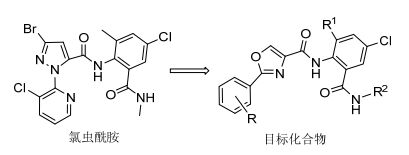
 图 1
目标化合物设计
Figure 1.
Design of target compounds
图 1
目标化合物设计
Figure 1.
Design of target compounds

Citation: Wang Mengmeng, Zhang Qingqing, Yue Kai, Li Qingshan, Xu Fengbo. Synthesis and Insecticidal Activity of o-Carboxamidobenzamide Compounds Containing 2-(Substituted phenyl)oxazole Group[J]. Chinese Journal of Organic Chemistry, 2017, 37(7): 1774-1780. doi: 10.6023/cjoc201612030

含2-(取代苯基)噁唑基的邻甲酰胺基苯甲酰胺类化合物合成及杀虫活性研究
-
关键词:
- 杀虫剂
- / 邻甲酰胺基苯甲酰胺类化合物
- / 苯基噁唑基化合物
English
Synthesis and Insecticidal Activity of o-Carboxamidobenzamide Compounds Containing 2-(Substituted phenyl)oxazole Group
-
Key words:
- insecticide
- / o-carboxamidobenzamide compounds
- / benzoxazole compounds
-
邻甲酰胺基苯甲酰胺类杀虫剂是以昆虫的鱼尼丁受体为靶标的杀虫剂, 其独特的作用靶标, 新颖的结构以及对鳞翅目的高活性, 使这类农药受到广泛关注[1]. 2000年美国杜邦公司在日本农药公司产品的基础上, 以氟虫酰胺为先导, 合成出了邻甲酰胺基苯甲酰胺类化合物-氯虫苯甲酰胺.该化合物特异性地作用于昆虫的鱼尼丁受体, 杀虫活性高, 在较低浓度下仍表现出较高的杀虫活性, 且对哺乳动物具有较高的安全性.
噁唑基团作为一种重要的含氧氮类杂环, 在医药、农药等多领域表现出广泛的应用潜力和开发价值[2].噁唑类化合物可与生物体内多种酶和受体作用而呈现出生物活性[3~5].自先正达成功开发出有机磷类杀虫剂甲基吡噁磷以来, 一些药物公司相继开发出数十个含噁唑环的农药新品种, 如杀菌剂恶霉灵、噁唑菌酮、羟哌噁酮等.作为生物电子等排体, 噁唑可广泛用于代替三唑、咪唑、吡唑、噻唑、酰胺等基团设计开发具有良好杀虫、除草、抗癌和抗菌等生物活性的新型药物[6~10].最新报道的苯基取代异噁唑和苯并噁唑类衍生物均具有较高生物活性[11, 12].
本文以氯虫苯甲酰胺结构为基础, 根据生物电子等排原理以及活性亚结构拼接技术, 将噁唑基引入到邻甲酰胺基苯甲酰胺类杀虫剂结构中, 替代吡唑环设计合成了一系列含2-(取代苯基)噁唑基的邻甲酰胺基苯甲酰胺类化合物(图 1), 合成路线见Scheme 1.
从本实验的研究结果来看, 该类化合物具有很大的结构改造空间, 其潜在的研究价值仍需要我们不断探索.
1 结果与讨论
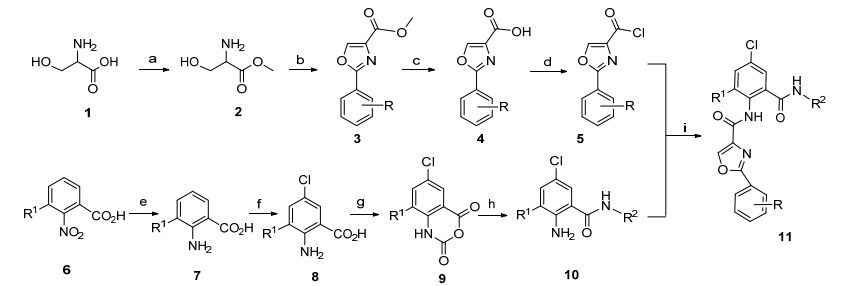
在合成中间体10过程中, 首先尝试的方法是先将取代苯甲酸酰氯化, 再和相应胺进行反应.在尝试使用不同投料比的缚酸剂情况下, 仍然存在过多副反应.于是尝试利用三光气缩合反应合环, 制备出化合物9, 再用甲氧胺或者甲胺开环的方法, 以较高的产率得到了中间体10.在目标化合物的合成过程中, 尝试了不同溶剂, 最终确定乙腈为反应溶剂, 乙腈作为溶剂对原料的溶解性很好, 对产物的溶解性较差.反应结束后, 产物以不溶物的形式存在于反应体系中, 直接过滤便能得到粗产物, 操作简单.
根据文献合成了4-噁唑甲酰氯化合物5[18]和邻氨基苯甲酰胺化合物10[16, 17], 通过二者在乙腈中反应, 方便地制备出一系列邻甲酰胺基苯甲酰胺类化合物11.对合成的化合物11a~11z和11a'~11c'通过1H NMR、13C NMR和HRMS做了详细表征.
我们对所合成出的29个目标化合物进行了杀虫活性的普筛测试.从表 1可以看出, 在样品浓度为50 mg/L时, 大部分目标化合物对东方粘虫表现中等的生物活性(10%~70%), 值得一提的是化合物11p在浓度50 mg/L时生物活性达到100%.总体上这些化合物与对照样氯虫苯甲酰胺相比, 尚有较大差距.杀虫活性受与噁唑基相连的苯环上取代基的影响较大, 其中苯环上含有卤素取代基团的结构活性最强, 整体表现为:溴代>氯代>氟代.当R1=CH3, R2=OCH3时, 溴取代基的位置对杀虫活性影响较大, 表现为:邻位>间位>对位; 但是当R1=CH3, R2=CH3时, 溴取代基位置对活性影响却刚好相反, 表现为:对位>间位>邻位, 但活性差距并不大. R1, R2的不同对化合物活性的影响并不明显.此系列化合物中邻位溴代结构化合物活性最高, 表现出了与氯虫苯甲酰胺相当的活性, 杀虫率达到了100%.实验结果为我们接下来的工作提供了方向.
编号 R R1 R2 杀虫活性/% (50 mg•L-1) 11a H CH3 CH3 20 11b α-Cl CH3 CH3 60 11c β-Cl CH3 CH3 25 11d γ-Cl CH3 CH3 70 11e α-Br CH3 CH3 25 11f β-Br CH3 CH3 30 11g γ-Br CH3 CH3 60 11h γ-F CH3 CH3 45 11i α-OCH3 CH3 CH3 12 11j β-OCH3 CH3 CH3 5 11k γ-OCH3 CH3 CH3 10 11l H CH3 OCH3 10 11m α-Cl CH3 OCH3 15 11n β-Cl CH3 OCH3 45 11o γ-Cl CH3 OCH3 30 11p α-Br CH3 OCH3 100 11q β-Br CH3 OCH3 40 11r γ-Br CH3 OCH3 10 11s α-F CH3 OCH3 40 11t β-F CH3 OCH3 10 11u γ-F CH3 OCH3 65 11v β-OCH3 CH3 OCH3 45 11w γ-OCH3 CH3 OCH3 10 11x α-Cl Cl OCH3 25 11y β-Cl Cl OCH3 50 11z α-F Cl OCH3 20 11a' β-F Cl OCH3 50 11b' γ-F Cl OCH3 15 11c' H Cl OCH3 30 100 2 结论
本实验采用简单易得的原料, 经过9步反应合成出含2-(取代苯基)噁唑基的邻甲酰胺基苯甲酰胺类杀虫活性化合物, 产率在30%~50%之间.通过对目标化合物进行活性筛选, 得到了与氯虫苯甲酰胺活性相当的化合物2-(2-溴苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11p), 在50 mg/L浓度时杀东方粘虫活性达到100%, 取得了较好效果.针对该类结构的化合物的结构修饰和杀虫活性测试有进一步研究的价值.
3 实验部分
3.1 仪器与试剂
Bruker AV 400型核磁共振仪(瑞士Bruker公司), 以DMSO-d6或CDCl3为溶剂, TMS为内标; Agilent 6520Q-TOF LC/MS高分辨质谱仪(美国Agilent公司); X-4型数字显示显微熔点仪(北京泰克仪器有限公司), 仪器未校正.
薄层层析用硅胶、柱层析硅胶(青岛海洋化工公司), L-丝氨酸(1)、3-氯-2-硝基苯甲酸(6a)、3-甲基-2-硝基苯甲酸(6b)(天津希恩思生化科技有限公司), 其余试剂都为市售分析纯或化学纯.
3.2 实验方法
化合物2[13]、化合物3[14, 15]、化合物4, 5和7[16]、化合物8[17]、化合物9[15, 17, 18]、化合物10[15, 17]参照文献方法合成, 其物理性质和波谱数据与文献数据一致.
3.2.1 邻-(4-噁唑甲酰胺基)苯甲酰胺类化合物11a~11z及11a'~11c'的制备
在50 mL单口烧瓶中加入1.50 mmol中间体10、20 mL乙腈、0.30 mL (2.25 mol)三乙胺, 室温搅拌至溶解, 再将中间体1.50 mmol 5的20 mL乙腈溶液缓慢滴入上述溶液中, 经5 h后反应结束, 溶液中有沉淀生成.抽滤, 滤饼洗涤干燥, 得到目标化合物.
2-苯基-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11a):产率30.5%. m.p. 198~200 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.27 (s, 1H, Ph-NH-CO), 8.88 (s, 1H, oxazole-H), 8.52 (d, J=4.5 Hz, 1H, CO-NH-CH3), 8.07 (dd, J=5.8, 2.9 Hz, 2H, Ph-H), 7.63~7.61 (t, J=5.8, 3H, Ph-H), 7.54 (d, J=1.8 Hz, 1H, Ph-H), 7.46 (d, J=1.8 Hz, 1H, Ph-H), 2.70 (d, J=4.5 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 167.1, 161.2, 158.6, 143.5, 138.7, 137.2, 134.1, 133.1, 132.1, 131.8, 130.6, 129.7, 126.8, 126.5, 125.8, 26.6, 18.9; HRMS calcd for C19H16ClN3O3K ([M+K]+) 408.0517, found 408.0518.
2-(2-氯苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11b):产率29.3%. m.p. 208~210 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.29 (s, 1H, Ph-NH-CO), 8.98 (s, 1H, oxazole-H), 8.52 (d, J=4.4 Hz, 1H, CO-NH-CH3), 8.05 (d, J=7.5 Hz, 1H, Ph-H), 7.72 (d, J=7.5 Hz, 1H, Ph-H), 7.64 (t, J=7.4 Hz, 1H, Ph-H), 7.58 (t, J=7.5 Hz, 1H, Ph-H), 7.54 (s, 1H, Ph-H), 7.46 (s, 1H, Ph-H), 2.71 (d, J=4.4 Hz, 3H, NH-CH3), 2.25 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 167.1, 161.2, 158.6, 143.5, 138.7, 137.2, 134.1, 133.1, 132.1, 131.8, 130.6, 129.7, 125.8, 26.6, 18.9; HRMS calcd for C19H15Cl2N3O3Na ([M+Na]+) 426.0388, found 426.0386.
2-(3-氯苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11c):产率32.1%. m.p. 235~237 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.26 (s, 1H, Ph-NH-CO), 8.92 (s, 1H, oxazole-H), 8.50 (d, J=4.6 Hz, 1H, CO-NH-CH3), 8.06 (s, 1H, Ph-H), 8.02 (d, J=7.5 Hz, 1H, ), 7.72~7.68 (m, 1H, Ph-H), 7.66 (d, J=7.6 Hz, 1H, Ph-H), 7.54 (d, J=2.1 Hz, 1H, Ph-H), 7.46 (d, J=2.1 Hz, 1H, Ph-H), 2.70 (d, J=4.6 Hz, 3H, NH-CH3), 2.23 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 167.1, 159.8, 158.5, 143.9, 138.7, 137.39, 134.3, 133.1, 132.1, 132.10~131.91 (m), 131.7, 130.7, 128.4, 126.3, 125.8, 125.4, 26.6, 18.9; HRMS calcd for C19H15Cl2N3O3Na ([M+Na]+) 426.0388, found 426.0385.
2-(4-氯苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11d):产率33.1%. m.p. 233~235 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.27 (s, 1H, Ph-NH-CO), 8.90 (s, 1H, oxazole-H), 8.51 (d, J=4.7 Hz, 1H, CO-NH-CH3), 8.08 (d, J=8.5 Hz, 2H, Ph-H), 7.70 (d, J=8.5 Hz, 2H, Ph-H), 7.55 (d, J=2.0 Hz, 1H, Ph-H), 7.47 (d, J=2.0 Hz, 1H, Ph-H), 2.71 (d, J=4.7 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 167.1, 162.4, 161.2, 160.4, 158.5, 148.5, 146.2, 143.9, 138.7, 137.3, 136.6, 134.0, 133.1, 132.2, 130.6, 130.0, 128.6, 125.9, 125.4, 26.6, 19.0; HRMS calcd for C19H15Cl2N3O3Na ([M+Na]+) 426.0388, found 426.0388.
2-(2-溴苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11e):产率34.2%. m.p. 231~233 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.28 (s, 1H, Ph-NH-CO), 8.98 (s, 1H, oxazole-H), 8.53 (d, J=4.5 Hz, 1H, CO-NH-CH3), 7.98 (d, J=7.7 Hz, 1H, Ph-H), 7.88 (d, J=8.0 Hz, 1H, Ph-H), 7.62 (t, J=7.6 Hz, 1H, Ph-H), 7.57~7.53 (m, 2H, Ph-H, Ph-H), 7.46 (s, 1H, Ph-H), 2.71 (d, J=4.6 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 159.8, 158.4, 144.1, 138.6, 136.9, 134.7, 134.1, 133.2, 133.1, 132.3, 132.1, 130.6, 128.6, 125.8, 26.6, 19.0; HRMS calcd for C19H15ClBrN3-O3Na ([M+Na]+) 469.9883, found 469.9883.
2-(3-溴苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11f):产率29.8%. m.p. 217~219 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.27 (s, 1H, Ph-NH-CO), 8.91 (s, 1H, oxazole-H), 8.51 (d, J=4.3 Hz, 1H, CO-NH-CH3), 8.20 (s, 1H, Ph-H), 8.06 (d, J=7.7 Hz, 1H, Ph-H), 7.82 (d, J=8.1 Hz, 1H, Ph-H), 7.61~7.56 (m, 1H, Ph-H), 7.54 (s, 1H, Ph-H), 7.46 (s, 1H, Ph-H), 2.71 (d, J=4.3 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 167.1, 162.5, 159.7, 158.5, 144.0, 138.7, 137.3, 134.5, 134.3, 133.1, 132.1, 132.1, 130.7, 129.2, 125.8, 125.8, 122.82, 26.6, 18.9; HRMS calcd for C19H15ClBrN3O3Na ([M+Na]+) 469.9883, found 469.9883.
2-(4-溴苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11g):产率29.7%. m.p. 227~229 ℃; 1H NMR (400 MHz, DMSO-d6)δ: 10.31 (s, 1H, Ph-NH-CO), 8.91 (s, 1H, oxazole-H), 8.56 (d, J=4.0 Hz, 1H, -CO-NH-CH3), 7.99 (d, J=8.3 Hz, 2H, Ph-H), 7.82 (d, J=8.3 Hz, 2H, Ph-H), 7.53 (s, 1H, Ph-H), 7.47 (s, 1H, Ph-H), 2.71 (d, J=4.0 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 167.1, 160.4, 158.5, 146.1, 143.8, 138.7, 137.3, 134.1, 132.8, 132.1, 130.6, 128.7, 125.8, 125.7, 125.4, 26.6, 18.9; HRMS calcd for C19H15ClBrN3O3Na ([M+Na]+) 469.9883, found 469.9883.
2-(4-氟苯基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11h):产率30.5%. m.p. 250~252 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.49 (s, 1H), 10.29 (s, 1H, Ph-NH-CO), 8.89 (s, 1H, oxazole-H), 8.56 (d, J=4.4 Hz, 1H, CO-NH-CH3), 8.11 (dd, J=8.6, 5.4 Hz, 2H, Ph-H), 7.53 (d, J=1.8 Hz, 1H, Ph-H), 7.47 (d, J=3.0 Hz, 2H, Ph-H), 2.70 (d, J=4.5 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 167.1, 163.1, 160.5, 158.6, 143.6, 138.7, 137.2, 134.1, 133.1, 132.2, 130.6, 129.5, 129.4, 125.9, 123.3, 117.1, 116.9, 26.6, 18.9; HRMS calcd for C19H15ClFN3O3Na ([M+Na]+) 410.0684, found 410.0684.
2-(2-甲氧基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11i):产率34.5%. m.p. 217~219 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.25 (s, 1H, Ph-NH-CO), 8.86 (s, 1H, oxazole-H), 8.53 (d, J=4.3 Hz, 1H, Ph-CO-NH), 7.92 (d, J=7.6 Hz, 1H, Ph-H), 7.59 (t, J=7.9 Hz, 1H, Ph-H), 7.53 (s, 1H, Ph-H), 7.46 (s, 1H, Ph-H), 7.26 (d, J=8.4 Hz, 1H, Ph-H), 7.14 (t, J=7.5 Hz, 1H, Ph-H), 3.92 (s, 3H, O-CH3), 2.71 (d, J=4.3 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 166.6, 159.9, 158.2, 157.4, 142.8, 138.19, 136.2, 133.5, 132.9, 132.6, 131.6, 130.4, 130.1, 125.3, 120.6, 115.1, 112.6, 55.8, 26.1, 18.5; HRMS calcd for C20H18ClN3O4Na ([M+Na]+) 422.0884, found 422.0885.
2-(3-甲氧基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11j):产率35.6%. m.p. 212~214 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.25 (s, 1H, Ph-NH-CO), 8.88 (s, 1H, oxazole-H), 8.52 (d, J=4.7 Hz, 1H, CO-NH-CH3), 7.66 (d, J=6.9 Hz, 1H, Ph-H), 7.56 (s, 1H, Ph-H), 7.54 (d, J=7.0 Hz, 2H, Ph-H), 7.46 (d, J=2.0 Hz, 1H, Ph-H), 7.19 (d, J=2.0 Hz, 1H, Ph-H), 3.87 (s, 3H, O-CH3), 2.70 (d, J=4.7 Hz, 3H, NH-CH3), 2.24 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 167.1, 161.1, 160.1, 158.6, 143.6, 138.7, 137.2, 134.2, 133.1, 132.1, 131.1, 130.6, 127.7, 125.8, 119.1, 117.8, 111.71, 55.8, 26.6, 18.9; HRMS calcd for C20H18ClN3O4Na ([M+Na]+) 422.0884, found 422.0883.
2-(4-甲氧基)-N-(4-氯-2-甲基氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11k):产率36.4%. m.p. 200~202 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.22 (s, 1H, Ph-NH-CO), 8.79 (s, 1H, oxazole-H), 8.50 (d, J=4.4 Hz, 1H, CO-NH-CH3), 8.00 (d, J=8.7 Hz, 2H, Ph-H), 7.53 (s, 1H, Ph-H), 7.46 (s, 1H, Ph-H), 7.16 (d, J=8.6 Hz, 2H, Ph-H), 3.86 (s, 3H, O-CH3), 2.70 (d, J=4.4 Hz, 3H, NH-CH3), 2.23 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 167.1, 162.1, 161.3, 158.7, 142.9, 138.7, 137.0, 134.0, 133.1, 132.1, 130.5, 128.6, 125.8, 119.1, 115.2, 55.96, 26.6, 18.9; HRMS calcd for C20H18ClN3O4Na ([M+Na]+) 422.0884, found 422.0880.
2-苯基-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11l):产率34.3%. m.p. 192~194 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.70 (s, 1H, CO-NH-O), 9.99 (s, 1H, Ph-NH-CO), 8.88 (s, 1H, oxazole-H), 8.08 (dd, J=6.5, 2.9 Hz, 2H, Ph-H), 7.62~7.61 (t, J=6.5, 3H, Ph-H), 7.58 (d, J=2.0 Hz, 1H, Ph-H), 7.38 (d, J=2.0 Hz, 1H, Ph-H), 3.63 (s, 3H, O-CH3), 2.26 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.5, 161.2, 158.9, 143.5, 139.2, 137.1, 133.1, 132.3, 131.8, 130.9, 129.8, 126.8, 126.5, 125.9, 63.6, 18.6; HRMS calcd for C19H16ClN3O4Na ([M+Na]+) 408.0727, found 408.0728.
2-(2-氯苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11m):产率33.2%. m.p. 200~202 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.65 (s, 1H, CO-NH-O), 9.98 (s, 1H, Ph-NH-CO), 8.96 (s, 1H, oxazole-H), 8.04 (d, J=7.7 Hz, 1H, Ph-H), 7.69 (d, J=7.7 Hz, 1H, Ph-H), 7.66~7.60 (m, 2H, Ph-H), 7.56 (d, J=7.4 Hz, 1H, Ph-H)7.37 (d, J=7.4 Hz, 1H, Ph-H), 3.64 (s, 3H, O-CH3), 2.26 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.5, 159.2, 158.7, 144.0, 139.3, 137.0, 133.1, 133.0, 132.9, 132.4 132.1, 131.9, 131.6, 131.0, 128.2, 125.9, 125.6, 63.6, 18.6; HRMS calcd for C19H15Cl2N3O4Na([M+Na]+)442.0337, found 442.0338.
2-(3-氯苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11n):产率41.2%. m.p. 215~217 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.64 (s, 1H, CO-NH-O), 9.98 (s, 1H, Ph-NH-CO), 8.91 (s, 1H, oxazole-H), 8.07 (s, 1H, Ph-H), 8.02 (d, J=7.4 Hz, 1H, Ph-H), 7.71~7.68 (m, 1H, Ph-H), 7.66 (d, J=7.6 Hz, 1H, Ph-H), 7.57 (s, 1H, Ph-H), 7.37 (s, 1H, Ph-H), 3.62 (s, 3H, O-CH3), 2.25 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.5, 159.8, 158.7, 143.9, 139.2, 137.34, 134.4, 133.0, 132.3, 131.9, 131.6, 130.9, 128.4, 126.3, 125.9, 125.4, 63.58, 18.5; HRMS calcd for C19H15Cl2N3-O4Na ([M+Na]+) 442.0337, found 442.0335.
2-(4-氯苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11o):产率50.1%. m.p. 206~208 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.70 (s, 1H, CO-NH-O), 10.00 (s, 1H, Ph-NH-CO), 8.90 (s, 1H, oxazole-H), 8.07 (d, J=7.7 Hz, 2H, Ph-H), 7.69 (d, J=7.6 Hz, 2H, Ph-H), 7.57 (s, 1H, Ph-H), 7.38 (s, 1H, Ph-H), 3.63 (s, 3H, O-CH3), 2.25 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.5, 160.3, 158.7, 143.7, 139.2, 137.2, 136.5, 133.1, 132.3, 130.9, 130.0, 128.6, 125.9, 125.4, 63.6, 18.6; HRMS calcd for C19H15Cl2N3O4Na ([M+Na]+) 442.0337, found 442.0333.
2-(2-溴苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11p):产率49.2%. m.p. 195~197 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.64 (s, 1H, CO-NH-O), 9.99 (s, 1H, Ph-NH-CO), 8.98 (s, 1H, oxazole-H), 7.98 (d, J=7.4 Hz, 1H, Ph-H), 7.88 (d, J=7.9 Hz, 1H, Ph-H), 7.62 (t, J=7.5 Hz, 1H, Ph-H), 7.56 (s, 1H, Ph-H), 7.53 (d, J=7.5 Hz, 1H, Ph-H), 7.39 (s, 1H, Ph-H), 3.64 (s, 3H, O-CH3), 2.26 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 159.9, 158.7, 143.9, 136.9, 134.7, 133.2, 133.1, 132.3, 128.6, 127.8, 125.9, 121.1, 63.5, 18.6; HRMS calcd for C19H15ClBrN3O4Na ([M+Na]+) 485.9832, found 485.9833).
2-(3-溴苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11q):产率39.8%. m.p. 209~211 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.60 (s, 1H, CO-NH-O), 9.99 (s, 1H, Ph-NH-CO), 8.90 (s, 1H, oxazole-H), 8.21 (s, 1H, Ph-H), 8.05 (d, J=7.7 Hz, 1H, Ph-H), 7.82 (d, J=7.9 Hz, 1H, Ph-H), 7.58 (m, 2H, Ph-H), 7.38 (d, J=1.8 Hz, 1H, Ph-H), 3.63 (s, 3H, O-CH3), 2.26 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 159.7, 158.7, 143.9, 139.3, 137.3, 134.5, 133.1, 132.3, 132.1, 131.0, 129.26, 128.6, 125.9, 125.7, 122.8, 63.5, 18.5; HRMS calcd for C19H15Cl-BrN3O4Na ([M+Na]+) 485.9832, found 485.9832.
2-(4-溴苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11r):产率42.5%. m.p. 216~218 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.73 (s, 1H, CO-NH-O), 9.99 (s, 1H, Ph-NH-CO), 8.93 (s, 1H, oxazole-H), 8.01 (d, J=8.2 Hz, 2H, Ph-H), 7.84 (d, J=8.3 Hz, 2H, Ph-H), 7.58 (d, J=12.6 Hz, 1H, Ph-H), 7.40 (d, J=12.9 Hz, 1H, Ph-H), 3.67 (s, 3H, O-CH3), 2.29 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.5, 160.4, 158.8, 143.7, 139.2, 137.3, 133.1, 132.9, 132.4, 130.9, 128.7, 125.9, 125.7, 125.4, 63.6, 18.6; HRMS calcd for C19H15ClBrN3O4Na ([M+Na]+) 485.9832, found 485.9829.
2-(2-氟苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11s):产率43.3%. m.p. 206~208 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.64 (s, 1H, CO-NH-O), 9.94 (s, 1H, Ph-NH-CO), 8.93 (s, 1H, oxazole-H), 8.12 (d, J=9.1 Hz, 1H, Ph-H), 7.67 (d, J=4.8 Hz, 1H, Ph-H), 7.57 (s, 1H, Ph-H), 7.50~7.43 (m, 2H, Ph-H), 7.38 (s, 1H, Ph-H), 3.63 (s, 3H, O-CH3), 2.26 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.5, 158.7, 158.5, 143.8, 139.3, 137.1, 134.1, 134.0, 133.1, 132.3, 130.9, 130.2, 125.8, 125.7, 117.7, 117.5, 114.67, 63.6, 18.6; HRMS calcd for C19H15ClFN3O4Na ([M+Na]+) 426.0633, found 426.0633.
2-(3-氟苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11t):产率46.7%. m.p. 202~204 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.66 (s, 1H, CO-NH-O), 9.98 (s, 1H, Ph-NH-CO), 8.92 (s, 1H, oxazole-H), 7.93 (d, J=6.1 Hz, 1H, Ph-H), 7.83 (d, J=9.5 Hz, 1H, Ph-H), 7.68 (s, 1H, Ph-H), 7.59 (s, 1H, Ph-H), 7.49 (t, J=8.0 Hz, 1H, Ph-H), 7.39 (s, 1H, Ph-H), 3.64 (s, 3H, O-CH3), 2.27 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ:163.5, 163.0, 161.1, 159.5, 158.3, 143.4, 138.8, 136.8, 132.6, 131.9, 131.8, 130.5, 129.8, 128.2, 125.4, 122.6, 118.4, 118.2, 63.1, 18.1; HRMS calcd for C19H15ClFN3O4Na ([M+Na]+) 426.0633, found 426.0635.
2-(4-氟苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11u):产率44.5%. m.p. 221~22 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.68 (s, 1H, Ph-NH-CO), 9.97 (s, 1H, oxazole-H), 8.87 (s, 1H, CO-NH-CH3), 8.16~8.08 (m, 2H, Ph-H), 7.58 (s, 1H, 2H, Ph-H), 7.46 (t, J=8.8 Hz, 2H, Ph-H), 7.38 (s, 1H, Ph-H), 3.63 (s, 3H, NH-CH3), 2.26 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 165.0, 163.0, 159.9, 158.3, 143.1, 138.8, 136.7, 132.6, 132.5, 131.9, 130.5, 129.0, 128.9, 125.4, 122.8, 116.6, 116.4, 63.1, 18.1; HRMS calcd for C19H15ClFN3O4Na ([M+Na]+) 426.0633, found 426.0630.
2-(3-甲氧基苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11v):产率42.5%. m.p. 181~183 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.63 (s, 1H, CO-NH-O), 9.95 (s, 1H, Ph-NH-CO), 8.86 (s, 1H, oxazole-H), 7.65 (d, J=7.6 Hz, 1H, Ph-H), 7.57 (s, 2H, Ph-H), 7.53 (t, J=7.9 Hz, 1H, Ph-H), 7.37 (s, 1H, Ph-H), 7.19 (d, J=8.3 Hz, 1H, Ph-H), 3.87 (s, 3H, O-CH3), 3.63 (s, 3H, O-CH3), 2.26 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.5, 161.1, 160.1, 158.9, 143.5, 139.3, 137.2, 133.1, 133.1, 132.3, 131.1, 130.9, 127.7, 125.9, 119.1, 117.8, 111.68, 63.6, 55.8, 18.5; HRMS calcd for C20H18ClN3O5Na ([M+Na]+) 438.0833, found 426.0832.
2-(4-甲氧基苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-甲基苯基)噁唑-4-甲酰胺(11w):产率35.7%. m.p. 200~202 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.57 (s, 1H, CO-NH-O), 9.93 (s, 1H, Ph-NH-CO), 8.79 (s, 1H, oxazole-H), 8.01 (d, J=8.8 Hz, 2H, Ph-H), 7.57 (d, J=2.1 Hz, 1H, Ph-H), 7.38 (d, J=2.1 Hz, 1H, Ph-H), 7.16 (d, J=8.9 Hz, 2H, Ph-H), 3.86 (s, 3H, O-CH3), 3.63 (s, 3H, O-CH3), 2.25 (s, 3H, Ph-CH3); 13C NMR (101 MHz, DMSO-d6)δ: 167.1, 162.1, 161.3, 158.7, 142.9, 138.7, 137.0, 134.0, 133.1, 132.1, 130.5, 128.6, 125.8, 119.1, 115.2, 63.6, 55.9, 18.9; HRMS calcd for C20H18ClN3O5Na ([M+Na]+) 438.0833, found 426.0830.
2-(2-氯苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-氯苯基)噁唑-4-甲酰胺(11x):产率37.8%. m.p. 192~194 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.68 (s, 1H, CO-NH-O), 10.04 (s, 1H, Ph-NH-CO), 9.00 (s, 1H, oxazole-H), 8.05 (d, J=7.6 Hz, 1H, Ph-H), 7.94 (s, 1H, Ph-H), 7.71 (d, J=7.9 Hz, 1H, Ph-H), 7.66~7.58 (m, 2H, Ph-H), 7.57 (s, 1H, Ph-H), 3.64 (s, 3H, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 162.2, 159.9, 158.9, 144.2, 137.0, 135.3, 134.4, 134.1, 132.2, 132.1, 131.8, 131.6, 131.3, 128.4, 127.6, 126.4, 125.45, 63.6; HRMS calcd for C18H12Cl3N3O4Na ([M+Na]+) 461.9791, found 461.9793.
2-(3-氯苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-氯苯基)噁唑-4-甲酰胺(11y):产率38.5%. m.p. 201~203 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.67 (s, 1H, CO-NH-O), 10.08 (s, 1H, Ph-NH-CO), 8.93 (s, 1H, oxazole-H), 8.08 (s, 1H, Ph-H), 8.02 (d, J=7.6 Hz, 1H, Ph-H), 7.94 (d, J=2.3 Hz, 1H, Ph-H), 7.70~7.67 (m, 1H, Ph-H), 7.66 (d, J=7.7 Hz, 1H, Ph-H), 7.56 (d, J=2.2 Hz, 1H, Ph-H), 3.63 (s, 3H, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 162.3, 159.8, 158.9, 144.2, 137.22, 135.3, 134.3, 134.2, 132.2, 132.2, 131.8, 131.5, 131.3, 128.4, 127.5, 126.4, 125.5, 63.7; HRMS calcd for C18H12Cl3N3O4Na ([M+Na]+) 461.9791, found 461.9790.
2-(2-氟苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-氯苯基)噁唑-4-甲酰胺(11z):产率40.2%. m.p. 220~222 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.66 (s, 1H, CO-NH-O), 10.00 (s, 1H, Ph-NH-CO), 8.96 (s, 1H, oxazole-H), 8.11 (d, J=6.4 Hz, 1H, Ph-H), 7.93 (s, 1H, Ph-H), 7.65 (d, J=8.7 Hz, 1H, Ph-H), 7.56 (d, J=2.0 Hz, 1H, Ph-H), 7.49 (d, J=10.8 Hz, 1H, Ph-H), 7.46 (d, J=7.3 Hz, 1H, Ph-H), 3.63 (s, 3H, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 162.2, 161.1, 158.9, 158.6, 157.8, 144.1, 136.8, 135.3, 134.1, 134.1, 132.2, 132.1, 131.4, 130.3, 127.6, 125.7, 117.7, 117.5, 63.7; HRMS calcd for C18H12Cl2FN3O4Na ([M+Na]+) 446.0087, found 446.0085.
2-(3-氟苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-氯苯基)噁唑-4-甲酰胺(11a'):产率45.2%. m.p. 229~231 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.68 (s, 1H, CO-NH-O), 10.07 (s, 1H, Ph-NH-CO), 8.94 (s, 1H, oxazole-H), 8.11 (s, 1H, Ph-H), 7.94 (s, 1H, Ph-H), 7.83 (d, J=9.0 Hz, 1H, Ph-H), 7.67 (d, J=6.0 Hz, 1H, Ph-H), 7.56 (s, 1H, Ph-H), 7.51~7.47 (m, 1H, Ph-H), 3.63 (s, 3H, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 164.1, 162.2, 158.9, 144.1, 137.0, 134.1, 132.2, 132.1, 131.3, 130.3, 127.6, 123.1, 118.9, 118.7, 113.6, 113.4, 112.31, 63.7; HRMS calcd for C18H12-Cl2FN3O4Na ([M+Na]+) 446.0087, found 446.0086.
2-(4-氟苯基)-N-(4-氯-2-甲氧氨基甲酰基-6-氯苯基)噁唑-4-甲酰胺(11b'):产率37.8%. m.p. 211~213 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.70 (s, 1H, CO-NH-O), 10.05 (s, 1H, Ph-NH-CO), 8.90 (s, 1H, oxazole-H), 8.12 (s, 2H, Ph-H), 7.93 (s, 1H, Ph-H), 7.56 (s, 1H, Ph-H), 7.46 (s, 2H, Ph-H), 3.63 (s, 3H, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 163.1, 162.1, 160.5, 159.0, 143.8, 136.9, 135.3, 134.1, 132.2, 132.1, 131.4, 129.5, 129.4, 127.6, 123.2, 117.1, 116.9, 63.7; HRMS calcd for C18H12Cl2FN3-O4Na ([M+Na]+) 446.0087, found 446.0088.
2-苯基-N-(4-氯-2-甲氧氨基甲酰基-6-氯苯基)噁唑-4-甲酰胺(11c'):产率38.4%. m.p. 198~200 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.68 (s, 1H, CO-NH-O), 10.04 (s, 1H, Ph-NH-CO), 8.90 (s, 1H, oxazole-H), 8.07 (s, 2H, Ph-H), 7.94 (s, 1H, Ph-H), 7.61 (s, 3H, Ph-H), 7.57 (s, 1H, Ph-H), 3.64 (s, 3H, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ: 162.2, 161.3, 159.1, 143.8, 136.8, 135.3, 134.1, 132.1, 132.1, 131.8, 131.3, 129.8, 127.6, 126.8, 126.5, 63.6; HRMS calcd for C18H13Cl2N3O4Na ([M+Na]+) 428.0181, found 428.0178.
3.3 生物活性测试
实验方法:采用浸液法, 以丙酮为溶剂, 将供试样品配成一系列浓度梯度的溶液, 把苗期玉米浸渍在上述溶液中, 晾干放入培养皿中.接入4龄阶段的实验幼虫, 并设用丙酮浸渍的玉米叶作空白对照组, 氯虫苯甲酰胺为阳性对照组, 重复2~4次, 24 h后开始添加新鲜玉米叶片.在48, 72 h分别观察实验结果.观察标准为粘虫的完全死亡.
辅助材料(Supporting Information) 中间体化合物2~5和7~10的合成, 邻-(4-噁唑甲酰胺基)苯甲酰胺类化合物11a~11z及11a'~11c'的1H NMR和13C NMR图谱.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
刘长令, 新农药, 2005, 44, 527.Liu, C. L. New Pestic. 2005, 44, 527(in Chinese).
-
[2]
Al-Terkait, F.; Charalambous, H. Chem. Mater. 2008, 20, 2829. doi: 10.1021/cm703641s
-
[3]
Hyoungsu, K.; Yongho, P.; Hong, J. Angew. Chem., Int. Ed. 2009, 48, 7577. doi: 10.1002/anie.v48:41
-
[4]
Nicolaou, K. C.; Jiang, X. F.; Lindsay-Scott, P. J.; Corbu, A.; Yamashiro, S.; Bacconi, A.; Fowler, V. M. Angew. Chem., Int. Ed. 2011, 50, 1139. doi: 10.1002/anie.v50.5
-
[5]
Skepper, C. K.; Quach, T.; Molinski, T. F. J. Am. Chem. Soc. 2010, 132, 10286. doi: 10.1021/ja1016975
-
[6]
张慧珍, 周成合, 耿蓉霞, 吉庆刚, 有机化学, 2011, 31, 1963. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract340559.shtmlZhang, H. Z.; Zhou, C. H.; Geng, R. X.; Ji, Q. G. Chin. J. Org. Chem. 2011, 31, 1963(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract340559.shtml
-
[7]
Nagori, K.; Singh, M. K.; Alexander, A.; Kumar, T.; Dewangan, D.; Badwaik, H.; Tripathi, D. K. J. Pharm. Res. 2011, 2991.
-
[8]
Zhao, Q. Q.; Liu, S. H.; Li, Y. H.; Wang, Q. M. J. Agric. Food Chem. 2009, 57, 2849. doi: 10.1021/jf803632t
-
[9]
Lu, X. Y.; Liu, X. B.; Wan, B. J.; You, Q. D. Eur. J. Med. Chem. 2012, 49, 164. doi: 10.1016/j.ejmech.2012.01.007
-
[10]
张成仁, 王柳, 葛燕丽, 巨修炼, 有机化学, 2007, 27, 1432. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract330187.shtmlZhang, C. R.; Wang, L.; Ge, Y. L.; Ju, X. L. Chin. J. Org. Chem. 2007, 27, 1432(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract330187.shtml
-
[11]
徐姗姗, 张敏, 张丽, 有机化学, 2015, 35, 2595. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345186.shtmlXu, S. S.; Zhang, M.; Zhang, L. Chin. J. Org. Chem. 2015, 35, 2595(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345186.shtml
-
[12]
谭月德, 何晓艳, 饶保奇, 有机化学, 2016, 36, 2449. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345589.shtmlTan, Y. D.; He, X. Y.; Rao, B. Q. Chin. J. Org. Chem. 2016, 36, 2449(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345589.shtml
-
[13]
Danel, M.; Akram, H.; Roland, B. Heterocycl. Commun. 2005, 11, 343.
-
[14]
Graham, T. H. Org. Lett. 2010, 12, 3614. doi: 10.1021/ol101346w
-
[15]
肖立伟, 张光霞, 景学敏, 周秋香, 冯茹, 有机化学, 2016, 36, 1000. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345375.shtmlXiao, L. W.; Zhang, G. X.; Jing, X. M.; Zhou, Q. X.; Feng, R. Chin. J. Org. Chem. 2016, 36, 1000(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345375.shtml
-
[16]
Lahm, G. P.; Selby, T. P.; Freudenberger, J. H.; Stevenson, T. M.; Myers, B. J.; Seburyamo, G.; Smith, B. K.; Flexner, L.; Clark. C. E.; Cordova, D. Bioorg. Med. Chem. Lett. 2005, 15, 4898. doi: 10.1016/j.bmcl.2005.08.034
-
[17]
Ou, J. J.; Zhu, X. K.; Wang, L.; Xu, C.; Liu, F.; Ren, L.; Xu, X. B.; Wang, Y.; Rui, C. H.; Liu, S. Z. J. Agricult. Food Chem. 2012, 60, 10942. doi: 10.1021/jf303376t
-
[18]
Eldo, J.; Heng, S.; Kantrowitz, E. R. Bioorg. Med. Chem. Lett. 2007, 17, 2086. doi: 10.1016/j.bmcl.2006.12.050
-
[1]
-
图式 1 合成路线
Scheme 1 Synthetic route
Reagents and conditions: (a) SOCl2, CH3OH, Ref. [13]; (b) CH3CHO, K2CO3/DMA, BrCCl3/DBU, Ref. [14, 15]; (c) NaOH, CH3OH, H2O, Ref. [16]; (d) SOCl2, CH2Cl2, Ref. [16]; (e) Fe, HCl, H2O, Ref.[16]; (f) H2O2, NaSO3, DMF, HCl, Ref. [17]; (g)三光气, 二氧六环, Ref. [15, 17, 18]; (h) R2NH2, CH3CO2H, Ref. [15, 17]; (ⅰ) Et3N, CH3CN, SOCl2, CH3OH
表 1 目标化合物结构及生物活性测定结果
Table 1. The structure and the insecticidal activities of target compounds
编号 R R1 R2 杀虫活性/% (50 mg•L-1) 11a H CH3 CH3 20 11b α-Cl CH3 CH3 60 11c β-Cl CH3 CH3 25 11d γ-Cl CH3 CH3 70 11e α-Br CH3 CH3 25 11f β-Br CH3 CH3 30 11g γ-Br CH3 CH3 60 11h γ-F CH3 CH3 45 11i α-OCH3 CH3 CH3 12 11j β-OCH3 CH3 CH3 5 11k γ-OCH3 CH3 CH3 10 11l H CH3 OCH3 10 11m α-Cl CH3 OCH3 15 11n β-Cl CH3 OCH3 45 11o γ-Cl CH3 OCH3 30 11p α-Br CH3 OCH3 100 11q β-Br CH3 OCH3 40 11r γ-Br CH3 OCH3 10 11s α-F CH3 OCH3 40 11t β-F CH3 OCH3 10 11u γ-F CH3 OCH3 65 11v β-OCH3 CH3 OCH3 45 11w γ-OCH3 CH3 OCH3 10 11x α-Cl Cl OCH3 25 11y β-Cl Cl OCH3 50 11z α-F Cl OCH3 20 11a' β-F Cl OCH3 50 11b' γ-F Cl OCH3 15 11c' H Cl OCH3 30 100 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 8
- 文章访问数: 1554
- HTML全文浏览量: 169


 下载:
下载:

 下载:
下载:

