丙氧基)甲基-2, 2'-二(甲氧基甲氧基)-1, 1'-联萘(8b), 并将其以氢氧化钾(KOH)为引发剂进行了阴离子聚合.然后在盐酸作用下脱去甲氧基甲氧基得到相应的聚醚配体Poly-A和Poly-B.具体合成路线见Scheme 1. Poly-A:产率85%, [α]36520 -342.4 (c 0.05, THF); Mn=5.2×103; PDI=Mw/Mn=2.02. Poly-B:产率82%, [α]36520-296.7 (c 0.05, THF); Mn=5.6×103; PDI=Mw/Mn=2.41.
1.2 苯乙炔与醛的不对称加成反应
1.2.1 苯乙炔与芳基醛反应的条件优化
参考蒲林的研究[36], 我们将苯乙炔与醛的加成反应分两个步骤: (1) 苯乙炔和二甲基锌制备炔基锌. (2) 依次加入配体Poly-A或Poly-B、钛酸异丙酯、醛.以n(苯乙炔):n(二甲基锌):n(苯甲醛)=4:4:1为模型反应进行了条件探索(表 1).首先, 对配体Poly-A和Poly-B进行了筛选(表 1, Entries 1, 2), 当配体由Poly-A换为Poly-B时, 反应的对映异构体选择性有所下降(Entry 2).接着考察了溶剂对反应的影响(表 1, Entries 1, 3~6), 当溶剂为四氢呋喃时, 产物的产率和ee值最好, 分别为94%和79% (Entry 1).然后考察了温度对反应的影响(表 1, Entries 1, 7), 当把温度降到0 ℃时, 虽然ee值(85%)有所提高, 但是产率急剧下降到30% (Entry 7), 因此选择25 ℃为最佳反应温度.随后对钛酸异丙酯的用量考察表明(Entries 1, 8, 9), 当钛酸异丙酯用量100%时, 得到91%产率和85% ee (Entry 8).最后考察了配体的用量, 发现反应产率会随着配体用量增加而降低(Entries 8, 10, 11), 当配体用量20%时, 产物的ee值最高90%和产率为87% (Entry 10).延长反应时间至24 h, 产率得到了提高(Entry 12).最终选定的最优条件为25 ℃下THF为溶剂反应24 h: n(醛):n[Ti(O-i-Pr)4]:n(Me2Zn):n(苯乙炔):n(配体Poly-A)=1.0:1.0:4.0:4.0:0.2.
 表 1
聚合物Poly-A或Poly-B催化苯乙炔与苯甲醛的不对称加成反应条件优化a
Table 1.
Optimization of asymmetric reaction of phenylacetylene with benzaldehyde catalyzed by Poly-A or Poly-B
表 1
聚合物Poly-A或Poly-B催化苯乙炔与苯甲醛的不对称加成反应条件优化a
Table 1.
Optimization of asymmetric reaction of phenylacetylene with benzaldehyde catalyzed by Poly-A or Poly-B

Entry Conditions for step 2 Ti(O-i-Pr)4/mol% Ligand (mol%) Yieldb/% eec/% Config.d Solvent T/℃ 1 THF 25 50 Poly-A (10) 94 79 R 2 THF 25 50 Poly-B (10) 90 67 R 3 Toluene 25 50 Poly-A (10) 90 38 R 4 Et2O 25 50 Poly-A (10) 87 18 R 5 DCE 25 50 Poly-A (10) 89 71 R 6 DCM 25 50 Poly-A (10) 92 47 R 7 THF 0 50 Poly-A (10) 30 85 R 8 THF 25 100 Poly-A (10) 91 85 R 9 THF 25 150 Poly-A (10) 84 82 R 10 THF 25 100 Poly-A (20) 87 90 R 11 THF 25 100 Poly-A (30) 82 88 R 12e THF 25 100 Poly-A (20) 90 91 R aConditions for step 1: 2 mL of toluene, reflux 5 h; phenylacetylene: 2.0 mmol; Me2Zn: 2.0 mmol; Conditions for step 2: 8 mL of solvent; benzaldehyde: 0.5 mmol; reaction time: 8 h. b Isolated yields. cDetermined by HPLC with a Chiralcel OD-H column. d The absolute configurations of the products were determined by comparison to the literature data[39]. e Reaction time: 24 h. 1.2.2 底物拓展
按照上述的最优条件, 我们将不同的醛应用于该反应中来, 得到了从75%~95% ee值(表 2).当底物是苯甲醛(Entry 1) 时产物ee值为94%, 当底物为带有给电子取代基OCH3时产物的ee值有所降低(Entries 2, 3), 其中邻位取代OCH3的更低一些(Entry 2);当底物为带有吸电子取代基的对氯苯甲醛(Entry 4)、对溴苯甲醛(Entry 5)、对氟苯甲醛(Entry 6)、对硝基苯甲醛(Entry 7) 时产物都有较高的ee值, 分别达到90%, 85%, 89%和95%;间位取代的NO2比对位取代的NO2表现出明显要低的对映选择性(Entry 8); 1-萘甲醛的加成产物(Entry 9) ee值比2-萘甲醛(Entry 10) 要低, 我们认为是由于二者空间位阻差异引起的; 值得一提的是, 当底物换成苯丙醛(Entry 11) 和正庚醛(Entry 12) 时, 配体Poly-A依然表现出不错的手性诱导作用, 它们的ee值分别有80%和75%, 且收率分别高达92%和95%.这表明配体Poly-A具有良好的底物适用性.
 表 2
聚合物Poly-A催化苯乙炔与各种醛的不对称加成反应a
Table 2.
Enantioselective addition of phenylacetylene to various aldehydes catalyzed by Poly-A
表 2
聚合物Poly-A催化苯乙炔与各种醛的不对称加成反应a
Table 2.
Enantioselective addition of phenylacetylene to various aldehydes catalyzed by Poly-A

Entry R Yieldb/% eec/% Config.d 1 Phenyl 92 91 R 2 2-Methoxyphenyl 82 81 R 3 3-Methoxyphenyl 83 86 R 4 4-Chlorophenyl 90 90 R 5 4-Bromophenyl 87 85 R 6 4-Fluorophenyl 91 89 R 7 4-Nitrophenyl 89 95 R 8 3-Nitrophenyl 86 85 R 9 1-Naphthyl 81 88 R 10 2-Naphthyl 82 92 R 11 3-Phenethyl 92 80 R 12 Hexyl 95 75 R a Conditions for step 1: 2 mL of toluene, reflux 5 h; phenylacetylene: 2.0 mmol; Me2Zn: 2.0 mmol; Conditions for step 2: reactions were carried out with 20 mol% of Poly-A (as a BINOL monomer), 0.5 mmol of aldehyde, 1.0 mmol of Ti(O-i-Pr)4 in 8 mL of THF at 25 ℃ for 24 h.bIsolated yield. cDetermined by HPLC using a Chiralcel OD-H column. d The absolute configurations of the products were determined by comparison to the literature data[52]. 1.2.3 Poly-A的回收重复利用
相对于联萘酚, 联萘酚衍生的聚醚在一些有机溶剂如甲醇、乙醇中的溶解度很差, 使其容易通过一个简单的沉降方法予以回收.我们测试聚合物Poly-A的循环再利用效率. Poly-A参与一次催化过程之后, 将回收的聚合物Poly-A (1.0 g)溶于20 mL二氯甲烷中, 加1 mL浓盐酸, 搅拌1 h后分出有机层.减压除去大部分溶剂后倒入100 mL甲醇中, 析出固体, 抽滤, 将滤渣用甲醇洗涤两次. 40 ℃真空干燥8 h即得回收配体, 配体回收利用6次的收率分别为96%、98%、99%、97%、98%、98%.通过6次循环使用结果(表 3)可以看出, 催化产物的ee值和产率没有发生明显变化.
 表 3
回收的Poly-A催化苯乙炔与苯甲醛的不对称加成反应a
Table 3.
Asymmetric reaction of phenylacetylene with benzaldehyde catalyzed by the recovered Poly-A
表 3
回收的Poly-A催化苯乙炔与苯甲醛的不对称加成反应a
Table 3.
Asymmetric reaction of phenylacetylene with benzaldehyde catalyzed by the recovered Poly-A
Cycle 1 2 3 4 5 6 Yieldb/% 92 91 90 91 89 91 eec/% 91 91 90 92 93 90 a Conditions for step 1: 2 mL of toluene, reflux 5 h; phenylacetylene: 2.0 mmol; Me2Zn: 2.0 mmol; Conditions for step 2: reactions were carried out with 20 mol% of Poly-A (as a BINOL monomer), 0.5 mmol of benzaldehyde, 1.0 mmol of Ti(O-i-Pr)4, in 8 mL of THF at 25 ℃ for 24 h.b Isolated yield. cDetermined by HPLC using a Chiralcel OD-H column. 2 结论
以手性环氧氯丙烷和联萘酚为起始物合成了两种环氧化合物, 并通过阴离子聚合以及脱MOM保护过程形成了对应的聚醚Poly-A和Poly-B.这两种聚合物被用于诱导苯乙炔与醛的不对称加成反应, 获得了最高95%的ee值的对映选择性.同时, 催化剂可以回收和重复使用多次而其催化活性没有明显损失.
3 实验部分
3.1 仪器与试剂
1H NMR和13C NMR谱图使用德国Bruker公司的AV-400核磁共振仪(以TMS为内标, CDCl3为溶剂)测得; 比旋光度测试于美国Perkin Elmer公司Polarmetermodel 341型旋光仪; 元素分析使用德国Elementar公司Vario EL Ⅲ型元素分析仪测得; 熔点测试使用予华X-5熔点测试仪; 数均分子量和分子量分布指数测试使用美国Waters 1515凝胶渗透色谱仪(以聚乙烯为标准物质, 流速1.0 mL/min); 产品ee值经高效液相色谱分析: Dionex P680 HPLC, 流动相为正己烷和异丙醇, 手性柱OD-H (Daicel Chemical Ind., Ltd, 25 cm×0.46 cm), 柱温25 ℃, 流速1.0 mL/min. (S)-2, 2'-二羟基-1, 1'-联萘来自阿拉丁试剂公司, 实验中的四氢呋喃、甲苯等溶剂经钠/二苯甲酮干燥, 二氯甲烷经氢化钙干燥后使用, 所有反应均在氩气保护下进行, 手性聚合物配体Poly-A和Poly-B参考文献[26]合成.
3.2 实验方法
3.2.1 苯乙炔和醛的不对称加成反应过程
氩气氛围下, 苯乙炔(220 μL, 2.0 mmol)和1.0 mol·L-1的二甲基锌(2.0 mL, 2.0 mmol)的甲苯溶液回流5 h, 生成白色固体.冷却至室温后, 依次加入聚合物配体(基于单体单元)(37.25 mg, 0.1 mmol)、钛酸异丙酯(0.3 mL, 1.0 mmol), 15 min后加入苯甲醛(51 μL, 0.5 mmol).反应24 h后, 饱和氯化铵淬灭反应, 分离有机层, 水层用10 mL乙酸乙酯萃取.合并有机相并用无水硫酸镁干燥, 有机相过滤后减压浓缩并用薄层色谱[V(石油醚):V(乙酸乙酯)=8:1]纯化得到炔醇产物.
3.2.2 (S)-2-羟基-2'-特戊酰氧基-1, 1'-联萘(2)的合成
在0 ℃条件下, 往溶有(S)-2, 2'-二羟基-1, 1'-联萘(1) (11.46 g, 40 mmol)和三乙胺(16.8 mL, 120 mmol)的乙腈(80 mL)溶液中逐滴滴加特戊酰氯(4.88 g, 40.4 mmol)的乙腈(40 mL)溶液.滴加完毕后, 将反应混合物升温至25 ℃并且在该温度下反应6 h.反应结束后将反应混合物减压浓缩除去溶剂后用乙醚(100 mL)溶解, 再将混合溶液依次用1 mol·L-1 HCl (20 mL×2) 以及饱和食盐水(30 mL×2) 洗涤、无水硫酸镁干燥.将有机相过滤, 滤液减压蒸馏除去溶剂后所得残余物经柱层析(V(石油醚):V(二氯甲烷)=3:1) 分离获得13.40 g白色固体2[53], 产率90%. m.p. 62~65 ℃(文献值[53] m.p. 63~65 ℃); [α]D25-56.4 (c 0.5, THF); 1H NMR (CDCl3, 400 MHz) δ: 8.10 (d, J=8.8 Hz, 1H), 8.01 (d, J=8.1 Hz, 1H), 7.91 (d, J=8.9 Hz, 1H), 7.83 (d, J=7.9 Hz, 1H), 7.52 (t, J=6.4 Hz, 1H), 7.40~7.31 (m, 6H), 7.08 (d, J=8.2 Hz, 1H), 5.16 (s, 1H), 0.79 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 177.78, 151.85, 148.37, 133.71, 133.57, 132.23, 130.67, 130.27, 129.08, 128.33, 127.91, 127.44, 126.66, 126.18, 125.68, 124.60, 123.52, 123.08, 121.85, 118.27, 114.28, 38.77, 26.49. Anal. calcd for C25H22O3 C 81.06, H 5.99; found C 81.05, H 5.96.
3.2.3 (S)-6-溴-2-羟基-2'-特戊酰氧基-1, 1'-联萘(3)的合成
在0 ℃条件下, 往溶有(S)-2-羟基-2'-特戊酰氧基-1, 1'-联萘(2) (29.64 g, 80 mmol)的乙腈(200 mL)溶液中缓慢滴加Br2(10 mL, 195.5 mmol), 滴加完后, 反应混合物在0 ℃继续反应10 h.往反应混合物中加入100 mL Na2S2O4(23 g, 132 mmol)水溶液, 将混合物用CH2Cl2(60 mL×3) 萃取, 合并萃取液并将其用饱和食盐水(40 mL×3) 洗涤、无水硫酸镁干燥.有机相过滤后经减压蒸馏除去溶剂, 所得剩余物经柱层析[V(二氯甲烷):V(石油醚)=1:2]分离纯化获得32.35 g白色固体3[53], 产率90%. m.p. 79~81 ℃; [α]D25+6.2 (c 0.5, THF); 1H NMR (CDCl3, 400 MHz) δ: 8.07 (d, J=8.8 Hz, 1H), 7.99 (m, 2H), 7.79 (d, J=8.8 Hz, 1H), 7.51 (td, J=7.2, 1.2 Hz, 1H), 7.25~7.40 (m, 5H), 6.92 (d, J=8.8 Hz, 1H), 5.22 (s, 1H), 0.81 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ: 177.89, 152.26, 148.37, 133.43, 132.28, 132.25, 131.05, 130.17, 129.98, 129.96, 129.43, 128.49, 127.67, 126.55, 126.40, 125.48, 122.48, 121.91, 119.52, 117.38, 114.56, 38.85, 26.59. Anal. calcd for C25H21BrO3 C 66.82, H 4.71; found C 66.85, H 4.75.
3.2.4 (S)-6-溴-2, 2'-二羟基-1, 1'-联萘(4)的合成
在Ar氛围下, 往溶有(S)-6-溴-2-羟基-2'-特戊酰氧基-1, 1'-联萘(3) (28.0 g, 62.4 mmol)的THF (100 mL)溶液中一次性加入KOH (10.4 g, 184 mmol)和H2O (80 mL), 将反应混合物在30 ℃反应20 h.将反应混合物通过减压浓缩除去溶剂, 所得剩余物用50 mL乙酸乙酯溶解, 分离出有机相, 并将有机相依次用1 mol·L-1 HCl (30 mL×2) 和饱和食盐水(15 mL×2) 洗涤、无水硫酸镁干燥.有机相过滤后减压浓缩除去溶剂获得22.30 g白色固体4[53], 产率98%. m.p. 83~86 ℃(文献值[53] m.p. 83~85 ℃); [α]D25 +6.5 (c 0.5, THF); 1H NMR (CDCl3, 400 MHz) δ: 7.01 (d, J=8.0 Hz, 1H), 7.09 (d, J=7.2 Hz, 1H), 7.31~7.42 (m, 5H), 7.39 (t, J=5.8 Hz, 1H), 7.86~7.90 (m, 2H), 7.98 (d, J=8.6 Hz, 1H), 8.17 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ: 153.16, 152.87, 133.49, 132.22, 131.75, 131.01, 130.82, 130.74, 130.48, 129.63, 128.62, 127.78, 126.29, 124.30, 124.17, 119.06, 118.01, 117.96, 111.58, 110.46. Anal. calcd for C20H13BrO2 C 65.77, H 3.59; found C 65.75, H 3.58.
3.2.5 (S)-6-溴-2, 2'-二甲氧甲氧基-1, 1'-联萘(5)的合成
在氩气保护以及0 ℃条件下, 往NaH (3.88 g, 80 mmol)和THF (80 mL)的混合物中缓慢滴加溶有(S)-6-溴-2, 2'-二羟基-1, 1'-联萘(4) (14.58 g, 40 mmol)的THF (80 mL)溶液. 30 min后往上述混合物中缓慢滴加氯甲基甲醚(6.4 g, 80 mmol), 滴加完后将混合物在常温下反应6 h.加入30 mL水淬灭反应, 分离出有机层, 水相用乙酸乙酯(40 mL×2) 萃取, 合并有机相并用无水硫酸镁干燥.将有机相过滤, 滤液减压浓缩除去溶剂获得的剩余物经柱层析分离获得16.28 g白色固体5[54], 产率90%. m.p. 78~79 ℃(文献值[54] m.p. 78~79 ℃); [α]D25-59.6 (c 0.01, THF); 1H NMR (CDCl3, 400 MHz) δ: 8.04 (s, 1H), 7.95 (d, J=9.0 Hz, 1H), 7.87 (t, J=8.2 Hz, 2H), 7.63~7.56 (m, 2H), 7.36 (t, J=7.3 Hz, 1H), 7.29 (s, 1H), 7.24 (d, J=7.1 Hz, 1H), 7.11 (d, J=8.4 Hz, 1H), 7.04 (d, J=9.0 Hz, 1H), 5.08 (t, J=6.0 Hz, 2H), 4.98 (d, J=6.1 Hz, 2H), 3.15 (s, 6H); 13C NMR (CDCl3, 100 MHz) δ: 153.07, 152.76, 133.94, 132.64, 131.01, 129.93, 129.81, 129.64, 129.57, 128.45, 127.95, 127.50, 126.45, 125.32, 124.15, 121.68, 120.62, 118.38, 117.95, 117.23, 95.32, 95.24, 55.84. Anal. calcd for C24H21BrO4 C 63.59, H 4.67; found C 63.51, H 4.71.
3.2.6 (S)-6-甲酰基-2, 2'-二甲氧甲氧基-1, 1'-联萘(6)的合成
在氩气保护以及-78 ℃条件下, 将n-BuLi (2.5 mol·L-1, 24.0 mL, 60 mmol)的正己烷溶液逐滴滴加到(S)-6-溴-2, 2'-二甲氧基甲氧基-1, 1'-联萘(5) (18.14 g, 40 mmol)的THF (100 mL)的溶液中, 滴加完毕后, 保持混合物在该温度下继续反应1 h.在-78 ℃下将DMF (10.2 mL, 128.2 mmol)缓慢滴加到上述混合物中, 滴加完后将混合物在-78 ℃反应3 h.将温度升至-20 ℃, 加入冰水(30 mL)用于淬灭反应, 将混合物减压蒸除溶剂后所得剩余物用二氯甲烷(50 mL)溶解, 并往混合物中加入50 mL水, 分离出有机相后水相用二氯甲烷(30 mL×2) 萃取, 合并有机相, 依次用饱和食盐水(30 mL×2) 洗涤有机相, 有机相经无水硫酸镁干燥后过滤除去干燥剂, 滤液减压浓缩除去溶剂得到的剩余物经柱层析[V(石油醚):V(乙酸乙酯)=6:1]分离纯化获得10.40 g白色固体6[55], 产率65%. m.p. 95~98 ℃; [α]D25-56.4 (c 0.01, THF); 1H NMR (CDCl3, 400 MHz) δ: 10.10 (s, 1H), 8.38 (s, 1H), 8.13 (d, J=9.1 Hz, 1H), 7.98 (d, J=9.1 Hz, 1H), 7.91 (d, J=8.2 Hz, 1H), 7.71 (d, J=9.1 Hz, 2H), 7.61 (d, J=9.1 Hz, 1H), 7.36 (t, J=7.2 Hz, 1H), 7.24 (d, J=9.0 Hz, 1H), 7.12 (d, J=8.4 Hz, 1H), 5.17 (d, J=6.9 Hz, 1H), 5.11 (d, J=6.8 Hz, 1H), 5.07 (d, J=6.9 Hz, 1H), 4.98 (d, J=6.7 Hz, 1H), 3.18 (d, J=17.6 Hz, 6H); 13C NMR (CDCl3, 100 MHz) δ: 192.12, 155.44, 152.71, 137.51, 134.81, 133.84, 132.65, 131.29, 129.90, 128.81, 128.15, 126.66, 126.60, 125.15, 124.26, 123.33, 121.53, 120.17, 117.62, 116.98, 95.06, 94.64, 56.04, 55.90. Anal. calcd for C25H22O5 C 74.62, H 5.53; found C 74.58, H 5.49.
3.2.7 (S)-6-羟甲基-2, 2'-二甲氧甲氧基-1, 1'-联萘(7)的合成
冰浴下将(S)-6-甲酰基-2, 2'-二甲氧甲氧基-1, 1'-联萘(6) (9.25 g, 23 mmol)充分溶解于THF (100 mL)中, 分批次加入NaBH4(1.73 g, 46 mmol).反应体系升至室温反应一个小时后将反应混合物倾倒入100 mL水中, 用乙酸乙酯萃取混合物, 获得的有机相用无水硫酸镁干燥.有机相过滤后减压浓缩得获得9.21 g无色液体7[26], 产率99%. [α]20 D-58.2 (c 0.05, THF); 1H NMR (CDCl3, 400 MHz) δ: 3.05 (s, 3H), 3.06 (s, 3H), 4.71 (s, 2H), 4.88 (d, J=6.7 Hz, 2H), 4.96 (d, J=3.4Hz, 1H), 4.99 (d, J=3.4 Hz, 1H), 7.02~7.17 (m, 4H), 7.23 (t, J=6.8 Hz, 1H), 7.46 (d, J=9.0 Hz, 2H), 7.76 (s, 1H), 7.78 (d, J=8.0 Hz, 1H), 7.82 (d, J=5.6 Hz, 1H), 7.87 (d, J=5.6 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ: 55.81, 65.42, 95.10, 95.21, 117.29, 117.38, 121.10, 121.22, 123.90, 125.30, 125.58, 125.57, 125.91, 126.20, 127.71, 129.27, 129.28, 129.69, 129.68, 133.39, 133.81, 136.29, 152.50, 152.61. Anal. calcd for C25H24O5 C 74.24, H 5.98; found C 74.26, H 6.01.
3.2.8 (S, S)/(S, R)-6-(2, 3-环氧丙氧基)甲基-2, 2'-二(甲氧基甲氧基)-1, 1'-联萘(8a/8b)的合成
将(S)-6-羟甲基-2, 2'-二甲氧甲氧基-1, 1'-联萘(7) (4.05 g, 10 mmol)溶解于无水的THF (30 mL)中, 随后往反应体系中加入粉末状氢氧化钾(400 mol%)和四丁基溴化铵(10 mol%).反应混合物在室温下搅拌2 h, 将(S)-环氧氯丙烷(200 mol%)滴加到反应中, 反应继续在室温下进行12 h后结束.往反应混合物中加入(40 mL)乙酸乙酯并用食盐水洗涤有机相.分离出的有机相用无水硫酸镁干燥, 有机相过滤后减压浓缩除去溶剂, 初产物用柱色谱分离纯化.获得3.78 g无色黏状液体8a[26], 产率82%. [α]20 365-154.5 (c 0.1, THF); 1H NMR (CDCl3, 400 MHz) δ: 7.96~7.92 (m, 2H, ArH), 7.88~7.83 (t, J=3.0 Hz, 2H, ArH), 7.58~7.56 (d, J=9.0 Hz, 2H, ArH), 7.35~7.33 (t, J=8.0 Hz, 1H, ArH), 7.22~7.12 (m, 4H, ArH), 5.08~5.05 (m, 2H, CH2O), 4.98~4.96 (m, 2H, CH2O), 4.70~4.67 (dd, J=2.4, 6.0 Hz, 2H, CH2), 3.77~3.76 (dd, J=2.8, 5.4 Hz, 1H, CH), 3.49~3.47 (m, 1H, CH2), 3.18 (s, 1H, CH2), 3.15 (s, 3H, CH3), 3.14 (s, 3H, CH3), 2.79~2.78 (t, J=3.2 Hz, 1H, CH2), 2.61 (s, 1H, CH2); 13C NMR (CDCl3, 100 MHz) δ: 152.92, 152.72, 129.38, 126.69, 126.26, 125.99, 125.56, 124.07, 117.65, 117.45, 95.38, 73.44, 70.89, 55.80, 50.84, 44.30. Anal. calcd for C28H28O6 C 73.03, H 6.13; found C 73.08, H 6.16.
经过上述同样的方法, (S, R)-6-(2, 3-环氧丙氧基)甲基-2, 2'-二(甲氧基甲氧基)-1, 1'-联萘(8b)由(S)-6-羟甲基-2, 2'-二甲氧甲氧基-1, 1'-联萘(7)和(R)-环氧氯丙烷反应制备, 获得3.68 g淡黄色黏状液体8b, 产率80%. [α]20 365-102.4 (c 0.1, THF); 1H NMR (CDCl3, 400 MHz) δ: 7.96~7.92 (m, 2H, ArH), 7.88~7.83 (t, J=3.2 Hz, 2H, ArH), 7.59~7.57 (d, J=9.0 Hz, 2H, ArH), 7.35~7.33 (t, J=8.0 Hz, 1H, ArH), 7.22~7.12 (m, 4H, ArH), 5.08~5.06 (m, 2H, CH2O), 4.98~4.96 (m, 2H, CH2O), 4.71~4.68 (dd, J=2.4, 6.0 Hz, 2H, CH2), 3.79~3.77 (dd, J=2.8, 5.4 Hz, 1H, CH), 3.50~3.48 (m, 1H, CH2), 3.17 (s, 1H, CH2), 3.16 (s, 3H, CH3), 3.15 (s, 3H, CH3), 2.79~2.78 (t, J=3.2 Hz, 1H, CH2), 2.62 (s, 1H, CH2); 13C NMR (CDCl3, 100 MHz) δ: 152.85, 152.75, 134.07, 133.67, 129.97, 129.76, 129.38, 127.86, 126.68, 126.27, 125.99, 125.57, 124.07, 117.55, 95.41, 73.44, 70.88, 55.81, 50.83, 44.30. Anal. calcd for C28H28O6: C 73.03, H 6.13; found C 73.06, H 6.18.
3.2.9 (S, S)/(S, R)-6-(2, 3-环氧丙氧基)甲基-2, 2'-二(甲氧基甲氧基)-1, 1'-联萘的聚合以及脱MOM保护基过程
将装有一定量的手性环氧单体(S, S)/(S, R)-6-(2, 3-环氧丙氧基)甲基-2, 2'-二(甲氧基甲氧基)-1, 1'-联萘(10 mmol)和1.5 mL无水的二甲苯的聚合试管用氩气置换三次, 在氩气氛围下加入0.34 mmol的颗粒状KOH.将聚合试管置于120 ℃的温度中搅拌20 h.在这过程中混合物变得越来越粘稠, 颜色也越来越深.将聚合试管冷却至室温, 用10 mL THF将聚合瓶中的混合物充分溶解, 将溶解液逐滴加入到大量的甲醇中(溶液体积的30倍), 不断有白色固体析出, 抽滤分离得聚合物.将聚合物用20 mL THF溶解, 0 ℃下往溶解液中依次加入2 mL的水和2 mL的浓盐酸.混合物连续搅拌24 h后逐滴加入到搅拌的甲醇(200 mL)中, 不断有白色固体析出.将白色固体抽滤后反复沉降3次.最后获得的聚合物置于50 ℃下真空干燥得白色固体[26], 产率82%~85%. 1H NMR (CDCl3, 400 MHz) δ: 8.02~6.76 [br, 10H, Ar-H], 4.57~4.19 [br, 2H, CH2O], 3.57~2.92 [br, 5H, >CHO, CH2, CH2O]. Poly-A:产率85%. [α]20 365-342.4 (c 0.05, THF); Mn=5.2×103; PDI=Mw/Mn=2.02. Poly-B:产率82%, [α]20 365-296.7 (c 0.05, THF); Mn=5.6×103; PDI=Mw/Mn=2.41.
辅助材料(Supporting Information) 部分化合物的1H NMR、13C NMR图谱以及催化产物的HPLC谱图.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
吴琼, 赵佳佳, 孙斯兵, 屠蔓苏, 石枫, 化学学报, 2016, 74, 576. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201607003&dbname=CJFD&dbcode=CJFQWu, Q.; Zhao, J.; Sun, S.; Tu, M.; Shi, F. Acta Chim. Sinica 2016, 74, 576(in Chinese). http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201607003&dbname=CJFD&dbcode=CJFQ
-
[2]
程清卿, 许唤, 朱守非, 周其林, 化学学报, 2015, 73, 326. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201504004&dbname=CJFD&dbcode=CJFQCheng, Q.; Xu, H.; Zhu, S.; Zhou, Q. Acta Chim. Sinica 2015, 73, 326(in Chinese). http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201504004&dbname=CJFD&dbcode=CJFQ
-
[3]
冯向青, 杜海峰, 有机化学, 2015, 35, 259. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344662.shtmlFeng, X.; Du, H. Chin. J. Org. Chem. 2015, 35, 259(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344662.shtml
-
[4]
周伟, 高利华, 陶梦娜, 宿晓, 赵庆杰, 张俊良, 化学学报, 2016, 74, 800. doi: 10.3866/PKU.WHXB201602171Zhou, W.; Gao, L.; Tao, M.; Su, X.; Zhao, Q.; Zhang, J. Acta Chim. Sinica 2016, 74, 800(in Chinese). doi: 10.3866/PKU.WHXB201602171
-
[5]
叶旭, 曾兴平, 周剑, 化学学报, 2016, 74, 984. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201612004&dbname=CJFD&dbcode=CJFQYe, X.; Zeng, X.; Zhou, J. Acta Chim. Sinica 2016, 74, 984(in Chinese). http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201612004&dbname=CJFD&dbcode=CJFQ
-
[6]
Zheng, Y.; Ma, H.; Ma, J-A. Chin. J. Chem. 2016, 34, 511. doi: 10.1002/cjoc.201500901
-
[7]
Li, J.; Du, D. Chin. J. Chem. 2015, 33, 418. doi: 10.1002/cjoc.v33.4
-
[8]
刘运林, 周剑, 化学学报, 2012, 70, 1451. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201213006&dbname=CJFD&dbcode=CJFQLiu, Y.; Zhou, J. Acta Chim. Sinica 2012, 70, 1451(in Chinese). http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hxxb201213006&dbname=CJFD&dbcode=CJFQ
-
[9]
Dupont, J.; de Souza, R. F.; Suarez, P. A. Z. Chem. Rev. 2002, 102, 3667. doi: 10.1021/cr010338r
-
[10]
Fan, Q.-H.; Li, Y.-M.; Chan, A. S. C. Chem. Rev. 2002, 102, 3385. doi: 10.1021/cr010341a
-
[11]
Song, C. E.; Lee, S.-G. Chem. Rev. 2002, 102, 3495. doi: 10.1021/cr0103625
-
[12]
Bergbreiter, D. E.; Tian, J.; Hongfa, C. Chem. Rev. 2009, 109, 530. doi: 10.1021/cr8004235
-
[13]
Fraile, J. M.; Garcia, J. I.; Herrerias, C. I.; Mayoral, J. A.; Pires, E. Chem. Soc. Rev. 2009, 38, 695. doi: 10.1039/B806643B
-
[14]
Fraile, J. M.; García, J. I.; Mayoral, J. A. Chem. Rev. 2009, 109, 360. doi: 10.1021/cr800363y
-
[15]
Trindade, A. F.; Gois, P. M. P.; Afonso, C. A. M. Chem. Rev. 2009, 109, 418. doi: 10.1021/cr800200t
-
[16]
Fan, Q. H.; Ren, C. Y.; Hu, W. H.; Chan, A. S. C. J. Am. Chem. Soc. 1999, 121, 7407. doi: 10.1021/ja9837497
-
[17]
Fan, Q. H.; Chen, Y. M.; Chen, X. M.; Jiang, D. Z.; Xi, F.; Chan, A. S. C. Chem. Commun. 2000, 31, 789. doi: 10.1002/chin.200033023/full
-
[18]
Huang, W. S.; Hu, Q. S.; Pu, L. J. Org. Chem. 1999, 64, 7940. doi: 10.1021/jo990992v
-
[19]
Hu, Q. S.; Pu, L. Polym. Prepr. 2000, 41, 16.
-
[20]
Yang, X. W.; Sheng, J. H.; Da, C. S.; Wang, H. S.; Su, W.; Wang, R.; Chan, A. S. C. J. Org. Chem. 2000, 65, 295. doi: 10.1021/jo990771p
-
[21]
刘大财, 朱维君, 张安林, 阳年发, 杨利文, 有机化学, 2015, 35, 1797. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344941.shtmlLiu, D.; Zhu, W.; Zhang, A.; Yang, N.; Yang, L. Chin. J. Org. Chem. 2015, 35, 1797(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344941.shtml
-
[22]
晏瑾懿, 阳珠生, 阳年发, 有机化学, 2016, 36, 812. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345356.shtmlYan, J.; Yang, Z.; Yang, N. Chin. J. Org. Chem. 2016, 36, 812(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345356.shtml
-
[23]
Huang, C.; Yang, N.; Zhang, A.; Yang, L. Polymer 2012, 53, 3514. doi: 10.1016/j.polymer.2012.05.054
-
[24]
Zhang, A.; Yang, N.; Yang, L.; Peng, D. Chem. Lett. 2013, 43, 462. http://ci.nii.ac.jp/naid/130004868063
-
[25]
Chen, Y.; Qin, G.; Yang, L.; Yang, N. Chin. J. Chem. 2015, 33, 463. doi: 10.1002/cjoc.v33.4
-
[26]
Zhang, A.-L.; Yu, Z.-d.; Yang, L.-W.; Yang, N.-F. Tetrahedron:Asymmetry 2015, 26, 173. doi: 10.1016/j.tetasy.2014.12.012
-
[27]
Zhang, A.-L.; Yu, Z.-D.; Yang, L.-W.; Yang, N.-F.; Peng, D. J. Mol. Catal. A:Chem. 2015, 398, 407. doi: 10.1016/j.molcata.2015.01.007
-
[28]
Liu, D.; Ouyang, K.; Yang, N. Tetrahedron 2016, 72, 1018. doi: 10.1016/j.tet.2015.12.076
-
[29]
Corey, E. J.; Cimprich, K. A. J. Am. Chem. Soc. 1994, 116, 3151. doi: 10.1021/ja00086a066
-
[30]
Roush, W. R.; Sciotti, R. J. J. Am. Chem. Soc. 1994, 116, 6457. doi: 10.1021/ja00093a065
-
[31]
Stang, P. J.; Diederich, F. Modern Acetylene Chemistry, VCH, Weinheim, 1995.
-
[32]
Myers, A. G.; Zheng, B. J. Am. Chem. Soc. 1996, 118, 4492. doi: 10.1021/ja960443w
-
[33]
Thompson, A.; Corley, E. G.; Huntington, M. F.; Grabowski, E. J. J.; Remenar, J. F.; Collum, D. B. J. Am. Chem. Soc. 1998, 120, 2028. doi: 10.1021/ja9713791
-
[34]
Fox, M. E.; Li, C.; Marino, J. P.; Overman, L. E. J. Am. Chem. Soc. 1999, 121, 5467. doi: 10.1021/ja990404v
-
[35]
Trost, B.; Krische, M. J. J. Am. Chem. Soc. 1999, 121, 6131. doi: 10.1021/ja990183t
-
[36]
Gao, G.; Moore, D.; Xie, R.-G.; Pu, L. Org. Lett. 2002, 4, 4143. doi: 10.1021/ol026921r
-
[37]
Ishizaki, M.; Hoshino, O. Tetrahedron:Asymmetry 1994, 5, 1901. doi: 10.1016/S0957-4166(00)86262-X
-
[38]
Anand, N. K.; Carreira, E. M. J. Am. Chem. Soc. 2001, 123, 9687. doi: 10.1021/ja016378u
-
[39]
Lu, G.; Li, X.; Zhou, Z.; Chan, W. L.; Chan, A. S. C. Tetrahedron:Asymmetry 2001, 12, 2147. doi: 10.1016/S0957-4166(01)00384-6
-
[40]
Pu, L.; Yu, H. B. Chem. Rev. 2001, 101, 757. doi: 10.1021/cr000411y
-
[41]
Lu, G.; Li, X.; Chan, W. L.; Chan, A. S. Chem. Commun. 2002, 2, 172. http://www.ncbi.nlm.nih.gov/pubmed/12120356
-
[42]
Chen, Z.-C.; Hui, X.-P.; Yin, C.; Huang, L.-N.; Xu, P.-F.; Yu, X.-X.; Cheng, S.-Y. J. Mol. Catal. A:Chem. 2007, 269, 179. doi: 10.1016/j.molcata.2007.01.025
-
[43]
Jiang, B.; Chen, Z. L.; Huang, H. Lett. Org. Chem. 2005, 2, 319. doi: 10.2174/1570178054038911
-
[44]
Wu, P.-Y.; Wu, H.-L.; Shen, Y.-Y.; Uang, B.-J. Tetrahedron:Asymmetry 2009, 20, 1837. doi: 10.1016/j.tetasy.2009.07.032
-
[45]
Rachwalski, M.; Leśniak, S.; Kiełbasiński, P. Tetrahedron:Asymmetry 2010, 21, 2687. doi: 10.1016/j.tetasy.2010.10.013
-
[46]
Bauer, T.; Smoliński, S.; Gaweł, P.; Jurczak, J. Tetrahedron Lett. 2011, 52, 4882. doi: 10.1002/chin.201202070/pdf
-
[47]
Marinova, M.; Kostova, K.; tzvetkova, P.; tavlinova-kirilova, M.; chimov, A.; nikolova, R.; shivachev, B.; dimitrov, V. Tetrahedron:Asymmetry 2013, 24, 1453. doi: 10.1016/j.tetasy.2013.09.023
-
[48]
Chen, C.; Huang, Q.; Zou, S.; Wang, L.; Luan, B.; Zhu, J.; Wang, Q.; Pu, L. Tetrahedron:Asymmetry 2014, 25, 199. doi: 10.1016/j.tetasy.2013.12.013
-
[49]
Rachwalski, M. Tetrahedron:Asymmetry 2014, 25, 219. doi: 10.1016/j.tetasy.2013.11.011
-
[50]
Jarzyński, S.; Leśniak, S.; Pieczonka, A. M.; Rachwalski, M. Tetrahedron:Asymmetry 2015, 26, 35. doi: 10.1016/j.tetasy.2014.11.016
-
[51]
Marshall, J. A.; Wang, X. J. J. Org. Chem. 1992, 57, 1242. doi: 10.1021/jo00030a036
-
[52]
Mao, J.; Bao, Z.; Guo, J.; Ji, S. Tetrahedron 2008, 64, 9901. doi: 10.1016/j.tet.2008.08.003
-
[53]
Yuan, X. Y.; Li, H. Y.; Hodge, P.; Kilner, M.; Tastard, C. Y.; Zhang, Z. P. Tetrahedron Asymmetry 2006, 17, 2401. doi: 10.1016/j.tetasy.2006.08.011
-
[54]
Wang, X.; Wang, X.; Guo, H.; Wang, Z.; & Ding, K. Chem. Eur. J. 2005, 11, 4078. doi: 10.1002/(ISSN)1521-3765
-
[55]
Liu, G. H.; Tang, W. J.; Fan, Q. H. Tetrahedron 2003, 59, 8603. doi: 10.1016/j.tet.2003.09.030
-
[1]
-
表 1 聚合物Poly-A或Poly-B催化苯乙炔与苯甲醛的不对称加成反应条件优化a
Table 1. Optimization of asymmetric reaction of phenylacetylene with benzaldehyde catalyzed by Poly-A or Poly-B

Entry Conditions for step 2 Ti(O-i-Pr)4/mol% Ligand (mol%) Yieldb/% eec/% Config.d Solvent T/℃ 1 THF 25 50 Poly-A (10) 94 79 R 2 THF 25 50 Poly-B (10) 90 67 R 3 Toluene 25 50 Poly-A (10) 90 38 R 4 Et2O 25 50 Poly-A (10) 87 18 R 5 DCE 25 50 Poly-A (10) 89 71 R 6 DCM 25 50 Poly-A (10) 92 47 R 7 THF 0 50 Poly-A (10) 30 85 R 8 THF 25 100 Poly-A (10) 91 85 R 9 THF 25 150 Poly-A (10) 84 82 R 10 THF 25 100 Poly-A (20) 87 90 R 11 THF 25 100 Poly-A (30) 82 88 R 12e THF 25 100 Poly-A (20) 90 91 R aConditions for step 1: 2 mL of toluene, reflux 5 h; phenylacetylene: 2.0 mmol; Me2Zn: 2.0 mmol; Conditions for step 2: 8 mL of solvent; benzaldehyde: 0.5 mmol; reaction time: 8 h. b Isolated yields. cDetermined by HPLC with a Chiralcel OD-H column. d The absolute configurations of the products were determined by comparison to the literature data[39]. e Reaction time: 24 h. 表 2 聚合物Poly-A催化苯乙炔与各种醛的不对称加成反应a
Table 2. Enantioselective addition of phenylacetylene to various aldehydes catalyzed by Poly-A

Entry R Yieldb/% eec/% Config.d 1 Phenyl 92 91 R 2 2-Methoxyphenyl 82 81 R 3 3-Methoxyphenyl 83 86 R 4 4-Chlorophenyl 90 90 R 5 4-Bromophenyl 87 85 R 6 4-Fluorophenyl 91 89 R 7 4-Nitrophenyl 89 95 R 8 3-Nitrophenyl 86 85 R 9 1-Naphthyl 81 88 R 10 2-Naphthyl 82 92 R 11 3-Phenethyl 92 80 R 12 Hexyl 95 75 R a Conditions for step 1: 2 mL of toluene, reflux 5 h; phenylacetylene: 2.0 mmol; Me2Zn: 2.0 mmol; Conditions for step 2: reactions were carried out with 20 mol% of Poly-A (as a BINOL monomer), 0.5 mmol of aldehyde, 1.0 mmol of Ti(O-i-Pr)4 in 8 mL of THF at 25 ℃ for 24 h.bIsolated yield. cDetermined by HPLC using a Chiralcel OD-H column. d The absolute configurations of the products were determined by comparison to the literature data[52]. 表 3 回收的Poly-A催化苯乙炔与苯甲醛的不对称加成反应a
Table 3. Asymmetric reaction of phenylacetylene with benzaldehyde catalyzed by the recovered Poly-A
Cycle 1 2 3 4 5 6 Yieldb/% 92 91 90 91 89 91 eec/% 91 91 90 92 93 90 a Conditions for step 1: 2 mL of toluene, reflux 5 h; phenylacetylene: 2.0 mmol; Me2Zn: 2.0 mmol; Conditions for step 2: reactions were carried out with 20 mol% of Poly-A (as a BINOL monomer), 0.5 mmol of benzaldehyde, 1.0 mmol of Ti(O-i-Pr)4, in 8 mL of THF at 25 ℃ for 24 h.b Isolated yield. cDetermined by HPLC using a Chiralcel OD-H column. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 4
- 文章访问数: 502
- HTML全文浏览量: 100


 下载:
下载:

 下载:
下载:

