 图Scheme 1
Typical neutral nickel catalysts for olefin polymerization
Scheme1.
Typical neutral nickel catalysts for olefin polymerization
图Scheme 1
Typical neutral nickel catalysts for olefin polymerization
Scheme1.
Typical neutral nickel catalysts for olefin polymerization

RCOO-取代的镍(Ⅱ)配合物单组分高效催化乙烯均聚和共聚反应
English
Nickel(Ⅱ) Complexes Containing RCOO-Substituent as Highly Active Single-Component Catalysts for Ethylene (Co)Polymerization
-
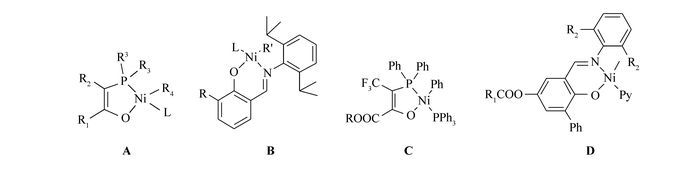
Shell higher olefin process(SHOP)-type catalysts[1] (Scheme 1, A) as the oldest neutral nickel catalysts have been successfully commercialized for the synthesis of linear α-olefins. However, no noticeable improvement has been made in this field until Grubbs et al.[2-3] covered the phenoxyiminato neutral nickel catalysts(Scheme 1, B). These complexes, with bulky groups at the ortho position of the phenoxy moiety, generate high relative molecular mass polymers with excellent activities comparable to the classic metallocenes, and show substantial tolerance toward various polar additives. Since then, nickel catalysts[4-10] have attracted much attention, and the electronic and steric effects of the auxiliary ligands have been extensively studied. Neutral nickel(Ⅱ) catalysts containing highly electron-withdrawing groups —CF3 and —COOR[11](Scheme 1, C) afford low relative molecular mass highly linear polyethylene(PE) with very high activity. Mecking group[12-13] reported the vital role of electron-donating groups in neutral nickel polymerization catalysts, which are responsible for the resultant hyperbranched ethylene oligomers. Generally speaking, electron-donating groups would enhance tolerance of the catalysts toward polar groups by increasing the electron density of nickel center, and facilitate polymerization initiation by promoting the dissociation of additional stabilizing ligand. As an electron-donating group, RCOO— has been rarely applied for nickel catalysts in olefin polymerization[14]. Thus, we designed a series of catalysts bearing RCOO— groups(Scheme 1, D) to study their properties in ethylene polymerization. Considering the vital role of effective blockage of the axial positions of nickel center, nickel complexes with 2, 6-(3, 5-tBu2C6H3)2C6H3NH2 was also synthesized.
 图Scheme 1
Typical neutral nickel catalysts for olefin polymerization
Scheme1.
Typical neutral nickel catalysts for olefin polymerization
图Scheme 1
Typical neutral nickel catalysts for olefin polymerization
Scheme1.
Typical neutral nickel catalysts for olefin polymerization
Moreover, —OC(O)—R—COO— as excellent bridges for binuclear catalyst[15] have gained great success in various catalytic reactions[16-19]. We thus also designed and synthesized novel binuclear neutral nickel catalyst Ni3 bearing OC(O)—R—COO—(R=o-C6H4) linkage to address their catalytic properties. Without any cocatalysts, all these complexes displayed very high activities up to 1.8×106 g of PE mol-1·Ni-1·h-1 even at low ethylene pressure(5×105 Pa), which are among the highest catalytic activities for phenoxyiminato neutral nickel catalysts in ethylene polymerization. These catalysts demonstrated good polar monomer tolerance, and were capable to catalyze the copolymerization of ethylene with 1, 5-hexadiene, 1, 7-octadiene, 6-bromo-1-hexene and methyl 10-undecenoate, yielding copolymers with moderate relative molecular mass at moderate activities.
1 Experimentals
1.1 Instruments and Reagents
All manipulations of air-and/or moisture-sensitive compounds were carried out using standard Schlenk techniques or in a Etelux lab2000 glovebox under a dry nitrogen atmosphere. Toluene, n-hexane, diethyl ether, dimethylformamide(DMF), and methylene dichloride were purified by an MBraun solvent purification system(SPS). Pyridine and N, N, N-triethylamine was distilled from sodium/benzophenone ketyl under nitrogen prior to use. BBr3, MeI, K2CO3, CH3COOH, CH3COONa, p-toluenesulfonate, 4-dimethylaminopyridine, benzoyl chloride and phthaloyl dichloride were purchased from Beijing Chemical Works and directly used without purification. 1, 5-Hexadiene, 1, 7-octadiene, and methyl 10-undecenoate were distilled after drying via calcium hydride for two days. These monomers were purchased from J&K Chemical. 2, 5-Dimethoxy-3-phenylbenzaldehyde[20], 2, 6-(3, 5-tBu2C6H3)2C6H3NH2[13], and (pyridine)2Ni(CH3)2[21] were synthesized according to reported literature. Ethylene(99.999%) was purchased from Changchun Juyang Corporation and was used without further purification.
The nuclear magnetic resonance(NMR) spectra of polyethylene samples were all recorded on a Varian Unity 400 MHz spectrometer(Bruker Corporation) with o-dichlorobenzene-d4 or 1, 1, 2, 2-tetrachloroethane-d2 as the solvent at 110 ℃. All 1H and 13C NMR spectra of small organic and organometallic compounds were obtained on a Bruker 300 MHz spectrometer, a Varian Unity 400 MHz spectrometer or a Bruker 500 MHz spectrometer at ambient temperature with CDCl3, C6D6 or dimethylsulfoxide-d6(DMSO-d6) as the solvent. The DSC measurements were performed on a Perkin-Elmer Pyris 1 differential scanning calorimeter at a heating rate of 20 ℃/min. The mass-average relative molecular mass(Mw) and the polydispersity index(PDI) of polyethylene samples were determined via high-temperature gel permeation chromatography(GPC) in which 1, 2, 4-trichlorobenzene was used as mobile phase at a flow rate of 1.0 mL/min. The calibration was made by the polystyrene standard Easi-Cal PS-1(PL Ltd., Agilen, Santa Clara, California, USA).
1.2 Synthesis of Compounds b~d
3-phenyl-2, 5-dihydroxy-benzaldehyde(b): In a flame dried two necked flask equipped with a pressure equalizing dropping funnel, 3-phenyl-2, 5-dimethoxy-benzaldehyde (a) (2.2 g, 9.0 mol) was dissolved in dry dichloromethane (DCM, 20 mL). The resulting solution was cooled to -78 ℃ and a solution of BBr3 in DCM(2 mol/L, 18 mL) was slowly added to the solution via the pressure equalizing dropping funnel in 20 min. The resulting solution was maintained at -78 ℃ for 40 min, then was allowed to warm up to room temperature and left with stirring overnight under a nitrogen atmosphere. After 10 hours, the solution was quenched with ice to neutralise the excess BBr3, and the DCM was removed under reduced pressure. The resulting mixture was extracted with ethyl acetate (EtOAc, 50 mL×3) and the combined organic extracts were washed twice with brine (50 mL×2), dried (MgSO4), and filtered. The solvent was removed in vacuo yielding 3-phenyl-2, 5-dihydroxy-benzaldehyde(1.7 g, 89%) as yellow powder. 1H NMR(400 MHz, DMSO-d6), δ:10.65(s, 1H), 10.02(s, 1H), 9.51(s, 1H), 7.54(d, J=7.0 Hz, 2H), 7.44(t, J=7.4 Hz, 2H), 7.36(t, J=7.3 Hz, 1H), 7.13(d, J=3.1 Hz, 1H), 7.09(d, J=3.1 Hz, 1H). 13C NMR(101 MHz, DMSO-d6), δ:196.89, 150.64, 150.28, 136.46, 130.78, 129.08, 128.24, 127.44, 125.26, 121.85, 116.67.
[(2, 6-iPr2-C6H3)-N=C(H)(3-Ph-2, 5-(OH)2-C6H2)](c): To an ethanol(5 mL) solution of compound b(0.63 g, 1.9 mmol) was added a catalytic amount of pyridinium p-toluenesulfonate(p-TSA) and 1.2 stoichiometric amount of 2, 6-diisopropylaniline. The mixture was stirred for 12 hours at room temperature. The yellow solid that precipitated was filtered, washed with cold ethanol and dried to afford the Schiff base in 99% yield. 1H NMR(400 MHz, CDCl3), δ:13.25(s, 1H, OH), 8.30(s, 1H, ArH), 7.67(d, J=7.4 Hz, 2H, ArH), 7.47(t, J=7.6 Hz, 2H, ArH), 7.37(t, J=7.0 Hz, 1H, ArH), 7.20(s, 3H, ArH), 7.08(s, 1H, ArH), 5.46(s, 1H, OH), 3.02(hept, J=6.8 Hz, 2H, CH(CH3)2), 1.18(d, J=6.9 Hz, 12H, CH(CH3)2). 13C NMR(101 MHz, CDCl3), δ:166.59, 152.73, 139.23, 137.01, 131.14, 129.44, 128.53, 127.76, 126.00, 123.49, 118.53, 116.88, 28.30, 23.73.
[(2, 6-(3, 5-tBu2C6H3)2C6H3)-N=C(H)(3-Ph-2, 5-(OH)2-C6H2)](d): Using the same method for synthesizing compound c, reaction of compound b with 2, 6-(3, 5-tBu2C6H3)2C6H3NH2 gave rise to compound d as yellow powder in 90% yield. 1H NMR(500 MHz, DMSO-d6), δ:12.49(s, 1H), 8.98(s, 1H), 8.08(s, 1H), 7.46(d, J=7.8 Hz, 2H), 7.42~7.33(m, 5H), 7.29(d, J=7.1 Hz, 1H), 7.26(t, J=1.7 Hz, 2H), 7.19(d, J=1.7 Hz, 4H), 6.77(d, J=2.8 Hz, 1H), 6.37(d, J=3.0 Hz, 1H), 1.19(s, 36H). 13C NMR(126 MHz, DMSO-d6), δ:169.97, 150.51, 149.93, 148.87, 145.13, 138.02, 137.43, 134.81, 129.63, 129.09, 128.77, 127.92, 126.89, 125.73, 124.16, 121.24, 120.24, 118.86, 116.72, 34.43, 31.07.
1.3 Synthesis of ligands L0~L3
[(2, 6-iPr2-C6H3)-N=C(H)(3-Ph-2-(OH)-C6H3)](L0): Ligand L0 was synthesized according to the reported literature[3]. 1H NMR(500 MHz, CDCl3), δ:8.37(s, 1H), 7.71(d, J=8.3 Hz, 2H), 7.53(d, J=7.6 Hz, 1H), 7.48(t, J=7.6 Hz, 2H), 7.37(t, J=7.1 Hz, 2H), 7.20(s, 3H), 7.06(t, J=7.6 Hz, 1H), 3.02 (hept, J=6.8 Hz, 2H), 1.18(d, J=6.9 Hz, 12H).
[(2, 6-iPr2-C6H3)-N=C(H)(3-Ph-5-PhCOO-2-(OH)-C6H2)](L1) General procedure for the preparation of ligands L1~L3. The appropriate acid chloride(1.0 mmol) was added to a solution of compounds c or d (1.1 stoichiometric amount for L1 and L2, 2.2 stoichiometric amount for L3), 4-dimethylaminepyridine(0.2 mmol), and triethylamine(3.0 mmol) in CH2Cl2(10 mL) at room temperature under N2 atmosphere. After complete consumption of the acid chloride detected by TLC, the mixture was concentrated under reduced pressure and purified by silica gel chromatography. Ligand L1 was afforded as yellow powder in 84% yield. 1H NMR(500 MHz, CDCl3), δ:8.34(s, 1H), 8.22(dd, J=8.3, 1.2 Hz, 2H), 7.73(d, J=7.1 Hz, 2H), 7.65(t, J=7.5 Hz, 1H), 7.53(t, J=7.8 Hz, 2H), 7.47(t, J=7.6 Hz, 2H), 7.40~7.35(m, 2H), 7.25(s, 1H), 7.19(s, 3H), 3.01(hept, J=6.8 Hz, 2H), 1.18(d, J=6.9 Hz, 12H). 13C NMR(126 MHz, CDCl3), δ:166.34, 165.79, 156.65, 145.92, 142.84, 138.90, 136.76, 133.91, 131.41, 130.34, 129.55, 129.45, 128.79, 128.43, 127.83, 127.68, 125.84, 123.79, 123.45, 118.88, 28.27, 23.73.
[(2, 6-(3, 5-tBu2C6H3)2C6H3-N=C(H)(3-Ph-5-PhCOO-2-(OH)-C6H2)](L2): Ligand L2 was afforded in 73% yield. 1H NMR(300 MHz, CDCl3), δ:8.14(d, J=7.3 Hz, 2H), 7.92(s, 1H), 7.62(t, J=7.4 Hz, 1H), 7.49(t, J=7.4 Hz, 6H), 7.35~7.39(m, 4H), 7.31(s, 2H), 7.23(d, J=1.6 Hz, 4H), 7.16(d, J=2.8 Hz, 1H), 6.60(d, J=2.8 Hz, 1H), 1.25(s, 36H). 13C NMR(126 MHz, CDCl3), δ:168.20, 165.30, 156.27, 150.76, 145.19, 142.35, 138.48, 137.02, 135.94, 133.70, 130.89, 130.22, 130.04, 129.64, 129.39, 128.70, 128.15, 127.49, 127.11, 125.98, 124.57, 123.07, 121.09, 119.08, 34.99, 31.51.
[C6H4(COO)2-{(2, 6-iPr2-C6H3)-N=C(H)(3-Ph-5-yl-2-(OH)-C6H2)}2](L3): Ligand L3 was afforded in 41% yield. 1H NMR(500 MHz, CDCl3), δ:8.27(s, 2H), 8.02(dd, J=5.7, 3.3 Hz, 2H), 7.73(dd, J=5.7, 3.3 Hz, 2H), 7.57(d, J=7.0 Hz, 2H), 7.38(dd, J=8.9, 6.1 Hz, 6H), 7.31(t, J=7.4 Hz, 2H), 7.28(d, J=2.9 Hz, 2H), 7.19(s, 6H), 2.96(hept, J=6.8 Hz, 4H), 1.14(d, J=6.9 Hz, 24H). 13C NMR(101 MHz, DMSO-d6), δ:167.83, 165.93, 156.01, 145.22, 142.19, 138.10, 135.92, 132.63, 130.63, 129.99, 129.62, 128.95, 128.15, 127.56, 127.12, 125.71, 124.31, 123.20, 118.82, 27.75, 23.23.
1.4 Synthesis of nickel complexes Ni0~Ni3
[[(2, 6-iPr2-C6H3)-N=C(H)(3-Ph-2-O-C6H3)-κ2-N, O]Ni(CH3)(pyridine)](Ni0): According to the literature[22], the nickel methyl pyridine complex Ni0 was prepared in excellent yield by adding dropwise the toluene solution of (pyridine)2NiMe2(0.295 g, 1.2 mmol) to toluene solution of ligand L0(0.36 g, 1.0 mmol) with vigorous stirring at room temperature. The mixture was stirred at room temperature to give a dark red solution as the reaction proceeded. After 6 hours, the mixture was filtrated to remove nickel black, and the filtrate was directly taken to dryness, affording complex Ni0 in 95% yield as dark red solid. 1H NMR(500 MHz, C6D6), δ:8.48(s, 2H), 7.61(s, 1H), 7.44(s, 3H), 7.08~7.16(m, 7H), 6.59(d, J=40.9 Hz, 2H), 6.18(s, 2H), 4.23(hept, J=6.8 Hz, 2H), 1.53(s, 6H), 1.11(s, 6H), -0.68(s, 3H, NiCH3). 13C NMR(126 MHz, C6D6), δ:166.59, 165.34, 151.97, 150.29, 141.21, 140.81, 135.51, 134.62, 133.91, 133.44, 129.97, 128.35, 127.51, 126.59, 123.65, 123.00, 120.73, 114.13, 28.60, 24.97, 23.23, -7.30(NiCH3). Anal. Calcd for C31H34N2NiO:C 73.11, H 6.73, N 5.50. Found:C 73.09, H 6.76, N 5.48.
[[(2, 6-iPr2-C6H3)-N=C(H)(3-Ph-5-PhCOO-2-O-C6H2)-κ2-N, O]Ni(CH3)(pyridine)](Ni1): Using the same method for synthesizing Ni0, complex Ni1 was obtained in 88% yield as yellow powder. 1H NMR(500 MHz, C6D6), δ:8.50(dd, J=6.4, 1.5 Hz, 2H), 8.31~8.26 (m, 1H), 7.53(s, 1H), 7.42(d, J=3.0 Hz, 1H), 7.37(dd, J=8.2, 1.1 Hz, 2H), 7.19~7.01(m, 6H), 6.93(t, J=7.6 Hz, 2H), 6.88(d, J=3.0 Hz, 1H), 6.64(tt, J=7.6, 1.6 Hz, 1H), 6.19(ddd, J=7.5, 5.3, 1.3 Hz, 2H), 4.21(hept, J=6.8 Hz, 2H), 1.56(d, J=6.9 Hz, 6H), 1.09(d, J=6.8 Hz, 6H), -0.65(s, 3H, NiCH3). 13C NMR(101 MHz, CDCl3), δ:166.04, 165.50, 163.40, 151.91, 150.13, 141.10, 139.82, 139.34, 135.55, 134.06, 133.15, 130.88, 130.33, 129.94, 128.82, 128.66, 127.47, 126.62, 126.08, 124.21, 123.62, 123.02, 119.60, 28.59, 24.92, 23.22, -7.23(NiCH3). Anal. Calcd for C38H38N2NiO3:C 72.51, H 6.09, N 4.45. Found:C 72.50, H 6.12, N 4.43.
[[(2, 6-(3, 5-tBu2C6H3)2C6H3-N=C(H)(3-Ph-5-PhCOO-2-C6H2)-κ2-N, O]Ni(CH3)(pyridine)](Ni2): Using the same method for synthesizing complexes Ni0, Ni2 was afforded in 91% yield as red powder. 1H NMR(500 MHz, C6D6), δ:8.21~8.16(m, 4H), 7.87(d, J=1.8 Hz, 4H), 7.65(t, J=1.7 Hz, 2H), 7.55(d, J=7.6 Hz, 2H), 7.34(dd, J=8.2, 1.2 Hz, 2H), 7.29 ~7.25(m, 2H), 7.22(t, J=7.6 Hz, 1H), 7.11(d, J=7.4 Hz, 1H), 7.05(dd, J=13.7, 6.6 Hz, 3H), 6.95(t, J=7.5 Hz, 2H), 6.65(t, J=7.6 Hz, 1H), 6.54(d, J=3.1 Hz, 1H), 6.29~6.25 (m, 2H), 1.45(s, 36H), -0.54(s, 3H, NiCH3). 13C NMR(101 MHz, CDCl3), δ:168.49, 165.23, 162.75, 151.52, 151.09, 150.89, 139.98, 139.91, 138.75, 137.45, 135.35, 133.27, 132.98, 130.98, 130.27, 129.87, 129.68, 128.58, 127.33, 126.36, 125.90, 124.12, 122.65, 121.04, 120.20, 35.28, 31.78, -7.96(NiCH3). Anal. Calcd for C60H66N2NiO3:C 78.17, H 7.22, N 3.04. Found:C 78.18, H 7.20, N 3.05.
[C6H4(COO)2-{[(2, 6-iPr2-C6H3N=CH)(3-Ph-5-yl-2-O-C6H2)-κ2-N, O]Ni(CH3)(pyridine)](Ni3): The nickel methyl pyridine complex Ni3 was prepared by adding dropwise the toluene solution of (pyridine)2NiMe2(0.15 g, 0.6 mmol) to toluene solution of ligand L3(0.36 g, 0.25 mmol) with vigorous stirring at room temperature. The mixture was stirred at room temperature for 6 hours, then the mixture was filtrated to remove nickel black, and the filtrate was directly taken to dryness, affording complex Ni3 in 70% yield as yellow powder. 1H NMR(400 MHz, C6D6), δ:8.52(d, J=5.4 Hz, 4H), 7.81~7.75(m, 2H), 7.53(d, J=2.9 Hz, 2H), 7.41(s, 2H), 7.25(d, J=7.6 Hz, 4H), 7.14~6.93(m, 16H), 6.65(t, J=7.2 Hz, 2H), 6.27~6.16(m, 4H), 4.15(hept, J=6.8 Hz, 4H), 1.57(d, J=6.8 Hz, 12H), 1.04(d, J=6.8 Hz, 12H), -0.66(s, 6H, NiCH3). 13C NMR(126 MHz, C6D6), δ:165.67, 165.05, 162.50, 150.96, 149.08, 140.10, 138.68, 138.35, 134.58, 133.10, 131.88, 130.35, 128.90, 128.34, 127.57, 126.55, 125.01, 124.70, 123.25, 122.59, 122.07, 118.68, 27.60, 23.95, 22.33, -8.07(NiCH3). Anal. Calcd for C70H70N4Ni2O6:C 71.21, H 5.98, N 4.75. Found:C 71.18, H 5.61, N 4.73.
1.5 X-ray Structure Determination
Single crystals suitable for X-ray diffraction analysis were grown within one day after layering a solution of these complexes in toluene with n-hexane in a glove box. The intensity data were collected with the ω scan mode(186 K) on a Bruker Smart APEX diffractometer with a CCD detector using MoKα radiation(λ=0.071073 nm). Absorption corrections were performed using the SADABS program. The crystal structures were solved using the SHELXTL program and refined using full matrix least-squares techniques. The positions of hydrogen atoms were calculated theoretically and included in the final cycles of refinement in a riding model along with attached carbons. Crystal data collection and refinement details are given in Table S1(see Supporting Information).
1.6 Procedure for Ethylene Polymerization
A 200 mL autoclave was heated under vacuum to 140 ℃ for 4 hours and was then cooled to the desired reaction temperature. The vessel was purged three times with ethylene and was charged with 30 mL toluene under ethylene pressure. A solution of nickel catalyst in 10 mL toluene was injected into the reactor. The reaction apparatus was then filled with ethylene and pressurized to the prescribed ethylene pressure immediately. The mixture was stirred for prescribed time for ethylene polymerization. After reaction, magnetic stirring was stopped, the reactor was vented, and the polymerization mixture was poured into 200 mL ethanol. The solid polymer was filtered, washed with ethanol several times, and dried to constant mass under vacuum.
1.7 Procedure for Ethylene Copolymerization
A 200 mL autoclave was heated under vacuum to 140 ℃ for 4 hours and was then cooled to the desired reaction temperature. The vessel was purged three times with ethylene and was charged with toluene under ethylene pressure. A solution of commoner was injected into the reactor before a toluene solution of nickel catalyst was added by using a dry syringe. The total reaction volume was 40 mL. The reaction apparatus was then filled with ethylene and pressurized to the prescribed ethylene pressure immediately. The mixture was stirred for 90 min under prescribed temperature. After reaction, magnetic stirring was stopped, the reactor was vented, and the polymerization mixture was poured into ethanol. The solid polymer was filtered, washed with ethanol several times, and dried to constant mass under vacuum.
2 Results and Discussion
2.1 Synthesis of Ligands and Complexes
2, 5-Dimethoxy-3-phenylbenzaldehyde(a) was synthesized at excellent yield from commercially available 2-hydroxy-5-methoxybenzaldehyde according to reported literature[20, 23]. The key intermediate 3-phenyl-2, 5-dihydroxy-benzaldehyde(b) was obtained after deprotection of compound a by BBr3(Scheme S1, see Supporting Information). Condensation of compound b with 2, 6-iPr2C6H3NH2 or 2, 6-(3, 5-tBu2C6H3)2C6H3NH2 gave rise to compounds c and d, respectively(Scheme S1).
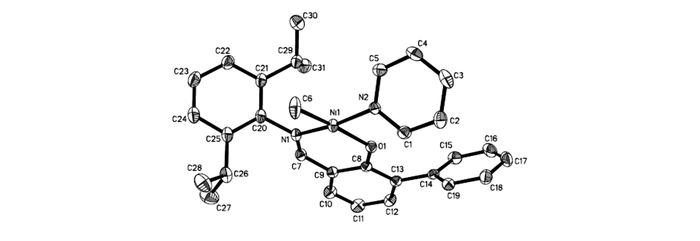
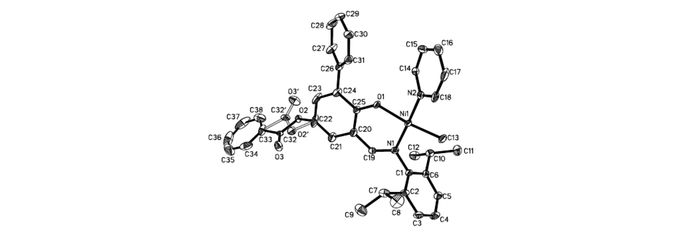
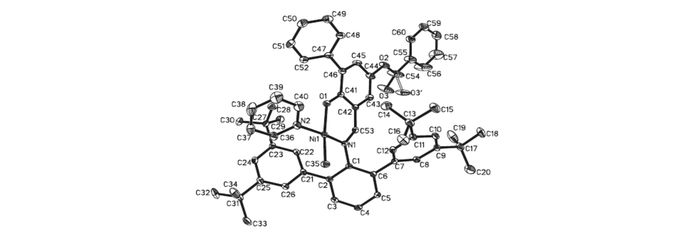
Ligands L1~L3 were synthesized by the reaction of compounds c or d with corresponding acyl chlorides(Scheme 2). Using the procedure reported by Mecking et al.[22], complexes Ni0~Ni3 were prepared by reaction of corresponding ligands with (pyridine)2Ni(Me)2 in 70%~95% yields(see Scheme 2). All these nickel complexes were characterized by 1H NMR, 13C NMR, and elemental analysis. Single crystals of Ni0, Ni1 and Ni2 were grown within one day after layering a solution of these complexes in toluene with n-hexane(1/3, volume ratio) and the structures of these complexes were determined by X-ray diffraction analysis(Fig. 1~Fig. 3). The coordination geometry at the nickel center is slightly distorted square planar, and the methyl groups are located trans. to the oxygen atoms. Obviously, the coordination environment of Ni2(see Fig. 3) was more congested than that of Ni1(Fig. 2). Due to the presence of bulkier substituted terphenyl imine moiety, the axial positions of the nickel center can be effectively shielded.
2.2 Ethylene polymerization
Ethylene polymerization trials were performed to determine the effects of polymerization temperature, reaction time, and additive triphenylphosphine (PPh3)(Table 1). All these nickel complexes(Ni0~Ni3) studied here can generate polyethylenes without cocatalysts such as expensive bis(1, 5-cyclooctadiene)nickel(0) Ni(COD)2 and B(C6F5)3. Relative molecular mass distributions of obtained polymers between 1.6 and 2.2 indicate that all these complexes were well-behaved single-site catalysts. Generally speaking, the relative molecular mass of polyethylenes produced by Ni0~Ni3 decreased at elevated temperatures due to more extensive chain transfer reactions. Accordingly, the Tm values of polymers decreased at elevated temperatures as well.
Entry Catalyst Temperature/℃ Yield/g Activity b Mwc/(kg·mol-1) Mw/Mnc Tmd/℃ 1 Ni0 40 0.19 1.5 45.4 2.2 114 2 Ni0 50 0.72 5.8 25.2 2.3 103 3 Ni0 60 1.23 9.8 15.1 2.2 96 4 Ni0 70 0.96 7.7 10.1 2.1 87 5 Ni1 40 0.22 1.8 51.7 2.2 115 6 Ni1 50 1.31 10.5 19.4 2.2 97 7 Ni1 60 0.82 6.6 11.6 2.2 89 8 Ni1 70 0.53 4.2 8.6 2.1 75 9e Ni1 50 2.24 6.0 17.0 2.2 92 10f Ni1 50 3.07 4.1 17.0 2.1 92 11g Ni1 50 0.016 0.13 4.9 1.8 - 12 Ni2 40 1.21 9.7 11.6 2.1 81 13 Ni2 50 1.91 15.3 6.9 2.0 68 14 Ni2 60 2.24 17.9 4.2 2.3 - 15 Ni2 70 1.75 14.0 3.5 1.1 - 16 Ni3 40 0.17 1.4 70.3 2.5 124 17 Ni3 50 1.11 8.9 27.3 2.4 99 18 Ni3 60 0.61 4.9 19.3 2.2 93 19 Ni3 70 trace - - - - 20e Ni3 50 2.52 3.4 16.6 2.1 91 21f Ni3 50 2.82 1.9 18.0 2.1 93 22g Ni3 50 0.021 0.17 5.4 1.9 - a.Polymerizations conditions:toluene, 40 mL; nickel, 5(mol; ethylene, 5×105 Pa; 15 min; b.in the unit of 105 g PE mol-1·Ni-1·h-1; c.determined by GPC vs polystyrene standards; d.determined by DSC; e.reaction time: 45 min; f.reaction time:90 min; g.25(mol PPh3 as additives. Compared with Ni0, catalyst Ni1 with PhCOO-remote from the nickel center exhibited higher activity at low reaction temperature(40 and 50 ℃), implying an easier initiation process. This may result from the weaker coordination of pyridine ligand to the less oxophilic nickel center due to the presence of electron-donating group PhCOO—. Catalyst Ni1 yields polyethylene with Mw=1.9×104 and 61 branches with the activity of 1.0×106 g of PE mol-1·Ni-1·h-1 at 50 ℃(entry 6, Table 1).
After introducing bulky terphenyl moiety, catalyst Ni2 generated PE with Mw=6900 and 81 branches per 1000 carbon atoms(C-1) at 1.5×106 g PE mol-1·Ni-1·h-1 at 50 ℃(entry 13, Table 1). With activities up to 1.8×106 g of PE mol-1·Ni-1·h-1 under 5×105 Pa ethylene pressure at 60 ℃(entry 14, Table 1), Ni2 is among the most active phenoxyiminato nickel catalysts for ethylene homopolymerization[3, 13, 22, 24-27]. The activity of Ni2 has doubled relative to that of Ni1, which is ascribed to the bulky substituted N-terphenyl moiety playing a vital role in preventing the forming of bis-ligated complex[28]. However, the amorphous polymer Mw obtained by Ni2 at 60 ℃ was much lower than that by Ni1 under the same reaction conditions(4200 vs 11600, entry 14 vs 7, Table 1). Electron-donating tert-butyl groups are responsible for the lower Mw due to extensive chain transfer reaction, in good agreement with the findings by Mecking group[12], that the electronic characteristics of the remote substituents of terphenyl moieties govern the polymerization behavior.
Binuclear Ni3 shows similar optimal activity relative to mononuclear Ni1(ca. 1.0×106 g of PE mol-1·Ni-1·h-1)(entry 6 vs 17, Table 1). Compared with mononuclear Ni1, binuclear catalyst Ni3 enhanced the Mw of polymer. This is consistent with the reported result of literatures[29-30] that binuclear catalysts enhanced polymer relative molecular mass. Catalyst Ni3 demonstrates slightly lower activity at low temperature than mononuclear Ni1, and generates only trace amount of polymer at elevated temperature(70 ℃, entry 19, Table 1), indicating an inferior thermal stability of binuclear catalyst Ni3 to mononuclear Ni1. It was reported by Grubbs et al. that bis-ligated nickel species was isolated as the main deactivation product when ligand frameworks were not sufficiently bulky[31]. We thus proposed that inactive bis-ligated complex was more readily generated for binuclear complex Ni3 in which two nickel centers were close to each other, especially at elevated temperatures[32]. The drop of the catalytic activity with polymerization time may partly reflect the stability of catalysts, we thus conducted ethylene polymerization for different polymerization time using catalysts Ni1 and Ni3. From 45 to 90 min, a 32% decrease in catalytic activity for Ni1 was observed and the value for binuclear Ni3 was 44%. This indicated a more serious catalyst deactivation with reaction time for binuclear Ni3, which was similar to the effect of reaction temperature.
To further investigate the different performances of these catalysts, extra PPh3 was added to the catalytic systems during the ethylene polymerization. In the presence of 5 stoichiometric amount of PPh3, the activity of mononuclear Ni1 was reduced to 1/80th of that without any additive due to the competitive coordination reaction of ethylene and PPh3[33]. For binuclear Ni3, the activity decreased sharply as well, but the activity in the presence of 5 stoichiometric amount of PPh3 was still 1/50th of the original value. The better tolerance toward PPh3 additives of the binuclear catalyst may be resulted from the close position of the nickel centers, which would prohibit simultaneous inhibition of the two metal centers by bulky PPh3 ligands[34-35].
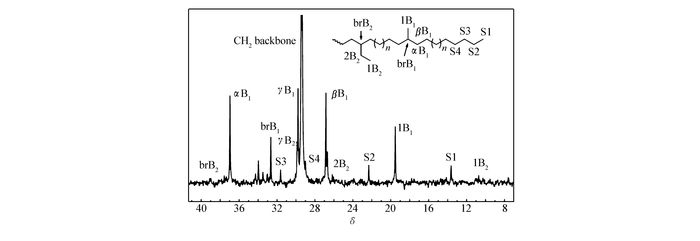
We also studied the microstructures of polyethylenes produced by these catalysts through 13C NMR. All these polymers obtained by Ni0~Ni3 at 50 ℃ possess similar branching pattern(Fig. 4). According to the 13C NMR, polymers generated by Ni1 contain main methyl branches and minor ethyl branches[36].
2.3 Ethylene Copolymerization
The typical results of ethylene copolymerization catalyzed by Ni1~Ni3 with 1, 5-hexadiene, 1, 7-octadiene, 6-bromo-1-hexene and methyl 10-undecenoate were summarized in Table 2. Both Ni1 and Ni3 could catalyze ethylene with 1, 5-hexdiene at moderate activities(1.8~2.0×104 g of PE mol-1·Ni-1·h-1), but the incorporation of the comonomer was low. In stark contrast, 1, 7-octadiene was efficiently incorporated into the polyethylenes by using Ni1 and Ni3. The resulting copolymers with pendant double bonds were clearly evidenced by the significant increase of external double bonds versus polyethylenes by 1H NMR measurement (Fig.S1, see Supporting Information). Additionally, binuclear Ni3 demonstrated slightly higher activity and polymer Mw than Ni1(entry 9 vs 2, Table 2).
Entry Catalyst Comonomer Yield/mg Activityb Mwc/(kg·mol-1) Mw/Mnc Tmd/℃ Xce 1 Ni1 
260 17.7 16.3 1.9 93 nd 2 Ni1 
230 15.3 16.6 2.0 94 nd 3 Ni1 
61 4.1 20.5 1.9 103 0.92 4 Ni1 
150 10.0 15.4 1.8 94 0.82 5 f Ni1 
49 3.3 16.5 2.2 97 1.01 6 Ni2 
18 1.2 6.0 1.7 - 0.16 7 Ni2 
101 6.7 6.7 1.7 - 0.25 8 Ni3 
301 20.1 14.9 2.0 92 nd 9 Ni3 
252 16.8 17.6 1.7 94 nd 10 Ni3 
72 4.8 16.1 1.9 96 0.91 11 Ni3 
182 12.1 16.5 1.9 95 0.64 12 f Ni3 
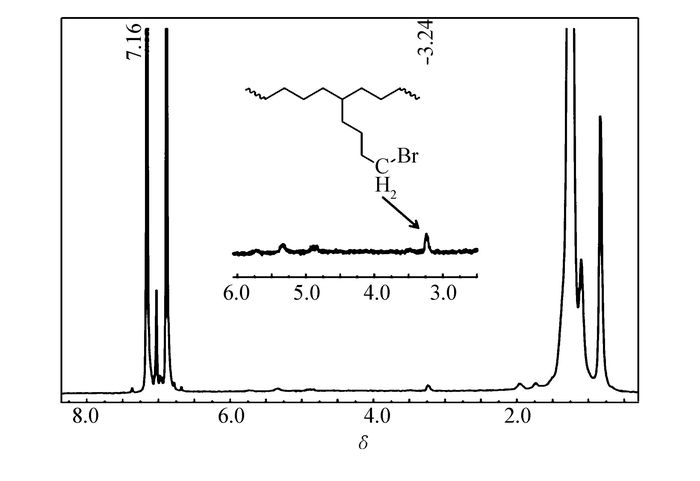
62 4.1 16.8 2.1 93 1.10 a.Polymerizations conditions:toluene, 40 mL; nickel, 10 (mol; ethylene, 5×105 Pa; 90 min, 600 stoichiometric amount of comonomer; b.in the unit of 103 g PE mol-1·Ni-1·h-1; c.determined by GPC vs. polystyrene standards; d.determined by DSC; e.incorporation ratio was calculated according to 1H NMR spectra; f.1000 stoichiometric amount of comonomer. 6-Bromo-1-hexene as a polar comonomer, could be efficiently incorporated into the copolymer backbone by Ni1~Ni3 (entry 3, 6, 10, Table 2) when copolymerizing with ethylene. According to the 1H NMR spectrum(Fig. 5) of copolymer obtained by Ni1 at 50 ℃, the signal at δ 3.24 is clearly assigned to the —CH2Br group[37]. The catalytic activity was much lower than that for ethylene homopolymerization, which is well consistent with that reported in literature [31]. The incorporation ratio by Ni1 is up to four times higher than that by Ni2 (entry 3 vs 6, Table 2). This may originate from the highly sterically hindered imine moiety of the nickel center in Ni2.
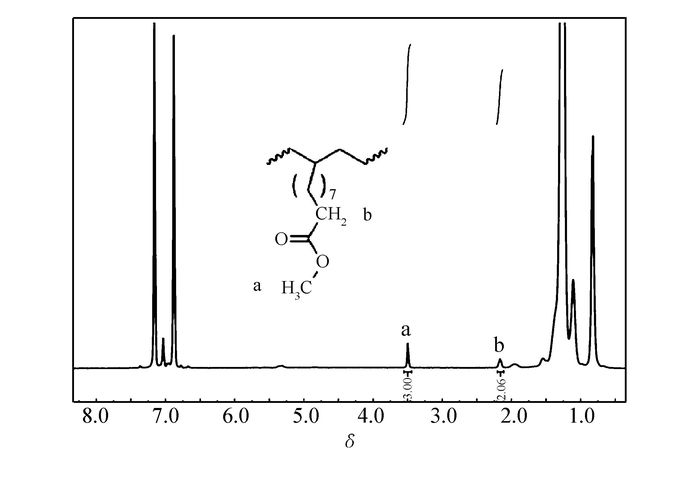
Complexes Ni1~Ni3 also could efficiently initiate the copolymerization of ethylene with the methyl 10-undecenoate. 1H NMR signals(Fig. 6) at 3.49 and 2.16, assigned to —COOCH3 and —CH2COO—, respectively, highlighted that methyl 10-undecenoate was effectively incorporated into the polymer backbone[38]. When the comonomer in the feed was increased from 600 to 1000 stoichiometric amount, Ni1 catalyzed the copolymerization with sharply decreased activity and higher comonomer incorporation(1.10%(molar fraction) vs 0.64%(molar fraction))(entry 5 vs 4, Table 2). In this aspect, binuclear catalyst Ni3 displayed similar tendency(entry 13, 14, Table 2). When compared with Ni2 bearing bulky imine moieties, the mononuclear Ni1 yielded copolymer with higher relative molecular mass(15400 vs 6700) and comonomer incorporation(0.82%(molar fraction)) vs 0.25%(molar fraction)). The bulky imine motif effectively shielded the axial positions of the nickel center, went against the coordination of comonomer with active center, and resulted in the sharply decreased incorporation ratio.
3 Conclusions
We have successfully synthesized and characterized a series of neutral nickel complexes containing benzoyloxy groups. Without any cocatalyst, they can promote ethylene polymerization at very high activities generating polyethylenes with moderate relative molecular mass. For catalyst Ni1, the electron-donating PhCOO— group promoted initiation of catalyst Ni1, showes better activity at low temperature compared to catalyst Ni0. Introducing bulky 2, 6-(3, 5-(t-Bu)2C6H3)2C6H3N-moiety, the activities of catalyst Ni2 up to 1.8×106 g of PE mol-1·Ni-1·h-1(at 5×105 Pa ethylene) are among the highest values using phenoxyimina to neutral nickel catalysts. However, lower relative molecular mass polymer was obtained due to the electron-donating 3, 5-(t-Bu)2C6H3 groups. The microstructures of the polymers generated by these catalysts contain main methyl branches and minor ethyl branches. Compared with catalyst Ni1, binuclear catalyst Ni3 produced polyethylene with higher Mw at similar activity and demonstrated better tolerance toward PPh3. These neutral nickel catalysts are also capable of copolymerizing ethylene with 1, 7-octadiene and polar comonomers such as 6-bromo-1-hexene and methyl-10-undecenoate to give various functional polyolefins
Supporting Information [Synthesis of important intermediates; Crystal data and structure refinement for complexes Ni0, Ni1 and Ni2; 1H NMR spectra of homo-and copolymers] is available free of charge at http://yyhx.ciac.jl.cn.
-
-
[1]
Keim W, Kowaldt F H, Goddard R. Novel Coordination of (Benzoylmethylene)triphenylphosphorane in a Nickel Oligomerization Catalyst[J]. Angew Chem Int Ed, 1978, 17(6): 466-467. doi: 10.1002/(ISSN)1521-3773
-
[2]
Wang C, Friedrich S, Younkin T R. Neutral Nickel(Ⅱ)-Based Catalysts for Ethylene Polymerization[J]. Organometallics, 1998, 17(15): 3149-3151. doi: 10.1021/om980176y
-
[3]
Younkin T R, Connor E F, Henderson J I. Neutral, Single-Component Nickel(Ⅱ) Polyolefin Catalysts that Tolerate Heteroatoms[J]. Science, 2000, 287(5452): 460-462. doi: 10.1126/science.287.5452.460
-
[4]
Makio H, Terao H, Iwashita A. FI Catalysts for Olefin Polymerization-A Comprehensive Treatment[J]. Chem Rev, 2011, 111(3): 2363-2449. doi: 10.1021/cr100294r
-
[5]
Delferro M, Marks T J. Multinuclear Olefin Polymerization Catalysts[J]. Chem Rev, 2011, 111(3): 2450-2485. doi: 10.1021/cr1003634
-
[6]
Mu H L, Pan L, Song D P. Neutral Nickel Catalysts for Olefin Homo-and Copolymerization:Relationships Between Catalyst Structures and Catalytic Properties[J]. Chem Rev, 2015, 115(22): 12091-12137. doi: 10.1021/cr500370f
-
[7]
Wang S, Sun W H, Redshaw C. Recent Progress on Nickel-Based Systems for Ethylene Oligo-/Polymerization Catalysis[J]. J Organomet Chem, 2014, 751: 717-741. doi: 10.1016/j.jorganchem.2013.08.021
-
[8]
Sun W H. Novel Polyethylenes via Late Transition Metal Complex Pre-catalysts[M]. In Polyolefins:50 Years after Ziegler and Natta Ⅱ:Polyolefins by Metallocenes and Other Single-Site Catalysts, Kaminsky, W., Ed, 2013, 258:163-178.
-
[9]
Gao R, Sun W H, Redshaw C. Nickel Complex Pre-Catalysts in Ethylene Polymerization:New Approaches to Elastomeric Materials[J]. Catal Sci Technol, 2013, 3(5): 1172-1179. doi: 10.1039/c3cy20691b
-
[10]
Zhang W, Zhang W J, Sun W H. Progress of Late Transition Metal Complexes for Ethylene Oligomerization and Polymerization[J]. Prog Chem, 2005, 17(2): 310-319.
-
[11]
Soula R, Broyer J P, Llauro M F. Very Active Neutral P, O-Chelated Nickel Catalysts for Ethylene Polymerization[J]. Macromolecules, 2001, 34(8): 2438-2442. doi: 10.1021/ma001714x
-
[12]
Wiedemann T, Voit G, Tchernook A. Monofunctional Hyperbranched Ethylene Oligomers[J]. J Am Chem Soc, 2014, 136(5): 2078-2085. doi: 10.1021/ja411945n
-
[13]
Zuideveld M A, Wehrmann P, Röhr C. Remote Substituents Controlling Catalytic Polymerization by Very Active and Robust Neutral Nickel(Ⅱ) Complexes[J]. Angew Chem, 2004, 116(7): 887-891. doi: 10.1002/(ISSN)1521-3757
-
[14]
Yakhvarov D G, Basvani K R, Kindermann M K. O-Acylated 2-Phosphanylphenol Derivatives-Useful Ligands in the Nickel-Catalyzed Polymerization of Ethylene[J]. Eur J Inorg Chem, 2009, 2009(9): 1234-1242. doi: 10.1002/ejic.v2009:9
-
[15]
Haak R M, Wezenberg S J, Kleij A W. Cooperative Multimetallic Catalysis Using Metallosalens[J]. Chem Commun, 2010, 46(16): 2713-2723. doi: 10.1039/c001392g
-
[16]
Konsler R G, Karl J, Jacobsen E N. Cooperative Asymmetric Catalysis with Dimeric Salen Complexes[J]. J Am Chem Soc, 1998, 120(41): 10780-10781. doi: 10.1021/ja982683c
-
[17]
Jacobsen E N. Asymmetric Catalysis of Epoxide Ring Opening Reactions[J]. Acc Chem Res, 2000, 33(6): 421-431. doi: 10.1021/ar960061v
-
[18]
Mazet C, Jacobsen E N. Dinuclear {(salen)Al} Complexes Display Expanded Scope in the Conjugate Cyanation of Alpha, Beta-Unsaturated Imides[J]. Angew Chem Int Ed, 2008, 47(9): 1762-1765. doi: 10.1002/(ISSN)1521-3773
-
[19]
Zhang Z, Wang Z, Zhang R. An Efficient Titanium Catalyst for Enantioselective Cyanation of Aldehydes:Cooperative Catalysis[J]. Angew Chem Int Ed, 2010, 49(38): 6746-6750. doi: 10.1002/anie.201002127
-
[20]
Evano G, Schaus J V, Panek J S. A Convergent Synthesis of the Macrocyclic Core of Cytotrienins:Application of RCM for Macrocyclization[J]. Org Lett, 2004, 6(4): 525-528. doi: 10.1021/ol036284k
-
[21]
Cámpora J, Del Mar Conejo M A, Mereiter K. Synthesis of Dialkyl, Diaryl and Metallacyclic Complexes of Ni and Pd Containing Pyridine, α-Diimines and Other Nitrogen Ligands:Crystal Structures of the Complexes Cis-NiR2Py2(R=benzyl, mesityl)[J]. J Organomet Chem, 2003, 683(1): 220-239. doi: 10.1016/S0022-328X(03)00691-0
-
[22]
Göttker-Schnetmann I, Wehrmann P, Röhr C. Substituent Effects in (κ2-N, O)-Salicylaldiminato Nickel(Ⅱ)-Methyl Pyridine Polymerization Catalysts:Terphenyls Controlling Polyethylene Microstructures[J]. Organometallics, 2007, 26(9): 2348-2362. doi: 10.1021/om0611498
-
[23]
Bedernjak A F, Zaytsev A V, Babolat M. Synthesis and Evaluation of Novel 7-and 8-Aminophenoxazinones for the Detection of Beta-Alanine Aminopeptidase Activity and the Reliable Identification of Pseudomonas aeruginosa in Clinical Samples[J]. J Med Chem, 2016, 59(10): 4476-4487. doi: 10.1021/acs.jmedchem.5b01591
-
[24]
Hu X, Dai S, Chen C. Ethylene Polymerization by Salicylaldimine Nickel(Ⅱ) Complexes Containing a Dibenzhydryl Moiety[J]. Dalton Trans, 2016, 45(4): 1496-1503. doi: 10.1039/C5DT04408A
-
[25]
Sujith S, Joe D J, Na S J. Ethylene/Polar Norbornene Copolymerizations by Bimetallic Salicylaldimine-Nickel Catalysts[J]. Macromolecules, 2005, 38(24): 10027-10033. doi: 10.1021/ma051344i
-
[26]
Mu H L, Ye W P, Song D P. Highly Active Single-Component Neutral Nickel Ethylene Polymerization Catalysts:The Influence of Electronic Effects and Spectator Ligands[J]. Organometallics, 2010, 29(23): 6282-6290. doi: 10.1021/om100658j
-
[27]
Chen Z, Mesgar M, White P S. Synthesis of Branched Ultrahigh-Molecular-Weight Polyethylene Using Highly Active Neutral, Single-Component Ni(Ⅱ) Catalysts[J]. ACS Catal, 2014, : 631-636.
-
[28]
Connor E F, Younkin T R, Henderson J I. Synthesis of Neutral Nickel Catalysts for Ethylene Polymerization-The Influence of Ligand Size on Catalyst Stability[J]. Chem Commun, 2003, (18): 2272-2273. doi: 10.1039/b306701g
-
[29]
Wehrmann P, Mecking S. Highly Active Binuclear Neutral Nickel(Ⅱ) Catalysts Affording High Molecular Weight Polyethylene[J]. Organometallics, 2008, 27(7): 1399-1408. doi: 10.1021/om700942z
-
[30]
Liu S, Motta A, Mouat A R. Very Large Cooperative Effects in Heterobimetallic Titanium-Chromium Catalysts for Ethylene Polymerization/Copolymerization[J]. J Am Chem Soc, 2014, 136(29): 10460-10469. doi: 10.1021/ja5046742
-
[31]
Connor E F, Younkin T R, Henderson J I. Linear Functionalized Polyethylene Prepared with Highly Active Neutral Ni(Ⅱ) Complexes[J]. J Polym Sci, Part A:Polym Chem, 2002, 40(16): 2842-2854. doi: 10.1002/pola.v40:16
-
[32]
Kuhn P, Semeril D, Jeunesse C. Catalytic Applications of Keto-Stabilised Phosphorus Ylides Based on a Macrocyclic Scaffold:Calixarenes with One or Two Pendant Ni(P, O)-Subunits as Ethylene Oligomerisation and Polymerisation Catalysts[J]. Dalton Trans, 2006, (30): 3647-3659. doi: 10.1039/B603861A
-
[33]
Song D P, Wu J Q, Ye W P. Accessible, Highly Active Single-Component Beta-Ketiminato Neutral Nickel(Ⅱ) Catalysts for Ethylene Polymerization[J]. Organometallics, 2010, 29(10): 2306-2314. doi: 10.1021/om100075u
-
[34]
Radlauer M R, Day M W, Agapie T. Bimetallic Effects on Ethylene Polymerization in the Presence of Amines:Inhibition of the Deactivation by Lewis Bases[J]. J Am Chem Soc, 2012, 134(3): 1478-1481. doi: 10.1021/ja210990t
-
[35]
Radlauer M R, Buckley A K, Henling L M. Bimetallic Coordination Insertion Polymerization of Unprotected Polar Monomers:Copolymerization of Amino Olefins and Ethylene by Dinickel Bisphenoxyiminato Catalysts[J]. J Am Chem Soc, 2013, 135(10): 3784-3787. doi: 10.1021/ja4004816
-
[36]
Galland G B, de Souza R F, Mauler R S. 13C NMR Determination of the Composition of Linear Low-Density Polyethylene Obtained with[η3-Methallyl-Nickel-Diimine]PF6 Complex[J]. Macromolecules, 1999, 32(5): 1620-1625. doi: 10.1021/ma981669h
-
[37]
Dai S, Chen C. Direct Synthesis of Functionalized High-Molecular-Weight Polyethylene by Copolymerization of Ethylene with Polar Monomers[J]. Angew Chem Int Ed, 2016, 55(42): 13281-13285. doi: 10.1002/anie.201607152
-
[38]
Takeuchi D, Chiba Y, Takano S. Double-Decker-Type Dinuclear Nickel Catalyst for Olefin Polymerization:Efficient Incorporation of Functional Co-monomers[J]. Angew Chem Int Ed, 2013, 52(48): 12536-12540. doi: 10.1002/anie.201307741
-
[1]
-
Figure 1 Molecular structure of complex Ni0
Thermal ellipsoids are drawn at the 30% probability level, and H atoms are omitted for clarity. Selected bond distances(nm) and angles(°):Ni1—N1=0.1888(2), Ni1—N2=0.1900(2), Ni1—O1=0.1922(2), Ni1—C6=0.1933(3), N1—Ni1—N2=171.08(9), N1—Ni1—O1=93.08(8), N1—Ni1—C6=9342(11), N2—Ni1—O1=86.31(8), N2—Ni1—C6=88.42(11), O1—Ni1—C6=170.11(12)
Figure 2 Molecular structure of complex Ni1
Thermal ellipsoids are drawn at the 30% probability level, and H atoms are omitted for clarity. Selected bond distances(nm) and angles(°):Ni1—N1=0.1901(5), Ni1—N2=0.1915(5), Ni1—O1=0.1907(4), Ni1—C13=0.1921(6), N1—Ni1—N2=176.2(2), N1—Ni1—O1=92.82(18), N1—Ni1—C13=93.2(2), N2—Ni1—O1=85.82(19), N2—Ni1—C13=88.3(2), O1—Ni1—C13=173.2(2)
Figure 3 Molecular structure of complex Ni2
Thermal ellipsoids are drawn at the 30% probability level, and H atoms are omitted for clarity. Selected bond distances(nm) and angles(°):Ni1—N1=0.1886(2), Ni1—N2=0.1904(3), Ni1—O1=0.1907(2), Ni1—C35=0.1935(3), N1—Ni1—N2=168.47(11), N1—Ni1—O1=93.87(10), N1—Ni1—C35=95.02(13), N2—Ni1—O1=85.55(11), N2—Ni1—C35=88.21(13), O1—Ni1—C35=164.62(13)
Figure 4 13C NMR spectrum of PE obtained by Ni1 at 50 ℃(entry 4, Table 1)
Figure 5 1H NMR spectrum(400 MHz, o-dichlorobenzene-d4, 110 ℃) of copolymer obtained by Ni1 at 50 ℃(entry 3, Table 2)
Figure 6 1H NMR spectrum(400 MHz, o-dichlorobenzene-d4, 110 ℃) of copolymer obtained by Ni1 at 50 ℃(entry 4, Table 2)
Table 1. Ethylene polymerization using neutral nickel catalysts Ni0~Ni3a
Entry Catalyst Temperature/℃ Yield/g Activity b Mwc/(kg·mol-1) Mw/Mnc Tmd/℃ 1 Ni0 40 0.19 1.5 45.4 2.2 114 2 Ni0 50 0.72 5.8 25.2 2.3 103 3 Ni0 60 1.23 9.8 15.1 2.2 96 4 Ni0 70 0.96 7.7 10.1 2.1 87 5 Ni1 40 0.22 1.8 51.7 2.2 115 6 Ni1 50 1.31 10.5 19.4 2.2 97 7 Ni1 60 0.82 6.6 11.6 2.2 89 8 Ni1 70 0.53 4.2 8.6 2.1 75 9e Ni1 50 2.24 6.0 17.0 2.2 92 10f Ni1 50 3.07 4.1 17.0 2.1 92 11g Ni1 50 0.016 0.13 4.9 1.8 - 12 Ni2 40 1.21 9.7 11.6 2.1 81 13 Ni2 50 1.91 15.3 6.9 2.0 68 14 Ni2 60 2.24 17.9 4.2 2.3 - 15 Ni2 70 1.75 14.0 3.5 1.1 - 16 Ni3 40 0.17 1.4 70.3 2.5 124 17 Ni3 50 1.11 8.9 27.3 2.4 99 18 Ni3 60 0.61 4.9 19.3 2.2 93 19 Ni3 70 trace - - - - 20e Ni3 50 2.52 3.4 16.6 2.1 91 21f Ni3 50 2.82 1.9 18.0 2.1 93 22g Ni3 50 0.021 0.17 5.4 1.9 - a.Polymerizations conditions:toluene, 40 mL; nickel, 5(mol; ethylene, 5×105 Pa; 15 min; b.in the unit of 105 g PE mol-1·Ni-1·h-1; c.determined by GPC vs polystyrene standards; d.determined by DSC; e.reaction time: 45 min; f.reaction time:90 min; g.25(mol PPh3 as additives. Table 2. Ethylene copolymerization using Ni1-Ni3 complexesa
Entry Catalyst Comonomer Yield/mg Activityb Mwc/(kg·mol-1) Mw/Mnc Tmd/℃ Xce 1 Ni1 
260 17.7 16.3 1.9 93 nd 2 Ni1 
230 15.3 16.6 2.0 94 nd 3 Ni1 
61 4.1 20.5 1.9 103 0.92 4 Ni1 
150 10.0 15.4 1.8 94 0.82 5 f Ni1 
49 3.3 16.5 2.2 97 1.01 6 Ni2 
18 1.2 6.0 1.7 - 0.16 7 Ni2 
101 6.7 6.7 1.7 - 0.25 8 Ni3 
301 20.1 14.9 2.0 92 nd 9 Ni3 
252 16.8 17.6 1.7 94 nd 10 Ni3 
72 4.8 16.1 1.9 96 0.91 11 Ni3 
182 12.1 16.5 1.9 95 0.64 12 f Ni3 
62 4.1 16.8 2.1 93 1.10 a.Polymerizations conditions:toluene, 40 mL; nickel, 10 (mol; ethylene, 5×105 Pa; 90 min, 600 stoichiometric amount of comonomer; b.in the unit of 103 g PE mol-1·Ni-1·h-1; c.determined by GPC vs. polystyrene standards; d.determined by DSC; e.incorporation ratio was calculated according to 1H NMR spectra; f.1000 stoichiometric amount of comonomer. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 1329
- HTML全文浏览量: 102


 下载:
下载:






 下载:
下载:

