 Figure Scheme1.
Mechanism of the activation of the target compound
Figure Scheme1.
Mechanism of the activation of the target compound

Synthesis and Crystal Structure of N1-(2-(4-Bromo-2-nitrophenoxy)-2-dimethyl acyloxymethyl)-5-fluorouracil①
English
Synthesis and Crystal Structure of N1-(2-(4-Bromo-2-nitrophenoxy)-2-dimethyl acyloxymethyl)-5-fluorouracil①
-
1 INTRODUCTION
It's known to all that the anti-metabolism drug 5-fluorouracil (5-Fu) was first introduced in 1957, which still remains an essential part of the treatment of a wide range of solid tumors, such as colorectal cancer, stomach cancer, breast cancer and so on[1, 2]. The action mechanism of 5-Fu is associated with noncompetitive inhibition of thymidylate synthase (TS) and the incorporation of 5-Fu into RNA and DNA[3]. As a pyrimidine analogue, it is transformed into different cytotoxic metabolites which are then incorporated into RNA and DNA, and finally induces cell cycle arrest and apoptosis by inhibiting the ability of cell to synthesize DNA. However, as an anticancer agent, 5-Fu also leads to several serious side effects including myelotoxicity, gastrointestinal reaction, leucopenia, thrombocytopenia, etc[4, 5]. In order to reduce such side effects, a lot of researches have been done by the scientific workers among the world, leding to a burst of some new drugs, such as Tegafur, Carmofur and Capecitabine. Although the new drugs showed better biological activities and lower toxicity, the lower selectivity to tumor cells limited their applications[6, 7]. Therefore, much more studies should be further carried out to overcome this deficiency.
Owing to the primitive state of tumor vasculature, solid tumors often develop regions of chronic or acute hypoxia as a result of chronic or transient deficiency of blood flow. And the nitroreductase (NTR) in the tumor cells had higher activity compared to the normal cells[8-11], so hypoxic tumor cells have greater capacity for reductive reactions as compared to the well-oxygenated normal cells. Based on these features of tumor cells, the selectivity of anticancer drugs to tumor cells could be improved[12-14]. The title compound was synthesized as a prodrug and scheme 1 shows the potential mechanism of the activation of the compound. The target compound was reduced in the tumor cells and then the lactam was generated by cyclization reaction. After that, the active moiety 5-Fu was released. Therefore, the selectivity of this series of compounds was improved. Herein, we report the synthesis, crystal structure and biological activities of the target compound.
2 EXPERIMENTAL
2.1 Instruments and reagents
1H-NMR and 13C-NMR (δ ppm) spectra were acquired on a Varian Mercury (400MHz) using TMS as the internal standard. Mass spectra were recorded on a VGZAB-HS (70 ev) spectrometer with ESI source as ionization. X-ray diffraction was performed using a Super Nova, Dual, Cu at zero, Eos diffractometer.
Unless otherwise noted, all materials were obtained from commercial suppliers and purified by standard procedures. All the reactions were monitored by analytical thin-layer chromatography (TLC) and silica gel F254 was used in TLC. The crystals for single-crystal X-ray analysis were grown by the slow evaporation of solution of methanol-chloroform (1:5 v/v).
2.2 Preparation of 2-(4-bromo-2- nitrophenoxy)-2-methylpropanoic acid (3)
Intermediate 3 was synthesized by the route shown in Scheme 2. The 4-bromo-2-nitrophenol (5 mmoL, 1.08 g) was treated with K2CO3 (7.5 mmoL, 1.04 g) in DMF for 30 minutes and then methyl 2-bromoisobutyrate (10 mmoL, 1.80 g) was added with the reaction temperature being kept at 120 ℃ for 6 h. During the reaction, the TLC was used to detect the process. After full reaction, the mixture was filtered and the filtrate was evaporated, and then compound 2 was obtained with an excellent yield. 2 was dissolved in THF (tetrahydrofuran, 40 mL) and 10% sodium hydroxide aqueous solution (40 mL), and the mixture was stirred at room temperature (r.t.) overnight. During the reaction, the pH of the solution was kept to 10 by 10% NaOH solution. After the reaction, the THF was removed by evaporation and the residual liquid was neutralized with concentrated hydrochloric acid (pH<2). Then, the precipitate was filtrated and washed by 1 moL/L HCl, and compound 3 was obtained after filtration (light yellow solid, 80% yield). 1H-NMR (400 MHz, DMSO-d6, TMS, ppm): δ 10.88(dr, 1H, COOH), 7.93(s, 1H, Ar-H3), 7.60(d, 1H, J = 8.8Hz, Ar-H5), 7.04(d, 1H, J = 8.8Hz, Ar-H6), 1.70(s, 6H, 2CH3). 13C-NMR (100 MHz, DMSO-d6, TMS, ppm): δ 178.90(COOH), 147.41(Ar-C1), 143.62(Ar-C2), 136.04(Ar-C5), 128.11(Ar-C3), 122.66(Ar-C6), 114.75(Ar-C4), 81.92(C (OC)), 24.83(2CH3).
2.3 Synthesis of the target compound (5)
Compound 5 was synthesized by the route shown in Scheme 3 [15, 16]. 5-Fu (3 mmol, 0.39 g) was dissolved in 37% aqueous formaldehyde (6.6 mmol, 0.54 g) for 2 h at 60 ℃. Subsequently, the solution was steamed out under reduced pressure, and compound 4 (1-hydroxymethyl-5-fluorouracil) was obtained. The compound was dissolved in THF (25 ml), after which compound 3 (3.9 mmol, 1.18 g), N, N΄-dicyclohexylcarbodiimide (DCC, 5.1 mmol, 1.05 g) and 4-dimethylaminopyridine (DMAP, 0.39 mmol, 0.05 g) were added to the solution. The mixture was stirred for 4 h at room temperature. After filtration, the solution was steamed out under reduced pressure and the residue was purified via silica gel column chromatography (eluent:dichloromethane/ acetone = 12/1). Then compound 5 (white solid, 50% yield) was obtained. 1H-NMR (400 MHz, DMSO-d6, TMS, ppm): δ 12.03(s, 1H, -NH-), 8.13(t, 2H, H-C-N, Ar-H3), 7.69(d, 1H, J = 6.8, Ar-H5), 7.06(d, 1H, J = 9.2, Ar-H6), 5.72(s, 2H), 1.57(s, 6H, 2CH3). 13C-NMR (100 MHz, DMSO-d6, TMS, ppm): δ 171.51(ArOCH2CO), 157.33(C4), 149.10(C2), 146.40(Ar-C1), 143.13(Ar-C2), 138.41(C5), 136.01(Ar-C5), 128.98(C6), 127.90(Ar-C3), 121.71(Ar-C6), 113.34(Ar-C4), 81.27(CH2N), 71.70(OC), 24.59(2CH3). ESI-MS: Calcd. for C15H13BrFN3O7 [M+H]+ 445.99, found 446.0046.
2.4 Structure determination
A clear light colourless crystal of the title compound with dimensions of 0.33mm × 0.28mm × 0.24mm was selected for X-ray diffraction analysis. The data were collected on a Super Nova, Dual, Cu at zero, Eos diffractometer equipped with a graphitemonochromatic Cu-Kα radiation (λ = 1.54184 Å) using an ω-scan mode in the range of 8.232≤2θ≤ 139.592° (-6≤h≤10, -12≤k≤12, -14≤l≤13) at 300.79 K[17]. The structure was solved with the ShelXS[18] structure solution program using direct methods and refined with the ShelXL[19] refinement package using least-squares minimisation. The hydrogen of idealised -CH3 was refined as the rotating group. Besides, the secondary CH2 and aromatic/ amide H were refined with riding coordinates. The non-hydrogen atoms were located from an E-map and refined anisotropically. The final R (reflections) = 0.0517, wR = 0.1385 (w = 1/[σ2(Fo 2) + (0.0800P)2 + 0.5197P], where P = (Fo 2 + 2Fc 2)/3), S = 1.059, (Δ/σ)max = 0.000, (Δ/ρ)max = 0.427 and (Δ/ρ)min = -0.679 e/Å3.
2.5 MTT assay to measure the cell growth inhibition in vitro
The inhibitory effects on cell proliferation of the target compound were investigated by the MTT assay[20]. Hela, A549, Hep-G2 and normal cell WI-38 were chosen for the experiment. The logarithm vegetal period cells were inoculated at an appropriate concentration of 5×103/mL into 96-well plates (100 μL per well). All cell lines were maintained at 37 ℃ in 5% CO2 in RPMI-1640 medium, supplemented with 10% fetal calf serum. The solution of the compound with different concentration was added into 96-well plates after 24 h with 5-Fu as the positive group. After 48 h, 10 μL of MTT solution (5 mg/mL in PBS) was added in each well and incubated for 4 h. Then, the medium was removed and 150 μL DMSO was added to dissolve the blue-coloured formazan. The absorbance was detected at 570 nm to calculate the inhibition rate (%) and the IC50 was defined using SPSS19.0.
3 RESULTS AND DISCUSSION
We synthesized N1-(2-(4-bromo-2-nitrophenoxy)- 2-dimethyl acyloxymethyl)-5-fluorouracil with 4-bromo-2-nitrophenol, methyl 2-bromoisobutyrate and 5-fluorouracil as the starting materials. The structure of the title compound was confirmed by 1H-NMR, 13C-NMR and ESI-MS.
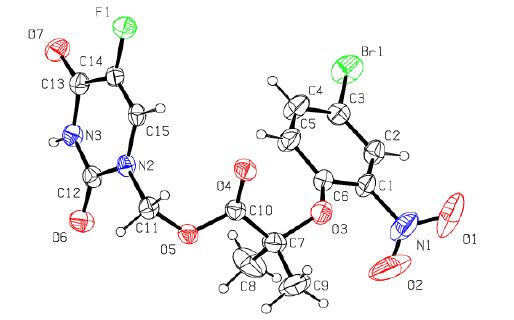
The structure of the title compound was finally confirmed by single-crystal X-ray diffraction, as shown in Fig. 1. The selected bond lengths, bond angles, π-π stacking interactions and the hydrogen bonds geometry are listed in Tables 1, 2 and 3, respectively. The hydrogen bonds and π-π stacking of the molecular structure of the title compound are shown in Fig. 2. Bond lengths and bond angles agree well with the values reported.
Bond Dist. Bond Dist. Br(1)-C(3) 1.881(3) N(2)-C(12) 1.379(3) F(1)-C(14) 1.348(3) N(2)-C(15) 1.385(4) O(3)-C(6) 1.361(3) N(3)-C(12) 1.369(4) O(3)-C(7) 1.444(4) N(3)-C(13) 1.387(4) O(4)-C(10) 1.194(4) C(1)-C(6) 1.384(5) O(5)-C(10) 1.348(4) C(1)-N(1) 1.454(5) O(5)-C(11) 1.433(4) C(5)-C(6) 1.375(5) O(6)-C(12) 1.220(4) C(7)-C(10) 1.519(5) O(7)-C(13) 1.210(4) O(1)-N(1) 1.220(6) N(2)-C(11) 1.442(4) O(2)-N(1) 1.127(7) Angle (°) Angle (°) C(6)-O(3)-C(7) 120.5(3) C(10)-C(7)-C(9) 106.9(3) C(10)-O(5)-C(11) 115.9(2) O(4)-C(10)-O(5) 123.3(3) C(12)-N(2)-C(11) 117.7(2) O(5)-C(11)-N(2) 111.1(2) C(12)-N(2)-C(15) 121.0(2) O(6)-C(12)-N(2) 121.5(3) C(15)-N(2)-C(11) 121.3(2) N(3)-C(12)-N(2) 115.5(3) C(12)-N(3)-C(13) 127.9(2) O(7)-C(13)-N(3) 122.2(3) C(6)-C(1)-N(1) 119.5(4) F(1)-C(14)-C(13) 115.7(3) C(2)-C(3)-Br(1) 119.9(3) C(14)-C(15)-N(2) 120.7(2) O(3)-C(7)-C(8) 111.9(3) O(2)-N(1)-O(1) 122.5(5) O(3)-C(7)-C(10) 109.0(3) Table 1. Selected Bond Lengths (Å) and B ond Angles (°) for the Target CompoundCg(I)Res(I)Cg(J) [ARU(J)] Cg-Cg Alpha Cgl Perp CgJ Perp Slippage Cg(2)[1]—Cg(2) [2576] 3.918(5) 0 3.575 3.575 1.605 Table 2. π-π Stacking Interactions (Å, °)D-H …A d(D-H) d(H-A) d(D-A) ZDHA N(3)-H(3)…O(6)#1 0.860 1.967 2.818 170.17 C(4)-H(4)-F(1)#2 0.930 2.573 3.312 136.64 C(9)-H(9C)…F(1)#3 0.960 2.620 3.579 177.72 C(11)-H(11A)…O(7)#4 0.970 2.559 3.352 138.96 C(15)-H(15)…O(4)#5 0.930 2.341 3.267 173.55 Symmetry codes: #1: –x+1, –y+1, –z; #2: –x+1, –y+2, –z; #3: –x, –y+2, –z; #4: x–1, y, z; #5: –x, –y+2, –z Table 3. Hydrogen Bond Lengths (Å) and Bond Angles (º)As shown in Fig. 1, the crystal structure contains two branched chains with its center placed at the midpoint of O. The phenyl ring (C(1)~C(6)) makes a dihedral angle of 85.3(1)° with the heterocyclic ring (N(2), C(12), N(3), C(13), C(14), C(15)), suggesting the two rings are non-coplanar. The bond length of C(12)-N(13) is 1.369 Å which is shorter than the normal length of C-N due to the presence of a p-π conjugative effect.
In the crystal packing, there are one intramolecular (C(5)-H(5)⋅⋅⋅O(4)) and five intermolecular (N(3)-H(3)⋅⋅⋅O(6), C(4)-H(4)⋅⋅⋅F(1), C(9)-H(9C)⋅⋅⋅F(1), C(11)-H(11A)⋅⋅⋅O(7), C(15)-H(15)⋅⋅⋅O(4)) hydrogen bonds. And two Cg2(C(1)~C(6)) rings of adjacent molecules are parallel with their centroid distances of 3.918 Å, which indicates the presence of π-π stacking in the packing. These hydrogen bonds and π-π stacking interactions make the molecules stack along the b axis. In addition, all the hydrogen bonds and π-π stacking play a major role in stabilizing the crystal and link the molecules into a three- dimensional network, as shown in Fig. 2.
The synthesized title compound was evaluated to get their anti-proliferative activity against three different kinds of tumor cells and a type of normal cell with 5-Fu as a reference compound, and the results are summarized in Table 4. It presents good inhibition profile against Hela, A549 and Hep-G2 but lower toxicity to normal cells (WI-38). What's more, the biological activity to A549 (18.09±0.6) was the best. According to the results, the toxicity to normal cells was much lower compared to the positive control. Therefore, to some extent, the target compound improved the selectivity and biological activity to cancer cells and reduced the toxicity to normal cells. Thus this series of compounds was worth exploring, and further studies are underway to research the structure activity relationships and structural modifications.
-
-
[1]
Longley, D. B.; Hapkin, D. P.; Johnston, P. G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003, 3, 330-338.
-
[2]
Shafiq S., Shakeel F., Talegaonkar S., Ahmad F. J., Khar R. K., Ali M.. Development and bioavailability assessment of ramipril nanoemulsion formulation[J]. Eur. J. Pharm. Biopharm., 2007, 66: 227-243. doi: 10.1016/j.ejpb.2006.10.014
-
[3]
Noordhuis, P.; Holwerda, U.; Van der Wilt, C. L.; Van Groeningen, C. J.; Smid, K.; Meijer, S.; Pinedo, H. M.; Peters, G. J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal. Cancers Ann. Oncol. 2004, 15, 1025-1032.
-
[4]
Malet-martino M., Martino R.. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review[J]. Oncologist., 2002, 79: 288-323.
-
[5]
Dönmez Y., Akhmetova L., İşeri Ö. D., Kars M. D., Gündüz U.. Effect of MDR modulators verapamil and promethazine on gene expression levels of MDR1 and MRP1 in doxorubicin-resistant MCF-7 cells[J]. Cancer. Chemoth. Pharm., 2011, 67: 823-828. doi: 10.1007/s00280-010-1385-y
-
[6]
Pan X. L., Wang C., Wang F., Li P., Hu Z., Shan Y., Zhang J.. Development of 5-fluorouracil derivatives as anticancer agents[J]. Curr. Med. Chem., 2011, 18: 4538-4556. doi: 10.2174/092986711797287584
-
[7]
Carrillo, E.; Navarro, S. A.; Ramírez, A.; García, M. Á.; Griñán-Lisón, C.; Perán, M.; Marchal, J. A. 5-Fluorouracil derivatives: a patent review (2012-2014). Eepert. Opin. Ther. Pat. 2015, 25, 1131-1344.
-
[8]
Dubowchik G. M., Walker M. A.. Receptor-mediated and enzyme-dependent targeting of cytotoxic anticancer drugs[J]. Pharmacol. Ther., 1999, 83: 67-123. doi: 10.1016/S0163-7258(99)00018-2
-
[9]
Rooseboom M., Commandur J. N., Vermenmeulen N. P.. Enzyme-catalyzed activation of anticancer prodrugs[J]. Pharmacol. Rev., 2004, 56: 53-102. doi: 10.1124/pr.56.1.3
-
[10]
Liu, B.; Hu, L. 5΄-(2-Nitrophenylalkanoyl)-2΄-deoxy-5-fluorouridines as potential prodrugs of FUDR for reductive activation. Bioorgan. Med. Chem. 2003, 11, 3889-3899.
-
[11]
Vaupel P. W., Frinak S., Bicher H. I.. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma[J]. Cancer. Res., 1981, 41: 2008-2013.
-
[12]
Bryant C., DeLuca M.. Purification and characterization of an oxygen-insensitive NAD (P) H nitroreductase from enterobacter cloacae[J]. Biol. Chem., 1991, 266: 4119-4125.
-
[13]
Whitmore G. F., Varghese A. J.. The biological properties of reduced nitroheterocyclics and possible underlying biochemical mechanisms[J]. Biochem. Pharmacol., 1986, 35: 97-103. doi: 10.1016/0006-2952(86)90565-4
-
[14]
Josephy P. D., Palcic B., Skarsgard L. D.. Reduction of misonidazole and its derivatives by xanthine oxidase[J]. Biochem. Pharmacol., 1981, 30: 849-853. doi: 10.1016/S0006-2952(81)80006-8
-
[15]
Dong Z., Zheng W., Xu Z., Yin Z.. Improved stability and tumor targeting of 5-fluorouracil by conjugation with hyaluronan[J]. J. Appl. Polym. Sci., 2013, 130: 927-932. doi: 10.1002/app.39247
-
[16]
Yang L., Wang M. J., Zhang Z. J., Morris-Natschke S. L., Goto M., Tian J., Liu Y. Q., Wang C. Y., Tian X., Yang X. M., Lee K. H.. Synthesis of novel spin-labeled derivatives of 5-Fu as potential antineoplastic agents[J]. Med. Chem. Res., 2014, 23: 3269-3273. doi: 10.1007/s00044-013-0906-8
-
[17]
Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K., Puschmann H... OLEX2: a complete structure solution, refinement and analysis program[J]. J. Appl. Cryst, 2009, 42: 339-341. doi: 10.1107/S0021889808042726
-
[18]
Sheldrick, G. M. SHELXS-97, Programm for the Solution of Crystal Structure. University of Göttingen 1997.
-
[19]
Sheldrick, G. M. SHELXL-97, Programm for the Refinement of Crystal Structure. University of Göttingen 1997.
-
[20]
Mueller H., Kassack M. U., Wiese M.. Comparison of the usefulness of the MTT, ATP, and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines[J]. J. Biomo. Screen., 2004, 9: 506-515. doi: 10.1177/1087057104265386
-
[1]
-
Table 1. Selected Bond Lengths (Å) and B ond Angles (°) for the Target Compound
Bond Dist. Bond Dist. Br(1)-C(3) 1.881(3) N(2)-C(12) 1.379(3) F(1)-C(14) 1.348(3) N(2)-C(15) 1.385(4) O(3)-C(6) 1.361(3) N(3)-C(12) 1.369(4) O(3)-C(7) 1.444(4) N(3)-C(13) 1.387(4) O(4)-C(10) 1.194(4) C(1)-C(6) 1.384(5) O(5)-C(10) 1.348(4) C(1)-N(1) 1.454(5) O(5)-C(11) 1.433(4) C(5)-C(6) 1.375(5) O(6)-C(12) 1.220(4) C(7)-C(10) 1.519(5) O(7)-C(13) 1.210(4) O(1)-N(1) 1.220(6) N(2)-C(11) 1.442(4) O(2)-N(1) 1.127(7) Angle (°) Angle (°) C(6)-O(3)-C(7) 120.5(3) C(10)-C(7)-C(9) 106.9(3) C(10)-O(5)-C(11) 115.9(2) O(4)-C(10)-O(5) 123.3(3) C(12)-N(2)-C(11) 117.7(2) O(5)-C(11)-N(2) 111.1(2) C(12)-N(2)-C(15) 121.0(2) O(6)-C(12)-N(2) 121.5(3) C(15)-N(2)-C(11) 121.3(2) N(3)-C(12)-N(2) 115.5(3) C(12)-N(3)-C(13) 127.9(2) O(7)-C(13)-N(3) 122.2(3) C(6)-C(1)-N(1) 119.5(4) F(1)-C(14)-C(13) 115.7(3) C(2)-C(3)-Br(1) 119.9(3) C(14)-C(15)-N(2) 120.7(2) O(3)-C(7)-C(8) 111.9(3) O(2)-N(1)-O(1) 122.5(5) O(3)-C(7)-C(10) 109.0(3) Table 2. π-π Stacking Interactions (Å, °)
Cg(I)Res(I)Cg(J) [ARU(J)] Cg-Cg Alpha Cgl Perp CgJ Perp Slippage Cg(2)[1]—Cg(2) [2576] 3.918(5) 0 3.575 3.575 1.605 Table 3. Hydrogen Bond Lengths (Å) and Bond Angles (º)
D-H …A d(D-H) d(H-A) d(D-A) ZDHA N(3)-H(3)…O(6)#1 0.860 1.967 2.818 170.17 C(4)-H(4)-F(1)#2 0.930 2.573 3.312 136.64 C(9)-H(9C)…F(1)#3 0.960 2.620 3.579 177.72 C(11)-H(11A)…O(7)#4 0.970 2.559 3.352 138.96 C(15)-H(15)…O(4)#5 0.930 2.341 3.267 173.55 Symmetry codes: #1: –x+1, –y+1, –z; #2: –x+1, –y+2, –z; #3: –x, –y+2, –z; #4: x–1, y, z; #5: –x, –y+2, –z Table 4. Inhibition of Cell Growth (μmol/L)
Compound IC50 (wmoL/L) HeLa A549 Hep-G2 WI-38 Compound 5 25.31±0.8 18.09±0.6 23.81±0.7 >200 Positive group (5-Fu) 32.89±0.6 68.35±0.6 32.05±1.0 135.79±1.8 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 1652
- HTML全文浏览量: 165



 下载:
下载:


 下载:
下载:

