
Citation: Chen Liang, Wang Baoqu, Zhao Yucheng, Yan Shengjiao, Lin Jun. One-Pot Synthesis of Multisubstituted Chromone-Fused Bicyclic Pyridine Compounds[J]. Chinese Journal of Organic Chemistry, 2017, 37(6): 1433-1442. doi: 10.6023/cjoc201612038

一锅法合成多取代色酮并双环吡啶类化合物
English
One-Pot Synthesis of Multisubstituted Chromone-Fused Bicyclic Pyridine Compounds
-
Key words:
- environment friendly
- / atom economy
- / catalyst-free
- / bicyclic pyridine
- / neonicotinoids
-
化工产品和药物的生产离不开化学合成.化学合成丰富人们物质世界的同时, 不可避免地带来很多问题.包括资源和能源的浪费, 以及环境污染等[1, 2].而如何应对由化学合成带来的污染已成为现代化学工作者急需思考和面对的问题.理想的化学合成应该是一个绿色环保和高效低碳的过程[3, 4], 美国著名化学家Trost[5]在1991年提出了“原子经济性”的原则, 认为在设计合成路线的时候应该尽可能地消除或者减少有害物质的产生, 美国环境保护组织(U.S. EPA)也倡导化学反应应满足“绿色化学”的要求[6].而本文旨在探索如何绿色、高效构筑C—C和C—N键并合成含色酮骨架结构的双环咪唑并吡啶类化合物的.含氮杂环化合物在合成药物[7, 8]及天然产物中扮演着十分重要的角色[9, 10].
含氮杂环化合物具有丰富多样的生物活性, 比如说抗癌[11]、抗病毒[12]、治疗心脑血管疾病[13]、镇静安眠等[14, 15].咪唑并吡啶作为含氮杂环化合物家族中较为重要的一员, 已经在很多临床药物中发挥着重要的作用[16, 17], 已经商品化的药物包括奥普力农[18]、法倔唑[19]、唑吡旦[20]等.
新烟碱型类似物(如吡虫啉[21]、6-Cl-PMNI[22]、哌虫啶[23]等)因其良好的杀虫活性以及较低的哺乳动物毒性, 迅速发展并成为继有机磷农药、氨基甲酸盐及除虫菊酯之后的第四代新型农药[24, 25].而新烟碱类似物, 因其特殊的结构特征: (1) 柔性的苄基侧链, (2) 强吸电子的硝基, (3) 芳香核, (4) 和双键相连的胺基.李忠课题组[26, 27]开展一系列新烟碱类似物的研究工作, 合成获得很多活性化合物.其中, 新烟碱类似物N-苄基硝基烯酮缩胺(NBN-KAs, 2)属于多功能合成砌块即杂环烯酮缩胺(HKAs)类化合物[28, 29]. NBNKAs在构建具有潜在生物活性的含氮杂环化合物方面有独特的优势[30, 31].
黄酮类药物在抗癌、抗衰老及治疗血管疾病方面发挥着重要作用[32, 33].黄酮类化合物均含有色酮母核.本文拟根据药物拼合原理, 把色酮结构及新烟碱结构拼合到目标化合物双环咪唑并吡啶类化合物中, 希望新杂合物具有更优、更广谱的生物活性, 为今后进行生物活性普筛打下基础.
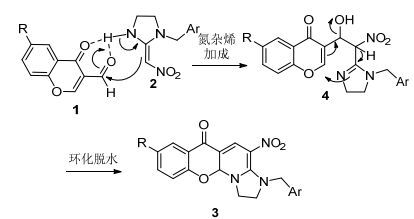
本文目标产物的合成用取代色酮-3-甲醛化合物1与2反应, 通过2 α-C选择性对色酮羰基加成脱水、氨基对色酮双键进行迈克尔加成得目标产物(Eq. 1).相对其他文献报道的杂环构筑方法[34], 本方法符合绿色合成原则, 操作简单, 反应条件温和, 反应时间短, 原子利用率高, 产率高.
1 结果与讨论
1.1 反应条件的筛选
称取1.1 mmol的色酮-3-甲醛1a和1.0 mmol的1-(4-硝基苄基)-2-(硝基亚甲基)咪唑烷(2a)加入到25 mL圆底烧瓶中, 加入15 mL溶剂搅拌, 之后对反应溶剂、催化剂、时间以及温度对产率的影响进行了筛选, 结果见表 1.

最初我们尝试了在室温下, 分别以EtOH和N, N-二甲基甲酰胺(DMF)为溶剂, 无催化剂条件下的反应, 以薄层色谱(TLC)作为检测手段, 结果显示(表 1, Entries 1~2), 两种情况下都有目标化合物3aa生成, 但是两种条件下, 原料都没有反应完.然后又考查了EtOH作为溶剂回流条件下的反应(表 1, Entry 3), 结果显示升高温度对反应是有利的, 能在较短时间(20 min)以较高产率(96%)获得目标化合物3aa.我们进一步考察了不同溶剂对反应的影响, 结果显示(表 1, Entries 4~9), 不论是质子性溶剂(EtOH, MeOH, H2O), 还是非质子性溶剂(DCM, toluene, 1, 4-dioxane)都能得到目标产物.但是产率存在一定的差异(72%~65%), 我们推测可能是由于目标产物最后都是以沉淀的形式从反应液中析出, 所以溶剂的极性对产率会造成影响, 对产物溶解性好的溶剂会使得产率下降, 但这并不意味着对产物溶解性差的溶剂就能给出高的产率(Entries 5, 9).部分溶剂由于没办法对底物进行有效地溶解, 使得底物无法充分接触, 反应受阻, 导致反应底物和产物混在一起, 影响后处理.在最优溶剂选择为EtOH的基础上, 进一步研究了催化剂对本反应的影响, 结果显示(表 1, Entries 10~13), 催化剂的加入反而降低了产率.
综合来看, 最优的合成条件为以乙醇作为溶剂, 底物1.1:1, 无催化剂回流20 min, 能够以优异的产率(96%)得目标化合物3aa.且反应结束后待反应液冷却到室温, 过滤后取沉淀干燥即可得纯的目标产物.
1.2 目标产物及产率
在最佳条件基础上, 为进一步的验证反应的普适性, 我们以色酮-3-甲醛1a~1d与N-苄基的硝基烯酮缩胺2a~2k反应, 成功合成30个双环咪唑并吡啶类化合物3aa~3dk.结果详见表 2.
实验过程中我们发现, 当色酮-3-甲醛的C(6) 位上是吸电子基团(比如F和Cl)时, 反应的速度明显比C(6) 位上是给电子基团CH3的时候快, 根据我们推测的反应机理(Scheme 1)来说, 有可能是色酮的苯环上有吸电子基团的时候, 能够提高色酮羰基碳的电正性, 有利于N-苄基的硝基烯酮缩胺α-C对其进攻, 所以造成反应速度加快.根据以上推测, 实际的反应速率顺序应为: F>Cl>H>CH3, 但是实际实验观察结果则是Cl>F>H>CH3.造成上述结果的原因可能是由C(6) 位是F的时候, F原子容易和NH里的H原子形成氢键, 干扰了NH中H原子与色酮羰基上氧原子氢键的形成.最终导致含F色酮的反应速度低于含Cl色酮.
通过的实验数据的分析可以发现, 不论是色酮-3-甲醛上取代基的种类还是N-苄基硝基烯酮缩胺的芳香环种类, 对反应的产率均无明显影响.结合最终产物以沉淀形式析出这一现象, 说明此反应有很好的热力学优势以及较为广泛的适用性.
1.3 反应机理
我们推测反应机理如Scheme 1所示. NBNKAs化合物氨基上的H原子首先和色酮-3-甲醛羰基氧原子形成分子间的氢键[35, 36].而氢键的形成提高了色酮-3-甲醛中羰基碳原子的电正性, 使得其亲电反应活性提高.另一方面, 分子间氢键的形成起到了定位作用, 有利于NBNKAs中双键α-C对色酮醛基亲核加成得中间体4.然后中间体4脱去一分子水, 而N进攻色酮的C(2) 位点, 经过双键位置的重排, 得到目标产物3.
2 结论
本文建立了一种绿色、高效一步法合成多取代色酮并双环吡啶类衍生物的方法.该方法利用C(6) 位不同取代基的色酮-3-甲醛与不同芳香取代的N-苄基硝基烯酮缩胺类化合物(NBNKAs)反应快速合成一系列含色酮骨架结构的咪唑并吡啶类化合物.且反应结束后, 经过简单过滤就能得到目标产物.反应过程符合绿色化学的理念, 溶剂环保, 整个反应只脱出一分子水, 反应原子利用率高, 产率高.另一方面, 合成所用两个原料本身就具有很好的生物活性, 这为后续对产物的活性筛选提供了良好的基础.
3 实验部分
3.1 仪器和试剂
IR:傅里叶红外光谱仪(Thermo Nicolet Avatar 360型); HRMS:高分辨质谱仪(Agilent CL/Msd TOF); NMR:核磁共振仪Bruck DRX500 (1H: 500 MHz, 13C: 125 MHz), Bruck DRX600 (1H: 600 MHz, 13C: 150 MHz); 熔点仪(XT-4A控温型显微熔点测定仪); 控温型电磁搅拌器. GF254高效薄层层析板, 分析纯或化学纯试剂, NBNKAs 2按文献[37]制备.
3.2 化合物3的合成
称取1.1 mmol色酮-3-甲醛1和1.0 mmol N-苄基硝基烯酮缩胺2共同加入到25 mL圆底烧瓶中, 加入15 mL乙醇, 充分搅拌并加热回流20 min, TLC监测直至原料消失, 停止反应.待反应液冷却至室温后过滤, 取沉淀在红外灯下干燥, 得到目标产物3aa~3dk, 产率为93%~98%.
4-硝基-3-(4-硝基苄基)-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3aa):黄色固体, m.p. 258~259 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.25 (d, J=9.0 Hz, 2H, ArH), 7.92 (s, 1H, CH), 7.83~7.85 (m, 1H, ArH), 7.68 (d, J=9.0 Hz, 2H, ArH), 7.61~7.65 (m, 1H, ArH), 7.17~7.20 (m, 1H, ArH), 7.11 (d, J=8.5 Hz, 2H, ArH), 6.47 (s, 1H, CH), 5.01 (d, J=17.3 Hz, 1H, CH2), 4.83 (d, J=17.5 Hz, 1H, CH2), 3.84~4.21 (m, 2H, CH2), 3.93~3.97 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.4, 156.6, 155.6, 147.3, 144.7, 136.6, 130.3, 128.8, 127.4, 124.0, 123.5, 123.2, 118.6, 112.4, 110.3, 85.9, 55.1, 51.3, 45.7; IR (KBr) ν: 1662, 1615, 1552, 1513, 1342, 1318, 1295, 1219, 622 cm-1; HRMS (TOF ES+) calcd for C21H17N4O6 [M+H]+ 421.1143, found 421.1140.
4-硝基-3-(4-(三氟甲基)苄基)-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ab):黄色固体, m.p. 240~241 ℃; 1H NMR (500 MHz, DMSO-d6)δ: 7.93 (s, 1H, CH), 7.83~7.84 (m, 1H, ArH), 7.75~7.77 (m, 2H, ArH), 7.62~7.63 (m, 3H, ArH), 7.17~7.20 (m, 1H, ArH), 7.11 (d, J=8.0 Hz, 1H, ArH), 6.46 (s, 1H, CH), 4.97 (d, J=16.5 Hz, 1H, CH2), 4.80 (d, J=16.5 Hz, 1H, CH2), 4.16~4.20 (m, 1H, CH2), 3.91~3.95 (m, 2H, CH2), 3.83~3.87 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 156.6, 155.5, 141.4, 136.5, 130.4, 128.5 (d, 2JC–F=32.5 Hz), 128.5, 127.4, 125.8 (d, 3JC–F=3.8 Hz), 123.2~123.7 (m), 118.6, 112.3, 110.3, 85.9, 55.0, 51.2, 45.6; IR (KBr) ν: 1657, 1616, 1557, 1460, 1326, 1295, 1114, 618 cm-1; HRMS (TOF ES+) calcd for C22H17F3N3-O4 [M+H]+ 444.1166, found 444.1167.
4-((4-硝基-6-氧代-1, 2-二氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-3(11aH)-基)甲基)苯甲腈(3ac):黄色固体, m.p. 243~244 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.91 (s, 1H, CH), 7.83~7.87 (m, 3H, ArH), 7.59~7.64 (m, 3H, ArH), 7.19 (d, J=7.5 Hz, 1H, ArH), 7.11 (d, J=8.0 Hz, 1H, ArH), 6.46 (s, 1H, CH), 4.96 (d, J=16.5 Hz, 1H, CH2), 4.79 (d, J=17.0 Hz, 1H, CH2), 4.18~4.19 (m, 1H, CH2), 3.91~3.95 (m, 2H, CH2), 3.85 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 156.6, 155.5, 142.5, 136.6, 132.8, 130.4, 128.6, 127.4, 123.5, 123.2, 119.2, 118.6, 112.3, 110.6, 110.3, 85.9, 55.2, 51.3, 45.6; IR (KBr) ν: 2222, 1661, 1615, 1553, 1460, 1307, 1293, 1220, 694 cm-1; HRMS (TOF ES+) calcd for C22H17N4O4[M+H]+ 401.1244, found 401.1243.
3-(4-氟苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ad):黄色固体, m.p. 234~235 ℃; 1H NMR (500 MHz, DMSO-d6)δ: 7.92 (s, 1H, CH), 7.82~7.84 (m, 1H, ArH), 7.60~7.64 (m, 1H, ArH), 7.43~7.46 (m, 2H, ArH), 7.16~7.24 (m, 3H, ArH), 7.10 (d, J=8.0 Hz, 1H, ArH), 6.43 (s, 1H, CH), 4.86 (d, J=15.5 Hz, 1H, CH2), 4.72 (d, J=15.5 Hz, 1H, CH2), 4.14~4.16 (m, 1H, CH2), 3.87~3.91 (m, 2H, CH2), 3.81~3.83 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 162.1 (d, 1JC–F=241.2 Hz), 156.6, 155.1, 136.5, 132.2, 130.2 (d, 3JC–F=8.8 Hz), 127.4, 123.5, 123.2, 118.6, 115.8 (d, 2JC–F=21.3 Hz), 112.2, 110.4, 85.9, 54.3, 50.8, 45.5; IR (KBr) ν: 3902, 1662, 1609, 1578, 1513, 1290, 1260, 1211, 1188, 624 cm-1; HRMS (TOF ES+) calcd for C21H17FN3O4 [M+H]+ 394.1198, found 394.1201.
3-(4-氯苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ae):黄色固体, m.p. 249~250 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.91 (s, 1H, CH), 7.82~7.84 (m, 1H, ArH), 7.61~7.64 (m, 1H, ArH), 7.41~7.46 (m, 4H, ArH), 7.17~7.20 (m, 1H, ArH), 7.10 (d, J=8.0 Hz, 1H, ArH), 6.44 (s, 1H, CH), 4.86 (d, J=16.0 Hz, 1H, CH2), 4.71 (d, J=16.0 Hz, 1H, CH2), 4.15~4.17 (m, 1H, CH2), 3.88~3.92 (m, 2H, CH2), 3.80~3.84 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 156.6, 155.2, 136.6, 135.3, 132.6, 130.4, 129.9, 128.9, 127.4, 123.5, 123.2, 118.6, 112.3, 110.3, 85.9, 54.4, 51.0, 45.5; IR (KBr) ν: 2975, 1663, 1612, 1462, 1289, 1211, 1187, 622 cm-1; HRMS (TOF ES+) calcd for C21H17ClN3O4[M+H]+ 410.0902, found 410.0900.
3-苄基-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3af):黄色固体, m.p. 242~243 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.91 (s, 1H, CH), 7.82~7.84 (m, 1H, ArH), 7.60~7.64 (m, 1H, ArH), 7.31~7.41 (m, 4H, ArH), 7.16~7.19 (m, 1H, ArH), 7.10 (d, J=8.5 Hz, 1H, ArH), 6.44 (s, 1H, CH), 4.88 (d, J=15.5 Hz, 1H, CH2), 4.75 (d, J=16.0 Hz, 1H, CH2), 4.15~4.16 (m, 1H, CH2), 3.88~3.92 (m, 2H, CH2), 3.79~3.83 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 156.6, 155.1, 136.5, 136.1, 130.5, 129.0, 128.1, 128.1, 127.4, 123.6, 123.2, 118.6, 112.2, 110.4, 85.9, 54.9, 51.0, 45.5; IR (KBr) ν: 3077, 1662, 1612, 1462, 1287, 1211, 1189, 1146, 1035, 625 cm-1; HRMS (TOF ES+) calcd for C21H18N3O4[M+H]+ 376.1292, found 376.1291.
3-(4-甲基苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ag):黄色固体, m.p. 219.5~220 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.91 (s, 1H, CH), 7.82~7.84 (m, 1H, ArH), 7.60~7.63 (m, 1H, ArH), 7.26 (d, J=8.0 Hz, 2H, ArH), 7.16~7.20 (m, 3H, ArH), 7.09 (d, J=8.0 Hz, 1H, ArH), 6.42 (s, 1H, CH), 4.82 (d, J=15.5 Hz, 1H, CH2), 4.71 (d, J=15.5 Hz, 1H, CH2), 4.13~4.14 (m, 1H, CH2), 3.86~3.90 (m, 2H, CH2), 3.79 (m, 1H, CH2), 2.30 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 156.6, 154.9, 136.4, 136.5, 132.9, 130.6, 129.6, 128.2, 127.3, 123.6, 123.2, 118.6, 112.1, 110.5, 85.9, 54.5, 50.8, 45.4, 21.2; IR (KBr) ν: 3075, 1662, 1611, 1557, 1462, 1288, 1211, 11190, 1145, 624 cm-1; HRMS (TOF ES+) calcd for C22H20N3O4[M+H]+ 390.1448, found 390.1448.
3-(4-甲氧基苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ah):黄色固体, m.p. 229~230 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 7.91 (s, 1H, CH), 7.82~7.83 (m, 1H, ArH), 7.60~7.63 (m, 1H, ArH), 7.31 (d, J=8.3 Hz, 2H, ArH), 7.16~7.19 (m, 1H, ArH), 7.08~7.10 (m, 1H, ArH), 6.95 (d, J=8.5 Hz, 2H, ArH), 6.42 (s, 1H, CH), 4.79 (d, J=15.2 Hz, 1H, CH2), 4.69 (d, J=15.2 Hz, 1H, CH2), 4.11~4.15 (m, 1H, CH2), 3.85~3.89 (m, 2H, CH2), 3.78~3.79 (m, 1H, CH2), 3.75 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6)δ: 179.3, 159.4, 156.6, 154.8, 136.5, 130.6, 129.7, 127.7, 127.3, 123.5, 123.2, 118.6, 114.5, 112.2, 110.5, 85.9, 55.6, 54.1, 50.6, 45.4; IR (KBr) ν: 3075, 2833, 1660, 1611, 1580, 1515, 1305, 1259, 1210, 1188, 623 cm-1; HRMS (TOF ES+) calcd for C22H20N3O5 [M+H]+ 406.1397, found 406.1398.
3-(3-氟苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ai):黄色固体, m.p. 218~219 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.92 (s, 1H, CH), 7.83~7.85 (m, 1H, ArH), 7.61~7.64 (m, 1H, ArH), 7.42~7.46 (m, 1H, ArH), 7.27 (d, J=10.0 Hz, 1H, ArH), 7.14~7.23 (m, 2H, ArH), 7.11 (d, J=8.0 Hz, 1H, ArH), 6.44 (s, 1H, CH), 4.90 (d, J=16.0 Hz, 1H, CH2), 4.72 (d, J=16.0 Hz, 1H, CH2), 4.15~4.18 (m, 1H, CH2), 3.90~3.94 (m, 2H, CH2), 3.81~3.85 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 162.8 (d, 1JC–F=241.3 Hz), 156.6, 155.3, 139.2 (d, 3JC–F=6.3 Hz), 136.5, 130.9 (d, 3JC–F=8.8 Hz), 130.5, 127.4, 123.9, 123.5, 123.2, 118.6, 114.9, 114.8 (d, 2JC–F=21.3 Hz), 112.3, 110.4, 85.9, 54.7, 51.1, 45.5; IR (KBr) ν: 3071, 1608, 1578, 1513, 1289, 1259, 1210, 1186, 1145, 623 cm-1; HRMS (TOF ES+) calcd for C21H17FN3O4[M+H]+ 394.1198, found 394.1201.
3-(3, 5-二氟苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3aj):黄色固体, m.p. 243.5~244 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.92 (s, 1H, CH), 7.83~7.85 (m, 1H, ArH), 7.61~7.63 (m, 1H, ArH), 7.16~7.20 (m, 3H, ArH), 7.11 (d, J=8.5 Hz, 1H, ArH), 6.45 (s, 1H, CH), 4.89 (d, J=16.5 Hz, 1H, CH2), 4.68 (d, J=17.0 Hz, 1H, CH2), 4.17~4.19 (m, 1H, CH2), 3.91~3.95 (m, 2H, CH2), 3.84 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6)δ: 179.3, 162.9 (dd, 1JC–F=246.3 Hz, 3JC–F=13.8 Hz), 156.6, 155.5, 141.4, 136.6, 130.4, 127.4, 123.6, 123.2, 118.6, 112.4, 111.0 (d, 2JC–F=25.0 Hz), 110.3, 103.3 (t, 2JC–F=26.3 Hz), 85.9, 54.8, 51.2, 45.6; IR (KBr) ν: 3076, 1665, 1619, 1551, 1452, 1272, 1222, 616 cm-1; HRMS (TOF ES+) calcd for C21H16F2-N3O4[M+H]+ 412.1103, found 412.1103.
3-((6-氯吡啶-3-基)甲基)-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ak):黄色固体, m.p. 230~231 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.46 (d, J=2.0 Hz, 1H, CH), 7.90~7.92 (m, 2H, ArH), 7.83~7.84 (m, 1H, ArH), 7.61~7.64 (m, 1H, ArH), 7.55 (d, J=8.5 Hz, 1H, ArH), 7.17~7.20 (m, 1H, ArH), 7.10 (d, J=8.0 Hz, 1H, ArH), 6.44 (s, 1H, CH), 4.90 (d, J=16.5 Hz, 1H, CH2), 4.72 (d, J=16.5 Hz, 1H, CH2), 4.15~4.20 (m, 1H, CH2), 3.92~3.96 (m, 2H, CH2), 3.79~3.85 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 156.6, 155.5, 149.9, 149.5, 139.5, 136.5, 131.8, 130.3, 127.4, 124.5, 123.5, 123.2, 118.6, 112.4, 110.3, 85.9, 52.6, 51.1, 45.6; IR (KBr) ν: 1659, 1614, 1549, 1460, 1328, 1293, 1278, 1219, 622 cm-1; HRMS (TOF ES+) calcd for C20H16ClN4O4[M+H]+ 411.0855, found 411.0854.
3-((2-氯噻唑-5-基)甲基)-4-硝基-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3al):黄色固体, m.p. 258~259 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.92 (s, 1H, CH), 7.82~7.84 (m, 1H, ArH), 7.78 (s, 1H, ArH), 7.61~7.64 (m, 1H, ArH), 7.17~7.20 (s, 1H, ArH), 7.10 (d, J=8.0 Hz, 1H, ArH), 6.43 (s, 1H, CH), 4.99 (d, J=16.0 Hz, 1H, CH2), 4.83 (d, J=16.0 Hz, 1H, CH2), 4.15~4.16 (m, 1H, CH2), 3.88~3.96 (m, 2H, CH2), 3.77~3.79 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 179.3, 156.5, 154.8, 152.3, 142.2, 136.7, 135.7, 130.0, 127.4, 123.4, 123.3, 118.6, 112.9, 110.3, 85.7, 50.4, 48.6, 45.4; IR (KBr) ν: 1613, 1563, 1294, 1267, 1215, 1052, 619 cm-1; HRMS (TOF ES+) calcd for C18H14ClN4O4S[M+H]+ 417.0419, found 417.0417.
8-氟-4-硝基-3-(4-(三氟甲基)苄基)-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bb):黄色固体, m.p. 233~234 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.95 (s, 1H, CH), 7.56 (d, J=8.0 Hz, 2H, ArH), 7.63 (d, J=8.0 Hz, 2H, ArH), 7.49~7.53 (m, 2H, ArH), 7.16~7.18 (m, 1H, ArH), 6.45 (s, 1H, CH), 4.97 (d, J=16.5 Hz, 1H, CH2), 4.81 (d, J=16.5 Hz, 1H, CH2), 4.15~4.20 (m, 1H, CH2), 3.92~3.95 (m, 2H, CH2), 3.81~3.87 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 157.8 (d, 1JC–F=238.8 Hz), 155.4, 152.8, 141.3, 131.1, 128.5 (d, 2JC–F=31.3 Hz), 128.5, 125.7 (d, 3JC–F=7.5 Hz), 124.4 (d, 3JC–F=7.5 Hz), 123.6, 123.7 (d, 2JC–F=23.8 Hz), 120.8 (d, 3JC–F=7.5 Hz), 112.3 (d, 2JC–F=23.8 Hz), 111.5, 110.5, 86.0, 55.0, 51.2, 45.6; IR (KBr) ν: 2891, 1656, 1601, 1555, 1479, 1324, 1275, 1258, 1126, 1065, 631 cm-1; HRMS (TOF ES+) calcd for C22H16F4N3O4[M+H]+ 462.1071, found 462.1069.
4-((8-氟-4-硝基-6-氧代-1, 2-二氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-3(11aH)-基)甲基)苯甲腈(3bc):黄色固体, m.p. 258~259 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.93 (s, 1H, CH), 7.87 (d, J=8.5 Hz, 2H, ArH), 7.60 (d, J=8.0 Hz, 2H, ArH), 7.49~7.53 (m, 2H, ArH), 7.16~7.19 (m, 1H, ArH), 6.45 (s, 1H, CH), 4.96 (d, J=17.0 Hz, 1H, CH2), 4.79 (d, J=17.0 Hz, 1H, CH2), 4.15~4.20 (m, 1H, CH2), 3.91~3.95 (m, 2H, CH2), 3.83~3.87 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 157.8 (d, 1JC–F=238.8 Hz), 155.5, 152.8, 142.4, 132.7, 131.0, 128.6, 124.4 (d, 3JC–F=7.5 Hz), 123.7 (d, 2JC–F=25.0 Hz), 120.8 (d, 3JC–F=8.8Hz), 119.3, 112.3 (d, 2JC–F=22.5 Hz), 111.5, 110.6, 110.5, 86.0, 55.2, 51.3, 45.7; IR (KBr) ν: 2225, 1656, 1603, 1554, 1479, 1317, 1280, 1258, 1218, 621 cm-1; HRMS (TOF ES+) calcd for C22H16FN4O4[M+H]+ 419.1150, found 419.1151.
8-氟-3-(4-氟苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bd):黄色固体, m.p. 204~205 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.94 (s, 1H, CH), 7.43~7.52 (m, 4H, ArH), 7.20~7.24 (m, 2H, ArH), 7.14~7.17 (m, 1H, ArH), 6.42 (s, 1H, CH), 4.85 (d, J=15.5 Hz, 1H, CH2), 4.72 (d, J=16.0 Hz, 1H, CH2), 4.12~4.16 (m, 1H, CH2), 3.87~3.91 (m, 2H, CH2), 3.76~3.82 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 162.1 (d, 1JC–F=242.5 Hz), 157.8 (d, 1JC–F=238.8 Hz), 155.0, 152.8, 132.2 (d, 4JC–F=2.5 Hz), 131.2, 130.2 (d, 3JC–F=7.5 Hz), 124.4 (d, 3JC–F=6.3 Hz), 123.7 (d, 2JC–F=23.8 Hz), 120.8 (d, 3JC–F=7.5 Hz), 115.8 (d, 2JC–F=21.3 Hz), 112.3 (d, 2JC–F=23.8 Hz), 111.4, 110.6, 86.1, 54.3, 50.9, 45.5; IR (KBr) ν: 3075, 1667, 1618, 1575, 1476, 1306, 1260, 1187, 952, 618 cm-1; HRMS (TOF ES+) calcd for C21H16F2N3O4[M+H]+ 412.1103, found 412.1102.
3-(4-氯苄基)-8-氟-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3be):黄色固体, m.p. 236~237 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.93 (s, 1H, CH), 7.48~7.53 (m, 2H, ArH), 7.41~7.46 (m, 4H, ArH), 7.15~7.17 (m, 1H, ArH), 6.42 (s, 1H, CH), 4.86 (d, J=16.0 Hz, 1H, CH2), 4.71 (d, J=16.0 Hz, 1H, CH2), 4.12~4.16 (m, 1H, CH2), 3.88~3.92 (m, 2H, CH2), 3.79~3.83 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 157.8 (d, 1JC–F=240.0 Hz), 155.1, 152.8, 135.2, 132.7, 131.1, 129.9, 128.9, 124.4 (d, 3JC–F=6.3 Hz), 123.7 (d, 2JC–F=23.8 Hz), 120.8 (d, 3JC–F=7.5 Hz), 112.3 (d, 2JC–F=23.8 Hz), 111.4, 110.6, 86.0, 54.4, 51.0, 45.6; IR (KBr) ν: 3081, 1655, 1603, 1557, 1479, 1280, 1260, 1216, 619 cm-1; HRMS (TOF ES+) calcd for C21H16ClFN3O4[M+H]+ 428.0808, found 428.0809.
3-苄基-8-氟-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bf):黄色固体, m.p. 231~232 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.94 (s, 1H, CH), 7.48~7.52 (m, 2H, ArH), 7.37~7.40 (m, 3H, ArH), 7.33~7.34 (m, 1H, ArH), 7.14~7.17 (m, 1H, ArH), 6.42 (s, 1H, CH), 4.88 (d, J=16.0 Hz, 1H, CH2), 4.75 (d, J=15.5 Hz, 1H, CH2), 4.13~4.15 (m, 1H, CH2), 3.89~3.92 (m, 2H, CH2), 3.79~3.83 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6)δ: 178.5, 157.8 (d, 1JC–F=238.8 Hz), 155.0, 152.8, 136.0, 131.2, 129.0, 128.1 (d, 3JC–F=8.8 Hz), 124.4 (d, 3JC–F=7.5 Hz), 123.7 (d, 2JC–F=25.0 Hz), 120.8 (d, 3JC–F=7.5 Hz), 112.3 (d, 2JC–F=22.5 Hz), 111.3, 110.7, 86.1, 54.9, 51.0, 45.5; IR (KBr) ν: 3068, 1665, 1609, 1548, 1481, 1287, 1254, 1200, 1024, 620 cm-1; HRMS (TOF ES+) calcd for C21H17FN3O4[M+H]+ 394.1198, found 394.1199.
8-氟-3-(4-甲基苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bg):黄色固体, m.p. 212~213 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.93 (s, 1H, CH), 7.48~7.52 (m, 2H, ArH), 7.26 (d, J=7.5 Hz, 2H, ArH), 7.20 (d, J=8.0 Hz, 2H, ArH), 7.14~7.17 (m, 1H, ArH), 6.41 (s, 1H, CH), 4.82 (d, J=15.5 Hz, 1H, CH2), 4.71 (d, J=15.5 Hz, 1H, CH2), 4.12~4.14 (m, 1H, CH2), 3.87~3.91 (m, 2H, CH2), 3.77~3.79 (m, 1H, CH2), 2.31 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6)δ: 178.5, 157.8 (d, 1JC–F=238.8 Hz), 154.8, 152.8, 137.4, 132.9, 131.2, 129.6, 128.2, 124.4 (d, 3JC–F=6.3Hz), 123.7 (d, 2JC–F=23.8 Hz), 120.8 (d, 3JC–F=7.5 Hz), 112.3 (d, 2JC–F=23.8 Hz), 111.3, 110.7, 86.1, 54.5, 50.8, 45.5, 21.2; IR (KBr) ν: 2924, 1666, 1609, 1575, 1311, 1259, 1187, 619 cm-1; HRMS (TOF ES+) calcd for C22H19FN3O4[M+H]+ 408.1354, found 408.1354.
8-氟-3-(4-甲氧基苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bh):黄色固体, m.p. 219~220 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.93 (s, 1H, CH), 7.47~7.52 (m, 2H, ArH), 7.30 (d, J=8.5 Hz, 2H, ArH), 7.14~7.17 (m, 1H, ArH), 6.95 (d, J=8.5 Hz, 2H, ArH), 6.41 (s, 1H, CH), 4.79 (d, J=15.5 Hz, 1H, CH2), 4.69 (d, J=15.5 Hz, 1H, CH2), 4.09~4.14 (m, 1H, CH2), 3.85~3.89 (m, 2H, CH2), 3.73~3.79 (m, 1H, CH2), 3.76 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6)δ: 178.5, 159.4, 157.8 (d, 1JC–F=238.8 Hz), 154.7, 152.8, 131.2, 129.7, 127.6, 124.4 (d, 3JC–F=7.5 Hz), 123.7 (d, 2JC–F=25.0 Hz), 120.8 (d, 3JC–F=7.5 Hz), 114.5, 112.3 (d, 2JC–F=23.8 Hz), 111.3, 110.7, 86.1, 55.6, 54.1, 50.7, 45.4; IR (KBr) ν: 3073, 1662, 1568, 1510, 1443, 1267, 1212, 617 cm-1; HRMS (TOF ES+) calcd for C22H19FN3O5 [M+H]+ 424.1303, found 424.1305.
8-氟-3-(3-氟苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bi):黄色固体, m.p. 237~238 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.94 (s, 1H, CH), 7.48~7.52 (m, 2H, ArH), 7.41~7.46 (m, 1H, ArH), 7.26~7.29 (m, 1H, ArH), 7.22 (d, J=8.0 Hz, 1H, ArH), 7.14~7.18 (m, 2H, ArH), 6.43 (s, 1H, CH), 4.89 (d, J=16.5 Hz, 1H, CH2), 4.72 (d, J=16.5 Hz, 1H, CH2), 4.13~4.17 (m, 1H, CH2), 3.90~3.94 (m, 2H, CH2), 3.81~3.85 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 162.8 (d, 1JC–F=241.3 Hz), 157.8 (d, 1JC–F=238.8 Hz), 155.2, 152.9, 139.2 (d, 3JC–F=7.5 Hz), 131.1, 130.9 (d, 3JC–F=8.8 Hz), 124.4 (d, 3JC–F=6.3 Hz), 123.9, 123.7 (d, 2JC–F=23.8 Hz), 120.8 (d, 3JC–F=7.5 Hz), 114.9, 114.7, 112.3 (d, 2JC–F=22.5 Hz), 111.4, 110.6, 86.1, 54.7, 51.1, 45.6; IR (KBr) ν: 3076, 1655, 1608, 1553, 1484, 1284, 1251, 1215, 949, 620 cm-1; HRMS (TOF ES+) calcd for C21H16F2N3O4[M+H]+ 412.1103, found 412.1101.
3-(3, 5-二氟苄基)-8-氟-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bj):黄色固体, m.p. 256~257 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.94 (s, 1H, CH), 7.49~7.53 (m, 2H, ArH), 7.16~7.17 (m, 4H, ArH), 6.44 (s, 1H, CH), 4.88 (d, J=17.0 Hz, 1H, CH2), 4.68 (d, J=16.5 Hz, 1H, CH2), 4.16~4.18 (m, 1H, CH2), 3.91~3.95 (m, 2H, CH2), 3.82~3.86 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 162.9 (dd, 1JC–F=244.4 Hz, 3JC–F=13.1 Hz), 162.9 (d, 1JC–F=245.0 Hz), 157.8 (d, 1JC–F=238.8 Hz), 155.5, 152.9, 141.3, 131.1, 124.4, 123.7 (d, 2JC–F=25.0 Hz), 120.8 (d, 3JC–F=7.5 Hz), 112.3 (d, 2JC–F=22.5 Hz), 111.5, 111.0 (d, 2JC–F=25.0 Hz), 110.5, 103.3 (m), 86.1, 54.7, 51.2, 45.7; 19F NMR (470 MHz, DMSO-d6) δ:-109.9, -119.8; IR (KBr) ν: 3080, 1657, 1598, 1554, 1482, 1299, 1252, 1217, 1119, 612 cm-1; HRMS (TOF ES+) calcd for C21H15F3N3O4[M+ H]+ 430.1009, found 430.1008.
3-((6-氯吡啶-3-基)甲基)-8-氟-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3bk):黄色固体, m.p. 218~219 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.46 (s, 1H, CH), 7.90~7.93 (m, 2H, ArH), 7.49~7.56 (m, 3H, ArH), 7.15~7.18 (m, 1H, ArH), 6.43 (s, 1H, CH), 4.90 (d, J=16.0 Hz, 1H, CH2), 4.73 (d, J=16.5 Hz, 1H, CH2), 4.14~4.17 (m, 1H, CH2), 3.92~3.96 (m, 2H, CH2), 3.81~3.85 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 157.8 (d, 1JC–F=240.0 Hz), 155.4, 152.9, 149.9, 149.4, 139.4, 131.7, 131.0, 124.5, 124.4 (d, 3JC–F=6.3 Hz), 123.7 (d, 2JC–F=23.8 Hz), 120.8 (d, 3JC–F=7.5 Hz), 112.3 (d, 2JC–F=23.8 Hz), 111.6, 110.5, 86.0, 52.6, 51.1, 45.7; IR (KBr) ν: 3074, 1661, 1575, 1560, 1481, 1315, 1257, 1211, 616 cm-1; HRMS (TOF ES+) calcd for C20H15ClFN4O4[M+H]+ 429.0760, found 429.0762.
3-((2-氯噻唑-5-基)甲基)-8-氟-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2a]吡啶-6-酮(3bl):黄色固体, m.p. 230~231 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.94 (s, 1H, CH), 7.79 (s, 1H, ArH), 7.49~7.52 (m, 2H, ArH), 7.15~7.17 (m, 1H, ArH), 6.42 (s, 1H, CH), 4.99 (d, J=15.5 Hz, 1H, CH2), 4.84 (d, J=16.0 Hz, 1H, CH2), 4.14~4.15 (m, 1H, CH2), 3.89~3.96 (m, 2H, CH2), 3.78~3.80 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 157.8 (d, 1JC–F=238.8 Hz), 154.7, 152.8, 152.3, 142.3, 135.6, 130.7, 124.3 (d, 3JC–F=7.5 Hz), 123.8 (d, 2JC–F=25.0 Hz), 120.8 (d, 3JC–F=7.5 Hz), 112.3 (d, 2JC–F=23.8 Hz), 112.1, 110.5, 85.8, 50.4, 48.6, 45.5; IR (KBr) ν: 3067, 1663, 1568, 1482, 1258, 1213, 1049, 615 cm-1; HRMS (TOF ES+) calcd for C18H13ClFN4O4S[M+H]+ 435.0325, found 435.0327.
8-氯-4-硝基-3-(4-(三氟甲基)苄基)-1, 2, 3, 11a-四氢-6H-苯并吡喃并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3cb):黄色固体, m.p. 243~244 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.94 (s, 1H, CH), 7.75~7.77 (m, 3H, ArH), 7.62~7.65 (m, 3H, ArH), 7.16 (d, J=8.5 Hz, 1H, ArH), 6.48 (s, 1H, CH), 4.97 (d, J=16.5 Hz, 1H, CH2), 4.81 (d, J=16.5 Hz, 1H, CH2), 4.17~4.18 (m, 1H, CH2), 3.92~3.95 (m, 2H, CH2), 3.83~3.87 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.5, 155.3, 155.2, 141.3, 136.0, 131.3, 128.5 (d, 3JC–F=31.3 Hz), 128.5, 127.3, 125.8 (m), 123.7~125.8 (m), 124.7, 121.0, 111.2, 110.6, 86.2, 55.0, 51.2, 45.7; IR (KBr) ν: 1655, 1614, 1557, 1478, 1327, 1264, 1121, 1066, 615 cm-1; HRMS (TOF ES+) calcd for C22H16ClF3N3O4[M+H]+ 478.0776, found 478.0776.
8-氯-3-(4-氟苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3cd):黄色固体, m.p. 242~243 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.93 (s, 1H, CH), 7.75 (d, J=2.5 Hz, 1H, ArH), 7.64~7.67 (m, 1H, ArH), 7.43~7.46 (m, 2H, ArH), 7.20~7.24 (m, 2H, ArH), 7.14 (d, J=9.0 Hz, 1H, ArH), 6.45 (s, 1H, CH), 4.85 (d, J=15.0 Hz, 1H, CH2), 4.72 (d, J=15.0 Hz, 1H, CH2), 4.13~4.15 (m, 1H, CH2), 3.87~3.91 (m, 2H, CH2), 3.81 (m, 1H, CH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.2, 162.1 (d, 1JC–F=242.5 Hz), 155.2, 154.9, 135.7, 132.1 (d, 4JC–F=2.5 Hz), 131.3, 130.2 (d, 3JC–F=7.5 Hz), 127.2, 126.3, 124.7, 121.0, 115.8 (d, 2JC–F=21.3 Hz), 111.1, 110.7, 86.2, 54.3, 50.9, 45.6; IR (KBr) ν: 3072, 1655, 1615, 1562, 1479, 1362, 1270, 1213, 619 cm-1; HRMS (TOF ES+) calcd for C21H16ClFN3O4[M+H]+ 428.0808, found 428.0810.
8-氯-3-(4-甲基苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3cg):黄色固体, m.p. 233~234 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.93 (s, 1H, CH), 7.75 (d, J=2.5 Hz, 1H, ArH), 7.64~7.67 (m, 1H, ArH), 7.14~7.27 (m, 5H, ArH), 6.45 (s, 1H, CH), 4.73~4.80 (m, 2H, CH2), 4.10~4.14 (m, 1H, CH2), 3.88~3.91 (m, 2H, CH2), 3.78~3.82 (m, 1H, CH2), 2.31 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6)δ: 178.1, 155.2, 154.8, 137.4, 136.0, 132.9, 129.6, 128.2, 127.2, 126.3, 124.7, 121.0, 111.1, 86.3, 54.5, 50.9, 45.5, 21.2; IR (KBr) ν: 3071, 1654, 1614, 1562, 1480, 1376, 1270, 1215, 619 cm-1; HRMS (TOF ES+) calcd for C22H19ClN3O4[M+H]+ 424.1059, found 424.1057.
8-氯-3-(4-甲氧基苄基)-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3ch):黄色固体, m.p. 218~219 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 7.93 (s, 1H, CH), 7.74 (d, J=3.0 Hz, 1H, ArH), 7.63~7.66 (m, 1H, ArH), 7.31 (d, J=8.5 Hz, 2H, ArH), 7.14 (d, J=8.5 Hz, 1H, ArH), 6.95 (d, J=8.5 Hz, 2H, ArH), 6.44 (s, 1H, CH), 4.78 (d, J=14.0 Hz, 1H, CH2), 4.69 (d, J=14.5 Hz, 1H, CH2), 4.11~4.12 (m, 1H, CH2), 3.86~3.89 (m, 2H, CH2), 3.74~3.78 (m, 1H, CH2), 3.76 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6)δ: 178.2, 159.4, 155.2, 154.6, 135.9, 131.4, 129.7, 127.6, 127.2, 126.3, 124.7, 121.0, 114.5, 111.0, 110.7, 86.2, 55.6, 54.1, 50.7, 45.5; IR (KBr) ν: 2932, 1651, 1610, 1557, 1509, 1279, 1245, 1221, 1119, 620 cm-1; HRMS (TOF ES+) calcd for C22H19Cl-N3O5[M+H]+ 440.1008, found 440.1008.
4-((8-甲基-4-硝基-6-氧代-1, 2-二氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-3(11aH)-基)甲基)苄腈(3dc):黄色固体, m.p. 290~291 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 7.90(s, 1H, CH), 7.87 (d, J=7.9 Hz, 1H, ArH), 7.59~7.63 (m, 3H, ArH), 7.45 (d, J=8.2 Hz, 1H, ArH), 7.02 (d, J=8.4 Hz, 1H, ArH), 6.40 (s, 1H, CH), 4.96 (d, J=17.0 Hz, 1H, CH2), 4.78 (d, J=16.9 Hz, 1H, CH2), 4.16~4.20 (m, 1H, CH2), 3.91~3.94 (m, 2H, CH2), 3.81~3.86 (m, 1H, CH2), 2.32 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6)δ: 179.4, 155.5, 154.6, 142.5, 137.3, 132.8, 132.4, 130.2, 128.6, 127.0, 123.2, 119.2, 118.5, 112.6, 110.6, 110.3, 85.7, 55.2, 51.3, 45.6, 20.6; IR (KBr) ν: 2223, 1658, 1622, 1554, 1507, 1485, 1307, 1288, 1218, 623 cm-1; HRMS (TOF ES+) calcd for C23H19N4O4 [M+H]+ 415.1401, found 415.1398.
(4-甲氧基苄基)-8-甲基-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3dh):黄色固体, m.p. 207~208 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 7.91 (s, 1H, CH), 7.62 (s, 1H, CH), 7.43 (d, J=8.3 Hz, 1H, ArH), 7.31 (d, J=7.9 Hz, 2H, ArH), 6.99 (d, J=8.3 Hz, 1H, ArH), 6.95 (d, J=8.22 Hz, 2H, ArH), 6.36 (s, 1H, CH), 4.80 (d, J=15.2 Hz, 1H, CH2), 4.69 (d, J=15.2 Hz, 1H, CH2), 4.09~4.13 (m, 1H, CH2), 3.85~3.88 (m, 2H, CH2), 3.74~3.78 (m, 1H, CH2), 3.76 (s, 3H, CH3), 2.31 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6) δ: 179.3, 159.3, 154.8, 154.6, 137.3, 132.3, 130.4, 129.7, 127.0, 123.2, 118.4, 114.5, 112.4, 110.4, 85.8, 55.6, 54.1, 50.6, 45.4, 20.6; IR (KBr) ν: 1655, 1617, 1554, 1504, 1311, 1275, 1212, 1195, 623 cm-1; HRMS (TOF ES+) calcd for C23H22N3O5[M+H]+ 420.1554, found 420.1557.
3-((6-氯吡啶-3-基)甲基)-8-甲基-4-硝基-1, 2, 3, 11a-四氢-6H-色烯并[3, 2-e]咪唑并[1, 2-a]吡啶-6-酮(3dk):黄色固体, m.p. 248~249 ℃; 1H NMR (600 MHz, DMSO-d6) δ: 8.46 (s, 1H, CH), 7.91 (s, 2H, ArH), 7.63 (s, 1H, ArH), 7.57 (d, J=7.5 Hz, 1H, ArH), 7.45 (d, J=7.0 Hz, 1H, ArH), 7.01 (d, J=7.5 Hz, 1H, ArH), 6.39 (s, 1H, CH), 4.90 (d, J=16.3 Hz, 1H, CH2), 4.72 (d, J=16.2 Hz, 1H, CH2), 4.16~4.17 (m, 1H, CH2), 3.93 (s, 2H, CH2), 3.81~4.83 (m, 1H, CH2), 2.32 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6)δ: 179.4, 155.5, 154.6, 149.8, 149.5, 139.5, 137.3, 132.4, 131.8, 130.2, 127.0, 124.5, 123.2, 118.5, 112.7, 110.3, 85.8, 52.6, 51.1, 45.6, 20.5; IR (KBr) ν: 1661, 1624, 1555, 1506, 1452, 1290, 1216, 623 cm-1; HRMS (TOF ES+) calcd for C21H18ClN4O4[M+H]+ 425.1008, found 425.1008.
辅助材料(Supporting Information) 化合物的1H NMR和13C NMR谱图.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
Hunt, A. J.; Sin, E. H. K.; Marriott, R.; Clark, J. H. CHemSusChem 2010, 3, 306. doi: 10.1002/cssc.200900169
-
[2]
Agana, B. A.; Reeve, D.; Orbell, J. D. J Environ. Manage. 2013, 114, 445. doi: 10.1016/j.jenvman.2012.10.047
-
[3]
Marr, P. C.; Marr, A. C. Green Chem. 2016, 18, 105. doi: 10.1039/C5GC02277K
-
[4]
Marteel-Parrish, A. E. J. Chem. Educ. 2014, 91, 1084. doi: 10.1021/ed400393b
-
[5]
Trost, B. M. Science 1991, 254, 1471. doi: 10.1126/science.1962206
-
[6]
Misono, M. Yuki Gosei Kagaku Kyokaishi 2003, 61, 406. doi: 10.5059/yukigoseikyokaishi.61.406
-
[7]
Hartman, G. J.; Jin, Q. Z.; Collins, G. J.; Lee, K. N.; Ho, C. T.; Chang, S. S. J. Agric. Food Chem. 1983, 31, 1030. doi: 10.1021/jf00119a027
-
[8]
Ren, T.; Liu, W.; Xue, Q.; Wang, H. Lubr. Sci. 1993, 5, 205. doi: 10.1002/(ISSN)1557-6833
-
[9]
Ueda, Y.; Connolly, T. P.; Kadow, J. F.; Meanwell, N. A.; Wang, T.; Chen, C.-P. H.; Yeung, K.-S.; Zhang, Z.; Leahy, D. K.; Pack, S. K.; Soundararajan, N.; Sirard, P.; Levesque, K.; Thoraval, D. US 20050209246, 2005[Chem. Abstr. 2005, 143, 306343].
-
[10]
Garuti, L.; Roberti, M.; Pizzirani, D. Mini-Rev. Med. Chem. 2007, 7, 481. doi: 10.2174/138955707780619626
-
[11]
Schultz, C.; Link, A.; Leost, M.; Zaharevitz, D. W.; Gussio, R.; Sausville, E. A.; Meijer, L.; Kunick, C. J. Med. Chem. 1999, 42, 2909. doi: 10.1021/jm9900570
-
[12]
Pluta, K.; Morak-Mlodawska, B.; Jelen, M. Eur. J. Med. Chem. 2011, 46, 3179. doi: 10.1016/j.ejmech.2011.05.013
-
[13]
Sujatha, K.; Shanmugam, P.; Perumal, P. T.; Muralidharan, D.; Rajendran, M. Bioorg. Med. Chem. Lett. 2006, 16, 4893. doi: 10.1016/j.bmcl.2006.06.059
-
[14]
Helal, C. J.; Kang, Z.; Hou, X.; Pandit, J.; Chappie, T. A.; Humphrey, J. M.; Marr, E. S.; Fennell, K. F.; Chenard, L. K.; Fox, C.; Schmidt, C. J.; Williams, R. D.; Chapin, D. S.; Siuciak, J.; Lebel, L.; Menniti, F.; Cianfrogna, J.; Fonseca, K. R.; Nelson, F. R.; O'Connor, R.; MacDougall, M.; McDowell, L.; Liras, S. J. Med. Chem. 2011, 54, 4536. doi: 10.1021/jm2001508
-
[15]
Kim, I. Y.; Kim, S. H. KR 2016006050, 2016[Chem. Abstr. 2016, 164, 225869].
-
[16]
Lhassani, M.; Chavignon, O.; Chezal, J.-M.; Teulade, J.-C.; Chapat, J.-P.; Snoeck, R.; Andrei, G.; Balzarini, J.; De Clercq, E.; Gueiffier, A. Eur. J. Med. Chem. 1999, 34, 271. doi: 10.1016/S0223-5234(99)80061-0
-
[17]
Alcarazo, M.; Roseblade, S. J.; Cowley, A. R.; Fernandez, R.; Brown, J. M.; Lassaletta, J. M. J. Am. Chem. Soc. 2005, 127, 3290. doi: 10.1021/ja0423769
-
[18]
Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Cardiovasc. Drug Rev. 2002, 20, 163.
-
[19]
Ankley, G. T.; Kahl, M. D.; Jensen, K. M.; Hornung, M. W.; Korte, J. J.; Makynen, E. A.; Leino, R. L. Toxicol. Sci. 2002, 67, 121. doi: 10.1093/toxsci/67.1.121
-
[20]
Veber, D. F.; Johnson, S. R.; Cheng, H.-Y.; Smith, B. R.; Ward, K. W.; Kopple, K. D. J. Med. Chem. 2002, 45, 2615. doi: 10.1021/jm020017n
-
[21]
Bai, D.; Lummis, S. C. R.; Leicht, W.; Breer, H.; Sattelle, D. B. Pestic. Sci. 1991, 33, 197. doi: 10.1002/ps.v33:2
-
[22]
Feng, X.-G.; Liu, X.-W.; Han, Z.-L.; Guan, L.-T.; Xu, L.-Z. J. Qingdao Univ. Sci. Technol., Nat. Sci. Ed. 2012, 33, 381.
-
[23]
Li, J.; Huang, T.; Li, L.; Ding, T.; Zhu, H.; Yang, B.; Ye, Q.; Gan, J. J. Agric. Food Chem. 2016, 64, 8109. doi: 10.1021/acs.jafc.6b03422
-
[24]
Tomizawa, M.; Casida, J. E. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247. doi: 10.1146/annurev.pharmtox.45.120403.095930
-
[25]
Bao, H.; Shao, X.; Zhang, Y.; Deng, Y.; Xu, X.; Liu, Z.; Li, Z. J. Agric. Food Chem. 2016, 64, 5148. doi: 10.1021/acs.jafc.6b01512
-
[26]
Lu, S.; Zhuang, Y.; Wu, N.; Feng, Y.; Cheng, J.; Li, Z.; Chen, J.; Yuan, J.; Xu, X. J. Agric. Food Chem. 2013, 61, 10858. doi: 10.1021/jf403272h
-
[27]
Shao, X.; Xu, Z.; Zhao, X.; Xu, X.; Tao, L.; Li, Z.; Qian, X. J. Agric. Food Chem. 2010, 58, 2690. doi: 10.1021/jf902513t
-
[28]
(a) Huang, Z.; Wang, M. Heterocycles 1994, 37, 1233.
(b) Kong, L.; Yang, R.; Du, X.; Yan, S.; Lin, J. Chin. J. Org. Chem. 2016, 36, 2437 (in Chinese).
(孔令斌, 杨瑞霞, 杜璇璇, 严胜骄, 林军, 有机化学, 2016, 36, 2437.)
(c) Peng, M.; Yang, R.; Liu, X.; Yan, S.; Lin, J. Chin. J. Org. Chem. 2015, 35, 1754 (in Chinese).
(彭美阳, 杨瑞霞, 刘昔敏, 严胜骄, 林军, 有机化学, 2015, 35, 1754.) -
[29]
(a) Chen, X.-B.; Liu, Z.-C.; Lin, X.-R.; Huang, R.; Yan, S.-J.; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 2391.
(b) Luo, D.; Cui, S.; Hu, X.; Yan, S.; Lin, J. Chin. J. Org. Chem. 2017, 37, 166 (in Chinese).
(罗大云, 崔时胜, 胡兴梅, 林军, 严胜骄, 有机化学, 2017, 37, 166.) -
[30]
Yu, F.-C.; Huang, R.; Ni, H.; Fan, J.; Yan, S.-J.; Lin, J. Green Chem. 2013, 15, 453. doi: 10.1039/C2GC36552A
-
[31]
Chen, X.-B.; Liu, Z.-C.; Yang, L.-F.; Yan, S.-J.; Lin, J. ACS Sustainable Chem. Eng. 2014, 2, 1155. doi: 10.1021/sc500170d
-
[32]
Xiao, X.; Wang, X.; Gui, X.; Chen, L.; Huang, B. Chem. Biodiversity 2016, 11, 1427.
-
[33]
Mir, S. A. Int. J. PharmTech Res. 2016, 9, 70.
-
[34]
(a) Ding, Z.-W.; Tan, Q.-T.; Liu, B.-X.; Xu, K.; Xu, B. Acta Chim. Sinica 2015, 73, 1302 (in Chinese).
(丁正伟, 谭启涛, 刘秉新, 张可, 许斌, 化学学报, 2015, 73, 1302.)
(b) Zhao, J.-B.; Zhang, Q. Acta Chim. Sinica 2015, 73, 1235 (in Chinese).
(赵金钵, 张前, 化学学报, 2015, 73, 1235.) -
[35]
Alizadeh, A.; Bayat, F.; Zhu, Z. Res. Chem. Intermed. 2016, 42, 5927. doi: 10.1007/s11164-015-2414-6
-
[36]
Yaqub, M.; Perveen, R.; Shafiq, Z.; Pervez, H.; Tahir, M. N. Synlett 2012, 23, 1755. doi: 10.1055/s-00000083
-
[37]
Nishiwaki, H.; Nakagawa, Y.; Takeda, D. Y.; Okazawa, A.; Akamatsu, M.; Miyagawa, H.; Ueno, T.; Nishimura, K. Pest Manage. Sci. 2000, 56, 875. doi: 10.1002/(ISSN)1526-4998
-
[1]
-
表 1 优化反应条件
Table 1. Optimization of reaction conditionsa

表 2 一锅法合成化合物3a
Table 2. One-pot protocol for the synthesis of compound 3

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 9
- 文章访问数: 2127
- HTML全文浏览量: 293


 下载:
下载:
 下载:
下载:

