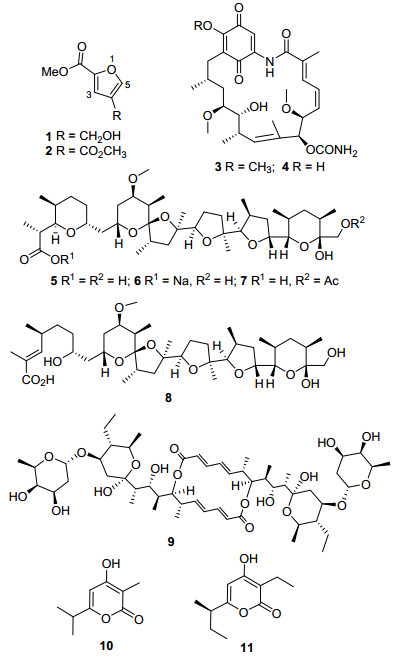
 图 1
化合物1~11的结构
Figure 1.
Structures of compounds 1~11
图 1
化合物1~11的结构
Figure 1.
Structures of compounds 1~11

Citation: Wang Cong, Wang Liping, Fan Jie, Sun Kunlai, Zhu Weiming. Cytotoxic Compounds from the DeepSea Sediment-Derived Streptomyces malaysiensis OUCMDZ-2167[J]. Chinese Journal of Organic Chemistry, 2017, 37(3): 658-666. doi: 10.6023/cjoc201609021

深海链霉菌Streptomyces malaysiensis OUCMDZ-2167来源的细胞毒性产物
English
Cytotoxic Compounds from the DeepSea Sediment-Derived Streptomyces malaysiensis OUCMDZ-2167
-
海洋链霉菌天然产物的研究始于1976年, 截止到2016年6月的40年时间内, 报道了547个海洋链霉菌天然产物[1], 是海洋放线菌天然产物的重要来源. 2010~2013年初报道的海洋放线菌新天然产物中, 约60%来源于海洋链霉菌[2].从海洋链霉菌的天然产物中寻找具有抗肿瘤、抗病毒、抗菌和抗糖尿病活性的化合物是作者的兴趣和目标, 并获得了一些新颖的活性化合物.如从海洋链霉菌Streptomyces sp. FMA[3]和Streptomyces fradiae 007M135[4]的发酵产物中获得了新颖的具有抗肿瘤活性的吲哚咔唑生物碱, 并对其进行半合成或结构修饰得到了具有蛋白激酶C (PKC) 抑制作用的衍生物[5, 6]; 而与Penicilliumsp. WC-29-5共培养时, S. fradiae007产生了具有细胞毒活性的聚酮类化合物9, 14-epoxy-11-deoxyfunicone[7].从Streptomyces sp. OUCMDZ-1703和Streptomyces sp. FXJ7.328的培养产物中分别获得了具有细胞毒活性的氯代多酚类化合物[8]以及具有抗甲型流感病毒H1N1活性的脱氢二酮哌嗪生物碱[9].为继续开展海洋链霉菌活性天然产物的研究, 作者从南海2061 m深的海洋沉积物样品中, 分离筛选到一株马来西亚链霉菌S. malaysiensis OUCMDZ-2167;其发酵产物在0.1 mg/mL浓度下, 对MCF-7、A549和K562细胞株的抑制率分别为 (86.0±3.3)%、(83.5±2.7)%和 (88.0±2.3)%[阿霉素adriamycin对应的抑制率分别为 (73.0±1.6)%、(75.0±2.5)%和 (74.0±1.2)%]; 并从其发酵产物中获得了11个聚酮类化合物, 包括2个呋喃衍生物1~2、2个格尔德霉素3~4、4个尼日利亚菌素5~8、1个大环内酯类化合物9和2个2-吡喃酮类化合物10~11, 其中化合物1为新化合物, 格尔德霉素3对A549、尼日利亚菌素5~8和洋橄榄叶素9对A549和K562肿瘤细胞株具有增殖抑制活性, IC50为0.48~9.60 μmol·L-1.
1 结果与讨论
1.1 菌株鉴定结果
OUCMDZ-2167的基因组的DNA电泳图及16S rDNA扩增产物电泳图谱, OUCMDZ-2167的基因组DNA片段在10000 bp左右, 16S rDNA扩增产物电泳图谱为1377 bp. OUCMDZ-2167与EzTaxon-e数据库中的菌株Streptomyces malaysiensis NBRC 16446 (T) 的同源性达99.78%, 亲缘关系最近, 在系统发育树上处于同一分支.故将菌株OUCMDZ-2167 (GenBank登录号KX394627) 初步鉴定为马来西亚链霉菌Streptomyces malaysiensis[10].进一步观察其形态特征, 在高氏Ⅰ号培养基上, OUCMDZ-2167菌株的菌落干燥, 气生菌丝生长良好, 产生螺旋孢子丝, 孢子呈球形, 在不同培养基 (ISP2、ISP3和高氏Ⅰ号培养基等) 的气生菌丝为灰色, 随着时间的延长, 基内菌丝逐渐由灰色变为褐色、黑色, 交织成网状; 在ISP2、ISP3等不同培养基也产生同样浅黄色的可溶性色素.这些特征与文献报道的S. malaysiensis NBRC 16446 (T) 的形态特征相一致[10, 11], 故鉴定OUCMDZ-2167为马来西亚链霉菌Streptomyces malaysiensis.
1.2 化合物的结构鉴定
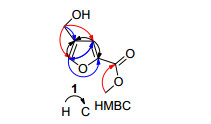
化合物1的正离子高分辨质谱HRESIMS给出m/z 157.0492 [M+H]+峰, 提示化合物的分子式是C7H8O4(对C7H9O4+的计算值为157.0495), 不饱和度为4.红外光谱给出羟基 (3410 cm-1)、羰基 (1680 cm-1) 等官能团的特征吸收峰. 1H NMR、13C NMR、DEPT和HMQC NMR给出1个羰基信号 (δC 158.4)、2个芳香季碳信号 (δC128.9、144.0)、2个芳香次甲基信号 (δC/H 118.2/7.26、143.7/7.84)、1个连氧的亚甲基信号 (δC/H54.3/4.39)、1个甲氧基信号 (δC/H 51.8/3.82) 及1个羟基信号 (δH 5.15) (表 1).这些NMR数据与已知的呋喃-2, 4-二甲酸二甲酯 (2) 很相似, 提示其为呋喃甲酸的衍生物.仔细比较发现, 化合物1中少了1个羰基和1个甲氧基信号, 但多了1个羟甲基信号, 表明化合物2中的1个甲氧羰基被还原为羟甲基.化合物1的HMBC谱图中出现了如下关键的氢-碳远程偶合相关 (图 2):即羟甲基质子 (δH4.39) 与2个芳香次甲基碳 (δC 118.2、143.7) 和1个芳香季碳信号 (δC128.9)、甲氧基质子 (δH3.82) 与羰基碳 (δC158.4) 的相关信号 (图 2、S6), 表明是化合物2中4-甲氧羰基还原为羟甲基.故化合物1的结构鉴定为4-羟甲基呋喃-2-甲酸甲酯[methyl 4-(hydroxymethyl) furan-2-carboxylate].
 表 1
化合物1的1H NMR、13C NMR和HMBC数据 (DMSO-d6)
Table 1.
1H NMR, 13C NMR and HMBC data of compound 1 (DMSO-d6)
表 1
化合物1的1H NMR、13C NMR和HMBC数据 (DMSO-d6)
Table 1.
1H NMR, 13C NMR and HMBC data of compound 1 (DMSO-d6)
Position δC, type δH (J in Hz) HMBC (1H→13C) 2 144.0, qC 3 118.2, CH 7.26 (s) C-2, C-4, C-5 4 128.9, qC 5 143.7, CH 7.84 (s) C-3, C-4 2-CO2CH3 158.4, qC 2-CO2CH3 51.8, CH3 3.82 (s) 2-CO2 4-CH2OH 54.3, CH2 4.39 (d, 5.0 Hz) C-3, C-4, C-5 4-CH2OH 5.15 (t, 5.0 Hz) 表 1 化合物1的1H NMR、13C NMR和HMBC数据 (DMSO-d6)
Table 1. 1H NMR, 13C NMR and HMBC data of compound 1 (DMSO-d6)经过分析波谱数据 (MS、NMR) 和比旋光值[3: [α]D25+53.5 (c 0.6, CHCl3), 4: [α]D25+15.2 (c 0.1, MeOH), 5: [α]D25+36.2 (c 0.8, CHCl3), 6: [α]D25+30.1 (c 0.8, CHCl3), 7: [α]D25+29.5 (c 0.78, CHCl3), 8: [α]D25+43 (c 0.03, CH3OH), 9: [α]D25-47 (c 0.1, CHCl3), 11: [α]D25+26 (c 0.04, CHCl3)], 并与文献比对, 化合物2~11的结构依次鉴定为:呋喃-2, 4-二甲酸二甲酯 (dimethyl furan-2, 4-dicarboxylate, 2)[12]、格尔德霉素 (geldanamycin, 3)[13]、17-O-去甲基格尔德霉素 (17-O-demethylgelda-namycin, 4)[14]、尼日利亚菌素 (nigericin, 5)[15]、尼日利亚菌素钠 (nigericin sodium salt, 6)[16, 17]、30-O-乙酰基尼日利亚菌素 (30-O-acetylnigericin, 7)[18]、奥布菌素 (abierixin, 8)[18, 19]、洋橄榄叶素 (elaiophylin, 9)[20, 21]、surugapyrone A (10)[22]和杀菌素A (germicidin A, 11)[23, 24].
1.3 化合物的肿瘤细胞毒活性
采用噻唑蓝 (MTT) 法评价了化合物对人乳腺浸润性导管癌细胞株MCF-7和人肺腺癌细胞株A549的细胞增殖抑制活性, 采用CCK-8法评价了化合物对人慢性髓性白血病细胞株K562的细胞增殖抑制活性.结果表明 (表 2), 格尔德霉素3、尼日利亚菌素5~8和洋橄榄叶素9对A549肿瘤细胞株的IC50值分别为2.56±0.30、0.96±0.03、0.96±0.04、0.81±0.05、7.11±0.90和4.09±0.70 μmol· L-1 (阿霉素adriamycin的IC50 0.81±0.04 μmol·L-1); 化合物5~9对K562肿瘤细胞株的IC50值分别为1.46±0.11、1.08±0.03、0.48±0.10、4.68±0.19和9.60±0.50 μmol·L-1 (阿霉素adriamycin的IC50 0.32±0.11 μmol· L-1); 化合物5、8和9对MCF-7肿瘤细胞株的IC50值分别为0.29±0.15、4.05±0.50和4.81±0.70 μmol·L-1 (阿霉素adriamycin的IC50 0.20±0.03 μmol·L-1).这是首次报道化合物5~8对K562和A549以及化合物5和8对MCF-7细胞株的细胞毒活性.
 表 2
化合物1~11对A549、MCF-7和K562细胞株的细胞毒活性
Table 2.
The cytotoxicities of compounds 1~11 against A549, MCF-7 and K562 cell lines
表 2
化合物1~11对A549、MCF-7和K562细胞株的细胞毒活性
Table 2.
The cytotoxicities of compounds 1~11 against A549, MCF-7 and K562 cell lines
化合物 A549 MCF-7 K562 抑制率/% IC50/(μmol·L-1) 抑制率/% IC50/(μmol·L-1) 抑制率/% IC50/(μmol·L-1) 1 -4.4±0.5 >10 3.2±0.3 >10 6.6±0.5 >10 2 7.3±0.7 >10 4.4±0.4 >10 9.7±1.0 >10 3 78.7±2.5 2.56±0.30 39.6±1.6 >10 68.7±2.3 >10 4 37.8±1.0 >10 8.7±0.4 >10 34.2±1.5 >10 5 83.0±1.1 0.96±0.03 78.4±2.1 0.29±0.15 84.6±0.8 1.46±0.11 6 86.9±3.3 0.96±0.04 21.4±0.4 >10 84.7±0.2 1.08±0.03 7 85.4±0.8 0.81±0.05 39.1±3.3 >10 84.3±1.2 0.48±0.10 8 73.8±0.9 7.11±0.90 67.2±5.0 4.05±0.50 77.9±0.5 4.68±0.19 9 55.6±2.3 4.09±0.70 69.5±3.6 4.81±0.70 80.5±0.6 9.60±0.50 10 2.0±0.4 >10 4.1±0.1 >10 -41.8±2.0 >10 11 4.4±0.5 >10 2.9±0.8 >10 -55.5±1.1 >10 阿霉素 77.6±0.05 0.81±0.04 72.1±0.3 0.20±0.03 81.9±0.2 0.32±0.11 表 2 化合物1~11对A549、MCF-7和K562细胞株的细胞毒活性
Table 2. The cytotoxicities of compounds 1~11 against A549, MCF-7 and K562 cell lines格尔德霉素和尼日利亚菌素分别属于安莎类和聚醚类抗生素, 主要来源于链霉菌[18, 25].格尔德霉素具有抗肿瘤、抗菌、抗原虫、抗病毒、抗炎、免疫调节和调节上皮氮氧合酶活性[25, 26], 报道最多的是抗肿瘤活性, 且作用靶点为Hsp90;但格尔德霉素有较强的肝脏毒性和较差的水溶性, 生物利用度也不高, 研究者也在不断寻找新的格尔德霉素的衍生物[26], 如17-位甲氧基被烯丙氨基取代的17-AAG已进入临床Ⅱ期[27].尼日利亚菌素具有抗菌、抗肿瘤、抗病毒、抗疟原虫以及抗疟疾等活性[17, 28], 在农业生产中用于治疗鸡球虫病以及牲畜生长促进剂[17]; 但有关尼日利亚菌素的抗肿瘤活性的报道较少, 仅有抗上皮肿瘤干细胞和肺癌细胞DLKP-A10的两篇报道[29, 30].本文首次报道了尼日利亚菌素类化合物5~8对K562和A549以及化合物5和8对MCF-7细胞株的细胞毒活性, 尤其是化合物5~7对A549细胞、化合物5对MCF-7细胞以及化合物7对K562细胞的活性最强, 半数抑制浓度达到了纳摩尔级, 为抗乳腺癌、肺癌和白血病药物的筛选提供了新的候选化合物.此外, 从海洋链霉菌的代谢产物中发现尼日利亚菌素的报道仅有2例, 即国内的张长生课题组[17]和国外的Andersen课题组[31]; 本文为第三例报道, 表明深海来源的海洋链霉菌具有代谢高活性天然产物的潜力, 具有进一步研究和开发的价值.
2 结论
从深海沉积物分离获得一株生产格尔德霉素和尼日利亚菌素的链霉菌S. malaysiensis OUCMDZ-2167, 并从其发酵产物中分离鉴定了2个格尔德霉素[格尔德霉素 (3)、17-O-去甲基格尔德霉素 (4)], 4个尼日利亚菌素[尼日利亚菌素 (5)、尼日利亚菌素钠 (6)、30-O-乙酰基尼日利亚菌素 (7)、奥布菌素 (8)], 1个大环内酯类化合物[洋橄榄叶素 (9)], 2个2-吡喃酮类化合物[surugapyrone A (10) 和杀菌素A (11)], 以及2个呋喃甲酸酯类化合物[4-羟甲基呋喃-2-甲酸甲酯 (1)、呋喃-2, 4-二甲酸二甲酯 (2)].其中化合物1为新化合物.格尔德霉素3和尼日利亚菌素5~8及大环内酯类化合物9对A549、K562和MCF-7肿瘤细胞株具有中等以上的细胞毒活性, IC50值为0.29~9.60 μmol·L-1; 其中化合物5~8对K562和A549以及化合物5和8对MCF-7的细胞毒活性为首次报道.
3 实验部分
3.1 仪器与试剂
高压灭菌锅 (LDZX-75KBS型立式压力蒸汽灭菌器); 恒温培养箱 (MIR-253);垂直层流洁净工作台HCB-1300V (松下电器有限公司上海分公司健康医疗公司); 柱层析及薄层层析用硅胶H (青岛海洋化工集团公司); Sephadex™ LH-20 (Pharmacia公司); 分析用高效液相色谱仪 (Waters 1525泵, Waters 2996二极管阵列检测器, Empower Ⅲ工作站, Capcell Pak C18柱: 5 mm, 4.6 mm×250 mm, Cosmosil Cholester柱: 5 mm, 4.6 mm×250 mm); 制备高效液相色谱仪 (Waters 1525泵, Waters 2487二极管阵列检测器, Breeze工作站, Cosmosil Cholester柱: 5 mm, 10 mm×250 mm; 紫外光谱仪 (Beck-man DUR640型); 旋光仪POLAX-L型 (美国ATAGO公司); X-4数字显示显微熔点测定仪; NMR用Agilent Pro Pulse 500型核磁共振波谱仪测定, 氢谱和碳谱用溶剂定标 (CHCl3δH/C 7.26/77.16, DMSO δH/C 2.50/39.52); ESIMS和高分辨ESIMS分别用Mariner API-TO型质谱仪和Q-TOF ULTIMA GLOBAL GAA076 LC高分辨质谱仪测定; IR用Nicolet NEXUS 470型红外分光光度计测定 (KBr压片); 细胞培养用MCO175型二氧化碳培养箱 (SANYO公司); 细胞毒测试用SpectraMax plus型酶标仪 (美国Molecular公司) 读取吸光度.
常规提取分离用乙酸乙酯、二氯甲烷、石油醚、甲醇等均为工业用化学纯产品, 高效液相色谱用乙腈和甲醇为色谱纯试剂, 洗脱剂添加的酸为分析纯三氟乙酸 (TFA).
3.2 菌株的来源、分离培养和鉴定
马来西亚链霉菌S. malaysiensis OUCMDZ-2167来自南海2061 m深的海洋沉积物样品 (2012年4月16日采自中国南海N18°01.854′、E113°01.41′):沉积物样品65 ℃下加热处理15 min, 取样品1 g溶于1 mL无菌水中, 用无菌水稀释100倍; 取0.3 mL稀释液均匀涂布于装有高氏Ⅰ号培养基的3个平板上 (其组成为: 20 g可溶性淀粉、1 g KNO3、0.5 g K2HPO4、0.5 g MgSO4、0.5 g NaCl、0.01 g FeSO4、1000 mL陈海水, pH=7.5, 20 g琼脂), 28 ℃倒置培养5 d, 挑取单菌落进行划线纯化, 将纯化好的菌株接入高氏Ⅰ号斜面培养基上, 保存于4 ℃冰箱. OUCMDZ-2167菌株形态特征观察:将菌种接种于高氏Ⅰ号平板培养基上, 28 ℃插片培养, 按照《链霉菌鉴定手册》对菌株初步鉴定[32]. 16S rDNA序列分析:按照生工SK8255-Ezup柱式细菌基因组DNA抽提试剂盒提取样品的基因DNA, 按照常规方法进行聚合酶链式反应 (PCR) 扩增. PCR扩增程序为: 94 ℃下5 min, 94 ℃下45 s, 55 ℃下45 s, 72 ℃下1 min, 30个循环, 延伸10 min. PCR产物经纯化由生工生物工程 (上海) 股份有限公司完成.将获得的16S rDNA基因序列提交EzTaxon-e数据库, BLAST比对, 利用软件MEGA4软件构建系统发育树[33].
3.3 发酵与提取
将平板培养3 d的菌株接种于装有150 mL培养液的500 mL三角瓶中, 培养液组成为: 20 g可溶性淀粉、1 g KNO3、0.5 g K2HPO4、0.5 g MgSO4、0.5 g NaCl、0.01 g FeSO4、1000 mL陈海水、pH=7.5, 置于28 ℃、180 rpm摇床上培养7 d, 发酵量为65 L.发酵液采用乙酸乙酯萃取3次, 得粗浸膏13 g.
3.4 发酵与提取活性产物的分离纯化
对浸膏进行减压硅胶柱层析, 采用 (石油醚/二氯甲烷/甲醇) 极性逐渐递增的梯度洗脱, 通过TLC、HPLC检测得到11个馏分 (Fr.1~Fr.11). Fr.4 (232 mg) 经凝胶Sephadex LH-20柱色谱分离[V(CH2Cl2):V(MeOH)=1:1洗脱]得到亚组分Fr.4-2和Fr.4-3, 其中Fr.4-2 (25 mg) 经半制备HPLC分离[Cosmosil-pack cholester柱, V(MeOH):V(水)=65:35+0.15%三氟乙酸 (TFA) 进行洗脱, 4 mL/min]得到化合物2 (tR=4.4 min; 1.4 mg). Fr.6 (2.3 g) 经凝胶Sephadex LH-20柱色谱分离[V(CH2Cl2):V(MeOH)=1:1洗脱]得到亚组分Fr.6-2、Fr.6-3、Fr.6-5和Fr.6-6; Fr.6-2 (450 mg) 经过Sephadex LH-20柱色谱分离[V(CH2Cl2):V(MeOH)=1:1洗脱]得到化合物6 (11 mg)、亚组分Fr.6-2-1和Fr.6-2-2; Fr.6-2-1 (227 mg) 再通过加压硅胶柱色谱分离[洗脱剂: V(石油醚):V(乙酸乙酯)=3:1]得到化合物5 (22 mg) 和化合物7 (20 mg). Fr.6-3 (290 mg) 经过半制备HPLC分离[Cosmosil-pack cholester柱, V(乙腈):V(水)=65:35+0.15% TFA洗脱, 4 mL/min]得到化合物3 (tR=7.1 min; 6.0 mg) 和4 (tR=5.2 min; 1.8 mg). Fr.6-5 (232 mg) 经半制备HPLC分离[Cosmosil-pack cholester柱, V(乙腈):V(水)=40:60洗脱, 4 mL/min]得到化合物10 (tR=5.8 min; 0.6 mg) 和11 (tR=7.1 min; 3.5 mg), Fr.6-6 (103 mg) 经半制备HPLC分离[Cosmosil-pack cholester柱, V(乙腈):V(水)=30:70洗脱, 4 mL/min]得到化合物1 (tR=5.8 min; 3.0 mg). Fr.7 (623 mg) 经凝胶Sephadex LH-20柱色谱分离[V(二氯甲烷):V(甲醇)=1:1洗脱]得到亚组分Fr.7-1和Fr.7-2, Fr.7-1 (398 mg) 通过加压硅胶柱色谱分离[V(二氯甲烷):V(甲醇)=50:1) 洗脱]得到化合物8(25 mg) 和9 (16 mg).
4-羟甲基呋喃-2-甲酸甲酯 (1):白色固体, m.p. 93~96 ℃; UV-vis (MeOH) λmax[log ε/(L・mol-1・cm-1)]: 258 (2.0) nm; 1H NMR (500 MHz, DMSO-d6) 和13C NMR (125 MHz, DMSO-d6) 见表 1; IR (KBr) νmax: 3410, 2926, 1680, 1406, 1151, 1082, 1024 cm-1; HRESIMS calcd for C7H9O4 [M+H]+157.0495, found 157.0492.
呋喃-2, 4-二甲酸二甲酯 (2):白色固体, m.p. 105~107 ℃(文献值[34] m.p. 104~107 ℃); 1H NMR (500 MHz, DMSO-d6) δ: 8.64 (s, 1H, H-5), 7.46 (s, 1H, H-3), 3.82 (s, 3H, 4-CO2CH3), 3.78 (s, 3H, 2-CO2CH3); 13C NMR (125 MHz, DMSO-d6) δ:158.3 (C, 2-CO2CH3), 152.2 (C, 4-CO2CH3), 145.3 (C, C-2), 143.7 (CH, C-5), 120.7 (C, C-4), 117.1 (CH, C-3), 52.7 (CH3, 2-CO2CH3), 52.4 (CH3, 4-CO2CH3); ESI-MS m/z: 185.0 [M+H]+.
格尔德霉素 (3):黄色粉末. m.p. 250~254 ℃(文献值[35] 252~255 ℃); [α]D25 +53.5 (c 0.6, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 8.71 (s, 1H, HN-22), 7.29 (s, 1H, H-19), 6.95 (d, J=12.2 Hz, 1H, H-3), 6.59 (t, J=11.3 Hz, 1H, H-4), 5.90 (t, J=10.4 Hz, 1H, H-5), 5.82 (d, J=9.5 Hz, 1H, H-9), 5.19 (s, 1H, H-7), 4.32 (d, J=9.4 Hz, 1H, H-6), 4.12 (s, 3H, CH3O-17), 3.54 (d, J=7.0 Hz, 1H, H-11), 3.88~3.39 (m, 1H, H-12), 3.36 (s, 3H, CH3O-12), 3.30 (s, 3H, CH3O-6), 2.77~2.80 (m, 1H, H-10), 2.45~2.53 (m, 2H, H-15), 2.03 (s, 3H, H-23), 1.79 (s, 3H, H-24), 1.76~1.77 (m, 2H, H-13), 1.66~1.68 (m, 1H, H-14), 0.99 (d, J=6.8 Hz, 3H, H-25), 0.96 (d, J=6.5 Hz, 3H, H-26); 13C NMR (125 MHz, CDCl3) δ: 185.0 (C, C-21), 184.1 (C, C-18), 168.2 (C, C-1), 157.0 (C, C-17), 155.9 (C, H2N-CO2-7), 138.0 (C, C-20), 136.4 (CH, C-5), 134.8 (C, C-2), 133.2 (C, C-8), 133.1 (CH, C-9), 127.5 (C, C-16), 127.2 (CH, C-3), 126.3 (CH, C-4), 111.7 (C, C-19), 81.7 (CH, C-7), 81.2 (CH, C-6), 81.0 (CH, C-12), 72.6 (CH, C-11), 61.7 (CH3, CH3O-17), 57.3 (CH3, CH3O-6), 56.7 (CH3, CH3O-12), 34.7 (CH2, C-13), 32.7 (CH2, C-15), 32.2 (CH, C-10), 27.9 (CH, C-14), 22.9 (CH3, C-26), 12.9 (CH3, C-24), 12.5 (CH3, C-23), 12.4 (CH3, C-25); ESI-MS m/z: 583.3 [M+Na]+.
17-O-去甲基格尔德霉素 (4):黄色针状晶体, m.p.>300 ℃(文献值[36] 353~357 ℃); [α]D25+15.2 (c 0.1, MeOH); 1H NMR (500 MHz, CDCl3)δ: 8.97 (s, 1H, HN-22), 7.39 (s, 1H, H-19), 6.98 (d, J=9.4 Hz, 1H, H-3), 6.60 (t, J=11.3 Hz, 1H, H-4), 5.91 (t, J=10.4 Hz, 1H, H-5), 5.82 (d, J=9.5 Hz, 1H, H-9), 5.18 (s, 1H, H-7), 4.32 (d, J=9.3 Hz, 1H, H-6), 3.54~3.56 (m, 1H, H-11), 3.35~3.36 (m, 1H, H-12), 3.30 (s, 3H, CH3O-12), 3.30 (s, 3H, CH3O-6), 2.78~2.80 (m, 1H, H-10), 2.45~2.48 (m, 2H, H-15), 2.03 (s, 3H, H-23), 1.80 (s, 3H, H-24), 1.77~1.78 (m, 2H, H-13), 1.75~1.76 (m, 1H, H-14), 0.99 (d, J=6.8 Hz, 3H, H-25), 0.98 (s, 3H, H-26); 13C NMR (125 MHz, CDCl3) δ: 184.3 (C, C-21), 183.1 (C, C-18), 168.1 (C, C-1), 156.0 (C, H2NCO2-7), 153.0 (C, C-17), 140.4 (C, C-20), 136.9 (CH, C-5), 134.6 (C, C-2), 133.3 (C, C-8), 133.1 (CH, C-9), 127.2 (CH, C-3), 126.2 (CH, C-4), 117.4 (C, C-16), 108.2 (C, C-19), 81.8 (CH, C-7), 81.4 (CH, C-6), 80.9 (CH, C-12), 72.6 (CH, C-11), 57.3 (CH3, CH3O-6), 56.7 (CH3, CH3O-12), 34.4 (CH2, C-13), 32.7 (CH2, C-15), 32.2 (CH, C-10), 28.0 (CH, C-14), 23.2 (CH3, C-26), 12.8 (CH3, C-24), 12.5 (CH3, C-23), 12.3 (CH3, C-25); ESI-MS m/z: 569.4 [M+Na]+, 585.3 [M+K]+.
尼日利亚菌素 (5):白色粉末. m.p. 181~184 ℃(文献值[37] 183~185℃); [α]D25+36.2 (c 0.8, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 4.30~4.33 (m, 1H, H-9), 4.11~4.15 (m, 1H, H-30a), 4.05~4.07 (m, 1H, H-7), 3.90 (dd, J=2.3, 10.2 Hz, 1H, H-24), 3.74~3.76 (m, 1H, H-25), 3.70~3.73 (m, 1H, H-30b), 3.44~3.48 (m, 1H, H-21), 3.44~3.45 (m, 1H, H-3), 3.36 (s, 1H, H-17), 3.32 (s, 3H, CH3O-11), 3.31~3.32 (m, 1H, H-11), 2.41 (dt, J=4.6, 13.7 Hz, 1H, H-2), 2.32~2.34 (m, 1H, H-23), 2.29~2.31 (m, 1H, H-14), 2.21~2.22 (m, 1H, H-10), 2.21~2.22 (m, 1H, H-22), 2.14~2.15 (m, 1H, H-19a), 2.10~2.12 (m, 1H, H-8a), 1.95~1.96 (m, 1H, H-5), 1.94~1.95 (m, 1H, H-6), 1.76~1.78 (m, 1H, H-28), 1.74~1.76 (m, 1H, H-4), 1.71~1.72 (m, 1H, H-15a), 1.57~1.59 (m, 1H, H-15b), 1.47~1.51 (m, 1H, H-18a), 1.44~1.47 (m, 1H, H-12), 1.41~1.43 (m, 1H, H-18b), 1.40~1.41 (m, 1H, H-5), 1.37~1.39 (m, 1H, H-10), 1.38 (s, 3H, H-35), 1.35~1.36 (m, 2H, H-27), 1.33~1.34 (m, 1H, H-26), 1.23~1.25 (m, 1H, H-19b), 1.10~1.13 (m, 1H, H-23), 1.10 (m, 1H, H-6), 1.04 (s, 3H, H-34), 1.02 (d, J=7.0 Hz, 1H, H-39), 0.98 (d, J=7.0 Hz, 1H, H-37), 0.91~0.92 (m, 1H, H-8b), 0.89 (d, J=6.9 Hz, 1H, H-36), 0.88 (d, J=6.5 Hz, 1H, H-38), 0.86 (d, J=6.2 Hz, 1H, H-33), 0.83 (d, J=6.5 Hz, 3H, H-31), 0.82 (d, J=4.2 Hz, 3H, H-32); 13C NMR (125 MHz, CDCl3) δ: 177.2 (C, C-1), 108.1 (C, C-13), 97.0 (C, C-29), 86.1 (CH, C-21), 83.7 (C, C-20), 82.3 (CH, C-17), 81.5 (C, C-16), 78.4 (CH, C-11), 77.3 (CH, C-25), 76.4 (CH, C-24), 73.1 (CH, C-3), 69.2 (CH, C-7), 68.2 (CH2, C-30), 60.3 (CH, C-9), 57.8 (CH3, CH3O-11), 44.1 (CH, C-2), 42.2 (CH2, C-15), 39.1 (CH, C-14), 37.4 (CH2, C-27), 37.1 (CH, C-28), 35.7 (CH, C-12), 35.4 (CH, C-22), 35.3 (CH2, C-8), 32.8 (CH2, C-10), 32.2 (CH2, C-23), 31.4 (CH, C-26), 30.8 (CH2, C-19), 27.7 (CH, C-4), 27.3 (CH3, C-35), 26.1 (CH2, C-5), 25.9 (CH2, C-18), 23.2 (CH2, C-6), 22.9 (CH3, C-34), 17.4 (CH3, C-32), 16.3 (CH3, C-31), 15.7 (CH3, C-33), 13.2 (CH3, C-36), 13.1 (CH3, C-37), 13.0 (CH3, C-39), 10.9 (CH3, C-38); ESI-MS m/z: 747.6 [M+Na]+, 763.6 [M+K]+.
尼日利亚菌素钠 (6):白色粉末状固体. m.p. 252~256 ℃; [α]D25+30.1 (c 0.8, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 4.34~4.37 (m, 1H, H-24), 4.33 (d, J=3.7 Hz, 1H, H-21), 4.24~4.27 (m, 1H, H-9), 4.24~4.27 (m, 1H, H-30a), 4.04 (d, J=13.6 Hz, 1H, H-7), 3.90~3.92 (m, 1H, H-30b), 3.73~3.74 (m, 1H, H-25), 3.69~3.70 (m, 1H, H-3), 3.66 (s, 1H, H-17), 3.61 (s, 3H, CH3O-11), 3.34~3.36 (m, 1H, H-11), 2.58~2.60 (m, 1H, H-8a), 2.36~2.38 (m, 1H, H-23), 2.36~2.38 (m, 1H, H-2), 2.30~2.31 (m, 1H, H-10), 2.28~2.29 (m, 1H, H-22), 2.18~2.21 (m, 1H, H-14), 2.16~2.17 (m, 1H, H-19a), 1.99~2.00 (m, 1H, H-5), 1.97~1.98 (m, 1H, H-6), 1.82~1.83 (m, 1H, H-18a), 1.78~1.79 (m, 1H, H-15a), 1.76~1.77 (m, 1H, H-12), 1.73~1.74 (m, 1H, H-4), 1.63~1.65 (m, 1H, H-15b), 1.61 (s, 3H, H-35), 1.52~1.53 (m, 1H, H-18b), 1.49~1.51 (m, 1H, H-28), 1.44~1.45 (m, 1H, H-5), 1.40~1.43 (m, 1H, H-23), 1.35~1.36 (m, 2H, H-27), 1.32~1.33 (m, 1H, H-26), 1.23~1.25 (m, 1H, H-19b), 1.12 (s, 3H, H-34), 1.10~1.11 (m, 1H, H-6), 1.02 (d, J=7.0 Hz, 3H, H-39), 0.99~1.01 (m, 1H, H-10), 0.95 (d, J=7.0 Hz, 3H, H-36), 0.93 (d, J=7.0 Hz, 3H, H-38), 0.92 (d, J=6.9 Hz, 3H, H-37), 0.90~0.91 (m, H, H-8b), 0.89 (d, J=6.5 Hz, 3H, H-33), 0.87 (d, J=6.5 Hz, 3H, H-31), 0.80 (d, J=4.2 Hz, 3H, H-32); 13C NMR (125 MHz, CDCl3) δ: 183.8 (C, C-1), 107.6 (C, C-13), 97.2 (C, C-29), 85.2 (CH, C-21), 84.8 (C, C-20), 81.5 (CH, C-17), 82.3 (C, C-16), 79.5 (CH, C-11), 76.9 (CH, C-25), 76.5 (CH, C-24), 73.2 (CH, C-3), 68.4 (CH, C-7), 67.1 (CH2, C-30), 60.4 (CH, C-9), 59.5 (CH3, CH3O-11), 45.9 (CH, C-2), 41.8 (CH2, C-15), 39.6 (CH, C-14), 37.2 (CH2, C-27), 36.8 (CH, C-28), 36.6 (CH, C-12), 35.2 (CH, C-22), 35.8 (CH2, C-8), 32.1 (CH2, C-10), 32.4 (CH2, C-23), 31.9 (CH, C-26), 29.6 (CH2, C-19), 27.7 (CH, C-4), 29.1 (CH3, C-35), 26.3 (CH2, C-5), 25.9 (CH2, C-18), 23.6 (CH2, C-6), 22.8 (CH3, C-34), 17.0 (CH3, C-32), 16.4 (CH3, C-31), 16.2 (CH3, C-33), 13.4 (CH3, C-36), 13.2 (CH3, C-37), 13.1 (CH3, C-39), 11.6 (CH3, C-38); ESI-MS m/z: 723.5 [M-Na]-.
30-O-乙酰基尼日利亚菌素 (7):无色油状. [α]D25+29.5 (c 0.78, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 4.32~4.33 (m, 1H, H-24), 4.29~4.30 (m, 1H, H-30a), 4.17 (m, 1H, H-9), 4.09~4.10 (m, 1H, H-7), 3.96 (d, J=3.5 Hz, 1H, H-21), 3.94~3.95 (m, 1H, H-25), 3.91 (d, J=10.2 Hz, 1H, H-30b), 3.77 (d, J=9.9 Hz, 1H, H-3), 3.52 (dd, J=5.1, 10.0 Hz, 1H, H-17), 3.39 (s, 3H, CH3O-11), 3.38 (m, 1H, H-11), 2.45~2.50 (m, 1H, H-8a), 2.33~2.35 (m, 1H, H-2), 2.29~2.31 (m, 2H, H-10), 2.24~2.25 (m, 1H, H-22), 2.22~2.23 (m, 1H, H-23a), 2.15~2.17 (m, 1H, H-19a), 2.09 (s, 3H, CH3CO2-30), 2.07~2.08 (m, 1H, H-14), 2.05~2.06 (m, 2H, H-6), 1.96~1.98 (m, 1H, H-5), 1.82~1.83 (m, 1H, H-4), 1.80~1.81 (m, 1H, H-18a), 1.78~1.79 (m, 1H, H-12), 1.75~1.76 (m, 1H, H-15a), 1.66~1.67 (m, 1H, H-5), 1.65 (d, J=6.3 Hz, 3H, H-31), 1.56~1.59 (m, 1H, H-15b), 1.48~1.49 (m, 1H, H-18b), 1.46~1.47 (m, 2H, H-27), 1.41~1.43 (m, 1H, H-28), 1.39 (s, 3H, H-35), 1.37~1.38 (m, 1H, H-26), 1.36~1.37 (m, 1H, H-19b), 1.35~1.36 (m, 1H, H-23b), 1.11 (s, 3H, H-34), 1.05 (d, J=11.3 Hz, 3H, H-39), 0.98 (d, J=7.0 Hz, 3H, H-36), 0.97~0.98 (m, 1H, H-8b), 0.93 (d, J=7.0 Hz, 3H, H-38), 0.89 (d, J=6.9 Hz, 3H, H-37), 0.86 (d, J=6.2 Hz, 3H, H-33), 0.84 (d, J=10.4 Hz, 3H, H-32); 13C NMR (125 MHz, CDCl3) δ: 176.3 (C, C-1), 171.2 (C, CH3CO2-30), 108.1 (C, C-13), 95.8 (C, C-29), 85.6 (CH, C-21), 83.6 (C, C-20), 82.5 (CH, C-17), 81.5 (C, C-16), 78.1 (CH, C-11), 77.2 (CH, C-24), 75.1 (CH, C-25), 73.2 (CH, C-3), 69.0 (CH, C-7), 68.2 (CH2, C-30), 60.3 (CH, C-9), 57.5 (CH3, CH3O-11), 44.1 (CH, C-2), 42.5 (CH2, C-15), 39.0 (CH, C-14), 37.1 (CH, C-12), 36.7 (CH2, C-27), 35.6 (CH, C-28), 35.5 (CH2, C-8), 35.2 (CH, C-22), 32.4 (CH, C-26), 32.3 (CH2, C-23), 31.8 (CH2, C-10), 30.8 (CH2, C-19), 27.7 (CH3, C-35), 27.6 (CH, C-4), 26.1 (CH2, C-18), 25.9 (CH2, C-5), 23.4 (CH2, C-6), 22.8 (CH3, C-34), 21.0 (CH3, CH3CO2-30), 17.4 (CH3, C-32), 16.2 (CH3, C-31), 15.9 (CH3, C-33), 13.2 (CH3, C-36), 13.1 (CH3, C-37), 12.9 (CH3, C-39), 10.9 (CH3, C-38); ESI-MS m/z: 789.7 [M+Na]+.
奥布菌素 (8):白色粉末. m.p. 81~84 ℃(文献值[38] 83~85 ℃); [α]D25+43 (c 0.03, CH3OH); 1H NMR (500 MHz, CDCl3) δ: 6.64 (d, J=10.0 Hz, 1H, H-3), 4.30 (dd, J=2.3, 10.2 Hz, 1H, H-24), 4.24~4.26 (m, 1H, H-9), 4.05 (d, J=3.7 Hz, 1H, H-21), 3.87 (d, J=2.6 Hz, 1H, H-7), 3.79~3.82 (m, 1H, H-25), 3.63 (d, J=11.0 Hz, 1H, H-30a), 3.57 (d, J=11.0 Hz, 1H, H-30b), 3.51 (s, 1H, H-17), 3.35 (s, 3H, CH3O-11), 3.27~3.30 (m, 1H, H-11), 2.50~2.51 (m, 1H, H-4), 2.27~2.28 (m, 1H, H-14), 2.25~2.26 (m, 1H, H-22), 2.21~2.22 (m, 1H, H-23), 2.14~2.15 (m, 1H, H-19a), 1.95~1.96 (m, 1H, H-15a), 1.84~1.85 (m, 2H, H-10), 1.81 (s, 3H, H-36), 1.77~1.78 (m, 1H, H-12), 1.76~1.77 (m, 1H, H-18a), 1.75~1.75 (m, 1H, H-5), 1.63~1.64 (m, 1H, H-28), 1.55~1.58 (m, 2H, H-6), 1.52~1.53 (m, 1H, H-18b), 1.47~1.48 (m, 1H, H-23), 1.45~1.46 (m, 1H, H-8a), 1.44~1.45 (m, 1H, H-15b), 1.42~1.43 (m, 2H, H-27), 1.40~1.41 (m, 1H, H-26), 1.40~1.41 (m, 1H, H-5), 1.40~1.41 (m, 1H, H-8b), 1.31 (s, 3H, H-35), 1.24~1.25 (m, 1H, H-19b), 1.02 (d, J=2.0 Hz, 3H, H-39), 1.00 (d, J=6.5 Hz, 3H, H-38), 0.99 (s, 3H, H-34), 0.90 (d, J=7.1 Hz, 3H, H-33), 0.89 (d, J=2.0 Hz, 1H, H-37), 0.87 (d, J=6.0 Hz, 3H, H-32), 0.83 (d, J=6.5 Hz, 1H, H-31); 13C NMR (125 MHz, CDCl3) δ: 172.2 (C, C-1), 149.9 (CH, C-3), 126.1 (C, C-2), 108.6 (C, C-13), 97.2 (C, C-29), 86.4 (CH, C-21), 84.1 (C, C-16), 84.0 (C, C-20), 82.0 (CH, C-17), 78.3 (CH, C-11), 77.4 (CH, C-25), 76.2 (CH, C-24), 71.5 (CH, C-7), 67.9 (CH2, C-30), 65.8 (CH, C-9), 58.2 (CH3, CH3O-11), 42.4 (CH2, C-6), 42.4 (CH2, C-15), 39.6 (CH, C-14), 37.0 (CH2, C-8), 37.0 (CH2, C-27), 36.9 (CH, C-12), 35.6 (CH, C-22), 34.9 (CH, C-28), 33.7 (CH, C-4), 33.4 (CH2, C-23), 33.1 (CH2, C-10), 32.7 (CH, C-26), 32.6 (CH2, C-5), 32.0 (CH2, C-19), 25.8 (CH3, C-35), 25.8 (CH2, C-18), 23.0 (CH3, C-34), 20.3 (CH3, C-38), 17.5 (CH3, C-32), 16.2 (CH3, C-37), 15.5 (CH3, C-33), 13.5 (CH3, C-31), 13.3 (CH3, C-39), 12.5 (CH3, C-36); ESI-MS m/z: 747.5 [M+Na]+.
洋橄榄叶素 (9):白色粉末. m.p. 176~182 ℃(文献值[39] 178~183 ℃); [α]D25-47 (c 0.1, CHCl3); 1H NMR (500 MHz, CDCl3) δ: 6.98 (dd, J=15.2, 11.2 Hz, 1H, H-3), 6.12 (dd, J=15.1, 11.2 Hz, 1H, H-4), 5.65 (d, J=15.2 Hz, 1H, H-2), 5.64 (dd, J=15.1, 10.2 Hz, 1H, H-5), 5.09 (brs, 1H, H-22), 4.74 (d, J=10.3 Hz, 1H, H-7), 4.14~4.16 (m, 1H, H-13), 4.02~4.03 (m, 1H, H-26), 4.00~4.01 (m, 1H, H-24), 3.98~3.99 (m, 1H, H-9), 3.98~3.99 (m, 1H, H-15), 3.65~3.66 (m, 1H, H-25), 2.52~2.55 (m, 1H, H-6), 2.39 (dd, J=11.5, 4.4 Hz, 1H, H-12), 1.95~1.96 (m, 1H, H-8), 1.83 (dd, J=11.5, 4.8 Hz, 2H, H-23), 1.74~1.75 (m, 1H, H-10), 1.64~1.66 (m, 1H, H-20), 1.45~1.48 (m, 1H, H-20), 1.27 (d, J=6.9 Hz, 3H, H-27), 1.20~1.22 (m, 1H, H-14), 1.10 (d, J=6.3 Hz, 3H, H-16), 1.05 (d, J=7.1 Hz, 3H, H-27), 1.05 (d, J=7.1 Hz, 3H, H-17), 1.00~1.03 (m, 1H, H-12), 0.99 (d, J=6.9 Hz, 3H, H-19), 0.85 (t, J=7.6 Hz, 3H, H-21), 0.84 (d, J=6.8 Hz, 3H, H-18); 13C NMR (125 MHz, CDCl3) δ: 170.2 (C, C-1), 145.2 (CH, C-5), 144.0 (CH, C-3), 132.0 (CH, C-4), 121.1 (CH, C-2), 99.2 (C, C-11), 93.4 (CH, C-22), 77.9 (CH, C-7), 71.5 (CH, C-25), 70.8 (CH, C-13), 70.2 (CH, C-9), 66.7 (CH, C-15), 66.1 (CH, C-26), 66.0 (CH, C-24), 48.5 (CH, C-14), 41.8 (CH, C-10), 41.2 (CH, C-6), 39.0 (CH2, C-12), 36.0 (CH, C-8), 33.6 (CH2, C-23), 19.5 (CH2, C-20), 19.3 (CH3, C-16), 16.9 (CH3, C-27), 15.1 (CH3, C-17), 9.3 (CH3, C-18), 8.9 (CH3, C-21), 7.0 (CH3, C-19);对称结构, NMR仅出现一半; ESI-MS m/z 1047.6 [M+Na]+.
Surugapyrone A (10):无色油状. 1H NMR (500 MHz, DMSO-d6) δ: 11.23 (s, 1H, 4-OH), 6.00 (s, 1H, H-5), 2.65~2.68 (m, 1H, H-7), 1.74 (s, 3H, H-10), 1.13 (d, J=6.9 Hz, 6H, H-8/9); 13C NMR (125 MHz, DMSO-d6) δ:167.4 (C, C-2/6), 165.5 (C, C-4), 97.5 (C, C-3), 97.1 (CH, C-5), 32.1 (CH, C-7), 20.3 (CH3, C-8/9), 8.9 (CH3, C-10); ESI-MS m/z: 169.1 [M+H]+.
杀菌素A (11):无色油状. [α]D25+26 (c 0.04, CHCl3); 1H NMR (500 MHz, DMSO-d6) δ: 11.27 (s, 1H, 4-OH), 5.99 (s, 1H, H-5), 2.43~2.45 (m, 1H, H-11), 2.27 (dd, J=7.3, 14.6 Hz, 1H, H-11), 1.54~1.57 (m, 1H, H-8a), 1.43~1.47 (m, 1H, H-8b), 1.10 (d, J=6.9 Hz, 3H, H-10), 0.95 (t, J=7.3 Hz, 3H, H-9), 0.82 (t, J=7.4 Hz, 3H, H-12); 13C NMR (125 MHz, DMSO-d6) δ: 166.6 (C, C-2), 164.7 (C, C-4/6), 102.8 (C, C-3), 98.5 (CH, C-5), 38.7 (CH, C-9), 26.8 (CH2, C-11), 17.6 (CH3, C-10), 16.1 (CH2, C-7), 12.6 (CH3, C-8), 11.4 (CH3, C-12); ESI-MS m/z: 195.1 [M-H]-.
3.5 化合物活性测试
以慢性髓性白血病K562、乳腺癌细胞MCF-7和肺癌细胞A549细胞为模型, 以阿霉素为阳性对照药.采用CCK-8和MTT法对化合物进行体外细胞增殖抑制活性测试.
MTT法[40, 41]:活性测试时, 取对数生长期的MCF-7和A549细胞, 用新鲜的RPMI-1640培养基配制成密度为每毫升3×104个细胞的细胞悬液, 按每孔100 μL接种于96孔板中, 在37 ℃下培养12 h后, 然后在每个孔中加100 μL稀释的待测样品 (做好阳性与阴性对照), 混匀后置于培养箱中培养72 h.然后每孔加20 μL MTT染色剂 (5 mg/L), 再培养4 h, 移出培养液后加入150 μL DMSO溶解formazan, 振荡10~15 min, 在570 nm处测定其吸收度.按照下式计算每个浓度下的细胞增殖抑制率:抑制率 (%)=(OD空白对照-OD样品)/OD空白对照×100%.对肿瘤细胞MCF-7和A549有较好抑制率的化合物 (10 μmol·L-1浓度时抑制率大于50%), 再设置不同梯度浓度计算化合物对肿瘤细胞的IC50, 每个样品的每个测量浓度设置3个平行.
CCK-8法[42, 43]:活性测试时, 取对数生长期的K562细胞, 用新鲜的RPMI-1640培养基配制成密度为每毫升3×104个细胞的细胞悬液, 按每孔100 μL接种于96孔板中, 在37 ℃下培养12 h后, 然后在每个孔中加100 μL稀释的待测样品 (做好阳性与阴性对照), 混匀后置于培养箱中培养72 h.然后加入10 μL CCK-8溶液, 再培养6 h, 在450 nm处测定其吸收度.按照公式:抑制率 (%)=(OD空白对照-OD样品)/OD空白对照×100%, 计算每个浓度下的细胞增殖抑制率.对肿瘤细胞K562细胞有较好抑制率的化合物 (10 μmol·L-1浓度时抑制率大于50%), 再设置不同梯度浓度计算化合物对肿瘤细胞的IC50, 每个样品的每个测量浓度设置3个平行.
辅助材料 (Supporting Information) 化合物1的原始谱图, 菌株S. malaysiensis OUCMDZ-2167的基因组的DNA电泳图, 扩增产物电泳图以及系统发育树状关系图.这些材料可以免费从本刊网站 (http://sioc-journal.cn/) 上下载.
-
-
[1]
王聪, 梅显贵, 朱伟明, 海洋科学集刊, 2016, 51, 86.Wang, C.; Mei, X.-G.; Zhu, W.-M. Stud. Mar. Sin. 2016, 51, 86 (in Chinese).
-
[2]
赵成英, 朱统汉, 朱伟明, 有机化学, 2013, 33, 1195. doi: 10.6023/cjoc201304039Zhao, C.; Zhu, T.; Zhu, W. Chin. J. Org. Chem. 2013, 33, 1195 (in Chinese). doi: 10.6023/cjoc201304039
-
[3]
Fu, P.; Yang, C.; Wang, Y.; Liu, P.; Ma, Y.; X, L.; Su, M.; Hong, K.; Zhu, W. Org. Lett. 2012, 14, 2422. doi: 10.1021/ol3008638
-
[4]
Fu, P.; Zhuang, Y.; Wang, Y.; Liu, P.; Qi, X.; Gu, K.; Zhang, D.; Zhu, W. Org. Lett. 2012, 14, 6194. doi: 10.1021/ol302940y
-
[5]
王立平, 庄义彬, 孙坤来, 朱伟明, 有机化学, 2014, 34, 1603. doi: 10.6023/cjoc201403046Wang, L.; Zhuang, Y.; Sun, K.; Zhu, W. Chin. J. Org. Chem. 2014, 34, 1603 (in Chinese). doi: 10.6023/cjoc201403046
-
[6]
Wang, L.; Mei, X.; Wang, C.; Zhu, W. Tetrahedron 2015, 71, 7990. doi: 10.1016/j.tet.2015.08.065
-
[7]
Wang, Y.; Wang, L.; Zhuang, Y.; Kong, F.; Zhang, C.; Zhu, W. Mar. Drugs 2014, 12, 2079. doi: 10.3390/md12042079
-
[8]
Fu, P.; Kong, F; Wang, Y.; Wang, Y.; Liu, P.; Zuo, G.; Zhu, W. Chin. J. Chem. 2013, 31, 100. doi: 10.1002/cjoc.201201062
-
[9]
Wang, P.; Xi, L.; Wang, Y.; Wang, W.; Huang, Y.; Zhu, W. Mar. Drugs 2013, 11, 1035. doi: 10.3390/md11041035
-
[10]
Al-Tai, A.; Kim, B.; Kim, S. B.; Manfio, G. P.; Googfellow, M. Int. J. Syst. Evol. Microbiol. 1999, 49, 1395.
-
[11]
刘为营, 殷瑜, 钱秀萍, 戈梅, 阮林高, 许激扬, 药物生物技术, 2011, 18, 322.Liu, W.-Y.; Yin, Y.; Qian, X.-P.; Ge, M.; Ruan, L.-G.; Xu, J.-Y. Pharm. J. Biotechnol. 2011, 18, 322 (in Chinese).
-
[12]
Simkhada, D.; Zhang, H.; Mori, S.; Williams, H.; Watanabe, C. M. H. Beilstein J. Org. Chem. 2013, 9, 1768. doi: 10.3762/bjoc.9.205
-
[13]
DeBoer, C.; Meulman, P. A.; Wnuk, R. J.; Peterson, D. H. J. Antibiot. 1970, 23, 442. doi: 10.7164/antibiotics.23.442
-
[14]
石妞妞, 王浩鑫, 鲁春华, 刘最, 沈月毛, 中国药学杂志, 2011, 46, 1317.Shi, N.-N.; Wang, H.-X.; Lu, C.-H.; Liu, Z.; Shen, Y.-M. Chin. Pharm. J. 2011, 46, 1317 (in Chinese).
-
[15]
Berrada, R.; Dauphin, G.; David, L. J. Org. Chem. 1987, 52, 2388. doi: 10.1021/jo00388a009
-
[16]
Alva, R.; Lugo-R. J. A.; Arzt, E.; Cerbón, J.; Rivera, B. E, Toro, M.; Estrada-O, S. J. Bioenerg. Biomembr. 1992, 24, 125. doi: 10.1007/BF00769539
-
[17]
牛四文, 李苏梅, 田新朋, 胡涛, 鞠建华, 杨晓红, 张偲, 张长生, 中国中药杂志, 2011, 36, 1763.Niu, S.; Li, S.; Tian, X.; Hu, T.; Ju, J.; Yang, X.; Zhang, S.; Zhang, C. Chin. Pharm. J. 2011, 36, 1763 (in Chinese).
-
[18]
Wu, Z.; Bai, L.; Wang, M.; Shen, Y. Chem. Nat. Compd. 2009, 45, 333. doi: 10.1007/s10600-009-9350-x
-
[19]
David, L.; Ayala, H. L. J. Antibiot. 1985, 38, 1655. doi: 10.7164/antibiotics.38.1655
-
[20]
龚雨梅, 陈军, 杨涛, 李宗珍, 李国友, 应用与环境生物学报, 2010, 16, 261. doi: 10.3724/SP.J.1145.2010.00261Gong, Y.; Chen, J.; Yang. T.; Li, Z.; Li, G. Chin. J. Appl. Environ. Biol. 2010, 16, 261 (in Chinese doi: 10.3724/SP.J.1145.2010.00261
-
[21]
张云, 周潇, 宋永相, 陈芸芸, 黄洪泼, 李杰, 华燕, 鞠建华, 天然产物研究与开发, 2013, 25, 185.Zhang, Y.; Zhou, X.; Song, Y.-X.; Chen, Y.-Y.; Huang, H.-P.; Li. J.; Hua, Y.; Ju, J.-H. Nat. Prod. Res. Dev. 2013, 25, 185 (in Chinese).
-
[22]
Sugiyama, Y.; Oya, A.; Kudo, T.; Hirota, A. J. Antibiot. 2010, 63, 365. doi: 10.1038/ja.2010.60
-
[23]
Song, L.; Barona-Gomez, F.; Corre, C.; Xiang, L.-K.; Udwary, D. W.; Austin. M. B.; Noel, J. P.; Moore, B. S.; Challis, G. L. J. Am. Chem. Soc. 2006, 128, 14754. doi: 10.1021/ja065247w
-
[24]
Aoki, Y.; Matsumoto, D.; Kawaide, H.; Natsume, M. J. Antibiot. 2011, 64, 607. doi: 10.1038/ja.2011.59
-
[25]
练富林, 林东海, 顾觉奋, 国外医药抗生素分册, 2010, 31, 203.Lian, F.-L.; Lin, D.-H.; Gu, J.-F. World Notes Antibiot. 2010, 31, 203 (in Chinese
-
[26]
Murphy, P.; Sharp, A.; Shin, J.; Gavrilyuk, V.; Russo, C. D.; Weinberg, G.; Sharp, F. R.; Lu, A.; Heneka, M. T.; Feinstein, D. L. J. Neurosci. Res. 2002, 67, 461. doi: 10.1002/(ISSN)1097-4547
-
[27]
Goetz, M. P.; Toft, D.; Reid, J.; Ames, M.; Stensgard, B.; Safgren, S.; Adjei, A. A.; Sloan, J.; Atherton, P.; Vasile, V.; Salazaar, S.; Adjei, A.; Croghan, G.; Erlichman, C. J. Clin. Oncol. 2005, 23, 1078. doi: 10.1200/JCO.2005.09.119
-
[28]
Adovelande, J.; Schrével, J. Life Sci. 1996, 59, PL309.
-
[29]
Gupta, P. B.; Onder, T. T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R. A.; Lande, E. S. Cell 2009, 138, 645. doi: 10.1016/j.cell.2009.06.034
-
[30]
Cleary, I.; Doherty, G; Moran, E.; Clynes, M. Biochem. Pharmacol. 1997, 53, 1493. doi: 10.1016/S0006-2952(97)00003-8
-
[31]
Dalisay, D. S.; Williams, D. E.; Wang, X.-L.; Centko, R.; Chen, J.; Andersen, R. J. PLoS One 2013, 8, e77078. doi: 10.1371/journal.pone.0077078
-
[32]
王聪, 刘培培, 王乂, 孙坤来, 贾海健, 朱伟明, 中国海洋药物, 2014, 33, 34.Wang, C.; Liu, P.-P.; Wang, Y.; Sun, K.-L.; Jia, H.-J.; Zhu, W.-M. Chin. J. Mar. Drug. 2014, 33, 34 (in Chinese).
-
[33]
Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. Mol. Biol. Evol. 2007, 24, 1596. doi: 10.1093/molbev/msm092
-
[34]
Richard, K.; Gerd, K. Cher. Ber, 1957, 90, 264. doi: 10.1002/(ISSN)1099-0682
-
[35]
朱宝泉, 胡海峰, 张琴, 尚坷, 刘宏伟, 王毅华, 中国天然产物, 2003, 1, 54.Zhu, B.-Q.; Hu, H.-F.; Zhang, Q.; Shang, K.; Liu, H.-W.; Wang, Y.-H. Chin. J. Nat. Med. 2003, 1, 54 (in Chinese).
-
[36]
Hong, Y. S.; Lee, D.; Kim, W.; Jeong, J. K.; Kim, C. G.; Sohng, J. K.; Lee, J. H.; Paik, S. J.; Lee, J.J. J. Am. Chem. Soc. 2004, 126, 11142. doi: 10.1021/ja047769m
-
[37]
Kubota, T.; Matsutani, S.; Shiro, M.; Koyama, H. Chem. Common. 1968, 23, 1541.
-
[38]
David, L.; Ayala, H. L.; Tabet, J. C. J. Antibiot. 1985, 38, 1655. doi: 10.7164/antibiotics.38.1655
-
[39]
严淑玲, 黄为一, 微生物学通报, 2002, 29, 103.Yan, S.-L.; Huang, W.-Y. Pharm. Acta Microbiol. Sin. 2002, 29, 103 (in Chinese).
-
[40]
Foongladda, S.; Roengsanthia, D.; Arjrattanakool, W.; Chuchottaworn, C.; Chaiprasert, A.; Franzblau, S. G. Int. J. Tuberc. Lung D 2002, 6, 1118.
-
[41]
王文玲, 王立平, 王聪, 刘海珊, 郝杰杰, 朱伟明, 中国海洋药物, 2015, 34, 1.Wang, W.-L.; Wang, L.-P.; Wang, C.; Liu, H.-S.; Hao, J.-J.; Zhu, W.-M. Chin. J. Mar. Drugs 2015, 34, 1 (in Chinese).
-
[42]
Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; . Suzuki, K.; Watanabe, M. Anal. Commun. 1999, 36, 47. doi: 10.1039/a809656b
-
[43]
侯春梅, 李新颖, 叶伟亮, 曹曦元, 肖鹤, 黎燕, 军事医学科学院院刊, 2009, 33, 400.Hou, C.-M.; Li, X.-Y.; Ye, W.-L.; Cao, X.-Y.; Xiao, H.; Li, Y. Bull. Acad. Milit. Med. Sci. 2009, 33, 400 (in Chinese).
-
[1]
-
表 1 化合物1的1H NMR、13C NMR和HMBC数据 (DMSO-d6)
Table 1. 1H NMR, 13C NMR and HMBC data of compound 1 (DMSO-d6)
Position δC, type δH (J in Hz) HMBC (1H→13C) 2 144.0, qC 3 118.2, CH 7.26 (s) C-2, C-4, C-5 4 128.9, qC 5 143.7, CH 7.84 (s) C-3, C-4 2-CO2CH3 158.4, qC 2-CO2CH3 51.8, CH3 3.82 (s) 2-CO2 4-CH2OH 54.3, CH2 4.39 (d, 5.0 Hz) C-3, C-4, C-5 4-CH2OH 5.15 (t, 5.0 Hz) 表 2 化合物1~11对A549、MCF-7和K562细胞株的细胞毒活性
Table 2. The cytotoxicities of compounds 1~11 against A549, MCF-7 and K562 cell lines
化合物 A549 MCF-7 K562 抑制率/% IC50/(μmol·L-1) 抑制率/% IC50/(μmol·L-1) 抑制率/% IC50/(μmol·L-1) 1 -4.4±0.5 >10 3.2±0.3 >10 6.6±0.5 >10 2 7.3±0.7 >10 4.4±0.4 >10 9.7±1.0 >10 3 78.7±2.5 2.56±0.30 39.6±1.6 >10 68.7±2.3 >10 4 37.8±1.0 >10 8.7±0.4 >10 34.2±1.5 >10 5 83.0±1.1 0.96±0.03 78.4±2.1 0.29±0.15 84.6±0.8 1.46±0.11 6 86.9±3.3 0.96±0.04 21.4±0.4 >10 84.7±0.2 1.08±0.03 7 85.4±0.8 0.81±0.05 39.1±3.3 >10 84.3±1.2 0.48±0.10 8 73.8±0.9 7.11±0.90 67.2±5.0 4.05±0.50 77.9±0.5 4.68±0.19 9 55.6±2.3 4.09±0.70 69.5±3.6 4.81±0.70 80.5±0.6 9.60±0.50 10 2.0±0.4 >10 4.1±0.1 >10 -41.8±2.0 >10 11 4.4±0.5 >10 2.9±0.8 >10 -55.5±1.1 >10 阿霉素 77.6±0.05 0.81±0.04 72.1±0.3 0.20±0.03 81.9±0.2 0.32±0.11 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 10
- 文章访问数: 2826
- HTML全文浏览量: 274


 下载:
下载:

 下载:
下载:

