 图Scheme1
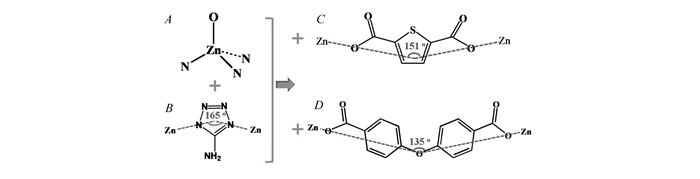
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9
Scheme1.
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9
图Scheme1
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9
Scheme1.
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9

利用羧酸配体合理设计沸石型四氮唑框架及其快速吸附碘
English
Rational Design of Zeolitic Tetrazolate Frameworks with Carboxylate Ligands for Rapid Accumulation of Iodine
-
Key words:
- zeolitic tetrazolate frameworks
- / assembly strategy
- / absorbing iodine
-
Owing to their intriguing architectures, large surface areas, and tunable pore size and shape, metal-organic frameworks(MOFs) have received tremendous attention over the past decade[1-12]. Through simulating the structure of zeolite, the assembly of zeolitic MOFs has attracted more extensive attention[13-17]. One key reason is that this kind of functional material not only shows the high porosity of MOF material but also possesses high thermal and chemical stability of zeolites[18]. As we know, employing the tetrahedrally coordinated divalent cations(M2+=Zn2+ or Co2+) and univalent imidazolate to replace TO4(T=Si4+, Al3+, or P5+, etc.) building blocks of zeolite is an effective strategy for fabricating zeolite-like structures. On the other hand, tailoring or altering organic component can often achieve awide exploration of diverse structures during the synthetic process of MOFs[19-23]. As a universal combination strategy, zeolitic MOFs are also constructed by using tetrahedrally coordinated divalent cation as the metal center and univalent imidazolate, triazolate or tetrazolate derivative as the linker[24-30]. On the other hand, as an important radioactive element, it is necessary to absorb I2 for purpose of avoiding serious environmental pollution. The irregular pores and little adjustable porosity of activated carbon and porous silica hinder their performances on adsorbing iodine species. As mentioned above, zeolitic MOFs are one type of the most promising materials because of high specific surface area, unique architecture, controlled pore size and regular shape of pore.
In our previous work, we successfully employed isonictinate as auxiliary ligand to construct tetrahedral imidazolate frameworks with dmp, dia and neb topology, respectively[22]. As a continuous work, we report here two tetrahedral tetrazolate frameworks(TTFs) with zeolitic BCT topology, namely, [N(CH3)4][Zn2(atz)3(thb)] guest(TTF-8, atz=5-amino-1H-tetrazole, thb=thiophene-2, 5-dicarboxylate) and [N(CH3)4][Zn2(atz)3(obb)] guest(TTF-9, H2obb=4, 4′-oxybisbenzoic acid)(Scheme 1). Notably, although these TTFs exhibit the same BCT topology, their frameworks and pore structures are entirely different. Remarkably, two TTFs materials display outstanding performance on rapidly enriching iodine.
 图Scheme1
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9
Scheme1.
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9
图Scheme1
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9
Scheme1.
The assembly strategy and bond angles of the linkers in TTF-8 and TTF-9
1 Experimental
1.1 Materials and Instruments
All reagents and solvents with analytic grade for the synthesis in this work were purchased from Energy Chemical Corporation and Beijing HWRK Chemical Co., Ltd in China and used as received.
Diffraction data were collected by using a computer-controlled XCalibur E CCD diffractometer(Agilent, America) with graphite-monochromated MoKα radiation(λMoKα=0.071073 nm) at T=293.2 K. The phase purity and the structural integrity of experimental samples were determined by MiniFlex2 X-ray diffractometer(XRD, Rigaku, Japan) using CuKα(λ=0.1542 nm) radiation. The diffractometer data were recorded for 2θ values from 3° to 50° at a scanning rate of 1°/min. Thermogravimetric analysis(TGA, Netzsch, Germany) was carried out on a Netzsch STA449C equipped with a platinum pan at a heating rate of 15 ℃/min in N2 atmosphere.
1.2 Synthesis of [N(CH3)4][Zn2(atz)3(thb)] guest(TTF-8) and [N(CH3)4][Zn2(atz)3(obb)](TTF-9)
A mixture of Zn(NO3)2·6H2O(0.1600 g, 0.54 mmol), pyrazine(0.0300 g, 0.37 mmol), 5-amino-1H-tetrazole(atz, 0.0500 g, 0.59 mmol), thiophene-2, 5-dicarboxylic acid(thb, 0.0560 g, 0.33 mmol), tetramethylammonium bromide(0.0300 g, 0.20 mmol), methanol(3 mL) and N, N-dimethylacetamide(DMA, 3 mL) in a 23 mL Teflon-lined stainless steel vessel was heated at 120 ℃ for 36 h, and then cooled to room temperature. The resulting transparent colorless crystals(TTF-8) were obtained, washed with acetone, and dried at room temperature. TTF-9 was obtained by the similar method as described for TTF-8 except for using 4, 4′-oxybisbenzoic acid(obb, 0.0800 g, 0.31 mmol) instead of thiophene-2, 5-dicarboxylic acid.
1.3 Crystal data for TTF-8
Tetragonal, M=553.1, a=b=2.15065(16) nm, c=1.02633(9) nm, V=4.7471(7) nm3, T=293(2) K, space group P4(2) mc, Z=4, 8440 reflections measured, 3862 independent reflections(Rint=0.1128). The final R1 values were 0.1128(I > 2σ(I)). The final wR(F2) values were 0.2765(I > 2σ(I)). The goodness of fit on F2 was 1.072.
1.4 Crystal data for TTF-9
Monoclinic, M=639.21, a=1.02628(4) nm, b=3.5575(4) nm, c=1.82706(15) nm, V=5.5614(8) nm3, T=293(2) K, space group P21/c, Z=4, 12063 reflections measured, 8132 independent reflections(Rint=0.0515). The final R1 value was 0.0771(I > 2σ(I)). The final wR(F2) value was 0.1872(I > 2σ(I)). The goodness of fit on F2 was 1.027.
2 Results and discussion
2.1 Crystal structures of TTF-8 and TTF-9
Colorless crystals of TTF-8 and TTF-9 were solvothermally prepared, respectively. With the purpose of achieving the charge-balancing of compounds, N(CH3)4+ is chosen as the counter-ion, which is very important for successfully obtaining TTF-8 and TTF-9. Their structures were characterized by single-crystal X-ray diffraction. Both of them exhibit anionic porous frameworks with large free voids occupied by the charge-balancing N(CH3)4+ cations and structurally disordered solvent molecules. The phase purity and thermal stability of TTF-8 and TTF-9 were measured by powder X-ray diffraction(PXRD) and TGA, respectively(see Fig.S1~Fig.S4 in Supporting Information).
TTF-8 crystallizes in a tetragonal space group of P42mc. In the structure of TTF-8, Zn(Ⅱ) ion adopts a tetrahedral coordination mode and is connected by three atz ligands and one thb ligand(Fig. 1A). The deprotonated atz ligand just uses two N donors to bridge two Zn(Ⅱ) sites, and the thb linker bridges two Zn centers. Six Zn centers are bridged by six atz ligands to generate a 6-membered ring, which is further linked by atz ligand to form a channel unit(Fig. 1B). Such Zn-atz channels are further linked by the thb ligands into a three-dimensional framework(Fig. 1C). It is worth noting that three types of channels are fabricated with vertex-sharing fashion to form the three-dimensional open framework of TTF-8.
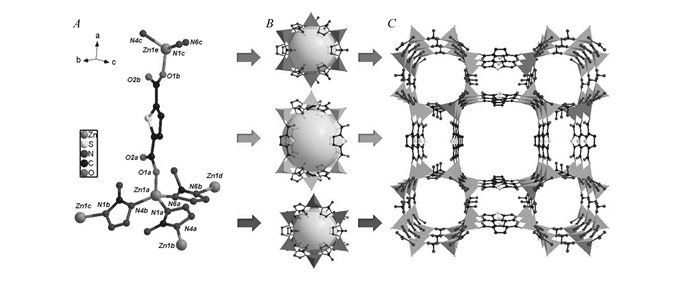 图1
The coordination environment of the zinc atom and bridging mode(A), the building block(B) and view of the 3D framework(C) of TTF-8. Hydrogen atoms were omitted for clarity
Figure1.
The coordination environment of the zinc atom and bridging mode(A), the building block(B) and view of the 3D framework(C) of TTF-8. Hydrogen atoms were omitted for clarity
图1
The coordination environment of the zinc atom and bridging mode(A), the building block(B) and view of the 3D framework(C) of TTF-8. Hydrogen atoms were omitted for clarity
Figure1.
The coordination environment of the zinc atom and bridging mode(A), the building block(B) and view of the 3D framework(C) of TTF-8. Hydrogen atoms were omitted for clarity
By employing obb to replace thb, another zeolitic framework TTF-9 is obtained under a similar reaction condition. Comparatively, the structure of TTF-9 shows low symmetry in monoclinic P21/c. TTF-9 exhibits the similar coodination environment of TTF-8, where Zn(Ⅱ) ion is 4-connected by three atz ligands and one obb ligand to form tetrahedron geometry(Fig. 2A). The deprotonated atz and obb ligand act as linear linkers to bridge one Zn1 and Zn2(Fig. 2B). If obb linkers serve as the pillars, atz ligands adopt a μ2-1, 4 bridging mode to link two Zn atoms and resulted in a wavy honeycomb-like Zn-atz layer along the b-axis(Fig. 2C). As shown in Fig. 2D, the adjacent layers are bridged by auxiliary obb linkers along the b-axis, which produces a three-dimensional network.
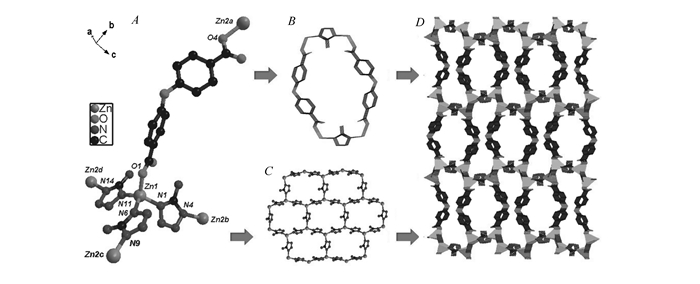 图2
The coordination environment of the zinc atom(A), bridging mode of ligands(B), layer unit(C) and view of the 3D framework(D) of TTF-9. Hydrogen atoms were omitted for clarity
Figure2.
The coordination environment of the zinc atom(A), bridging mode of ligands(B), layer unit(C) and view of the 3D framework(D) of TTF-9. Hydrogen atoms were omitted for clarity
图2
The coordination environment of the zinc atom(A), bridging mode of ligands(B), layer unit(C) and view of the 3D framework(D) of TTF-9. Hydrogen atoms were omitted for clarity
Figure2.
The coordination environment of the zinc atom(A), bridging mode of ligands(B), layer unit(C) and view of the 3D framework(D) of TTF-9. Hydrogen atoms were omitted for clarity
2.2 Structure differences between TTF-8 and TTF-9
It is obvious that there are many structural differences between TTF-8 and TTF-9. Although their coordination environment and bridging mode are similar, further analysis reveals that the Zn-atz-Zn angles are entirely different. The Zn-atz-Zn angles in TTF-8 are 112.7°, 112.7° and 113.7°, respectively, which lead to a one-dimensional channel as building block(Fig. 3A and Fig. 3C). Different Zn-atz-Zn angles(109.2°, 109.9° and 114.2°) in TTF-9 bring a layer as unit(Fig. 3B and 3D). The thb and obb link these two types of units, respectively, which produce two different structural features with the same topology. Both TTF framework topologies are identified as the 4-connected net with the symbol BCT(vertex symbol:4·65) by reducing each Zn site as the 4-connected node(Fig. 3E and Fig. 3F).
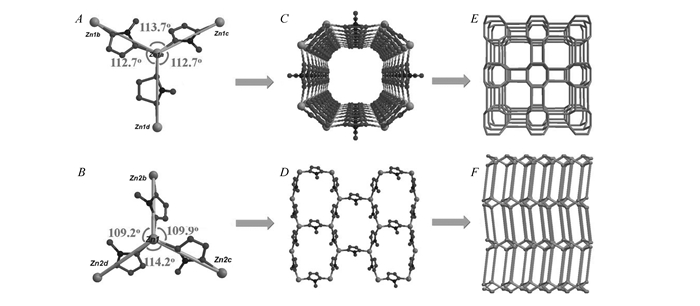 图3
The angle among Zn atoms bridged by atz in TTF-8(A) and TTF-9(B); the building block of TTF-8(C) and TTF-9(D); the BCT topology of TTF-8(E) and TTF-9(F)
Figure3.
The angle among Zn atoms bridged by atz in TTF-8(A) and TTF-9(B); the building block of TTF-8(C) and TTF-9(D); the BCT topology of TTF-8(E) and TTF-9(F)
图3
The angle among Zn atoms bridged by atz in TTF-8(A) and TTF-9(B); the building block of TTF-8(C) and TTF-9(D); the BCT topology of TTF-8(E) and TTF-9(F)
Figure3.
The angle among Zn atoms bridged by atz in TTF-8(A) and TTF-9(B); the building block of TTF-8(C) and TTF-9(D); the BCT topology of TTF-8(E) and TTF-9(F)
2.3 The performance of absorbing I2
Investigating the performance of absorbing I2 was finished by means of immersing 100 mg of single crystals of TTF-8 and TTF-9 in a cyclohexane solution of I2(0.1 mol/L), respectively. The dark red solutions of I2 faded slowly to pale brown with the colour of crystals changing from colorless to dark brown(Fig. 4A and Fig.S5A in Supporting Information). The mass of TTF-8 and TTF-9 after loading iodine increased by ca. mass fraction 23.3% and 18.1%, respectively. As depicted in Fig. 4B(and Fig.S5B in Supporting Information), when the compounds with encapsulated I2 were put into the ethanol(EtOH), the color of EtOH extract changed from colorless to pale brown, which shows that I2 sorption is reversible. The releasing process of I2 was further investigated by the following method with a non-aqueous solution. Very little I2-loaded single crystals of TTF-8 or TTF-9 were placed in 9 mL ethanol, and the iodine content was estimated by UV/Vis spectroscopy at room temperature(Fig. 4C and Fig.S5C in Supporting Information). The absorbance of I2 extracted into ethanol at 288 nm and 360 nm normally increases within 50 minutes. The dynamic equilibrium of the release and adsorption of I2 is reached about 1 hour(Fig.S6 and Fig.S7 in Supporting Information)
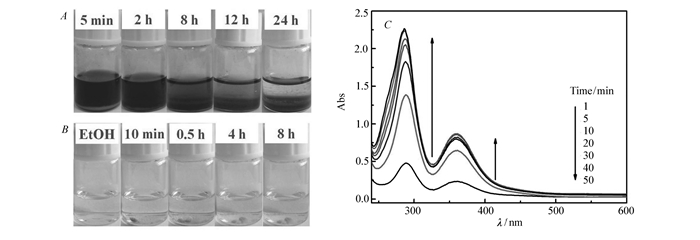 图4
(A)Photos of the iodine recovery process with 100 mg of crystals of TTF-8 soaked in cyclohexane solution of I2(0.1 mol/L, 1.5 mL); (B)iodine releasing process of TTF-8(10 mg) soaked in 1.5 mL of EtOH; (C)temporal evolution of UV/vis absorption spectra for the I2 releasing from TTF-8 in 9 mL of EtOH
Figure4.
(A)Photos of the iodine recovery process with 100 mg of crystals of TTF-8 soaked in cyclohexane solution of I2(0.1 mol/L, 1.5 mL); (B)iodine releasing process of TTF-8(10 mg) soaked in 1.5 mL of EtOH; (C)temporal evolution of UV/vis absorption spectra for the I2 releasing from TTF-8 in 9 mL of EtOH
图4
(A)Photos of the iodine recovery process with 100 mg of crystals of TTF-8 soaked in cyclohexane solution of I2(0.1 mol/L, 1.5 mL); (B)iodine releasing process of TTF-8(10 mg) soaked in 1.5 mL of EtOH; (C)temporal evolution of UV/vis absorption spectra for the I2 releasing from TTF-8 in 9 mL of EtOH
Figure4.
(A)Photos of the iodine recovery process with 100 mg of crystals of TTF-8 soaked in cyclohexane solution of I2(0.1 mol/L, 1.5 mL); (B)iodine releasing process of TTF-8(10 mg) soaked in 1.5 mL of EtOH; (C)temporal evolution of UV/vis absorption spectra for the I2 releasing from TTF-8 in 9 mL of EtOH
3 Conclusions
In summary, the synthesis of zeolitic framework with BCT topology is implemented via the strategy of using dicarboxylic acid as auxiliary ligand. The different structural feature deriving same coordination environment and bridging mode proves that secondary ligands(thb and obb) can alter the structures of TTFs. Both TTF-8 and TTF-9 display performance of rapid accumulation of iodine. This work provides a new approach toward the construction of novel zeolite-type framework materials.
The Supporting Information [PXRD, TGA, Releasing I2 of TTF-8 and TTF-9] is available free of charge on the website of Chinese Journal of Applied Chemistry(http://yyhx.ciac.jl.cn)
-
-
[1]
Wu H H, Gong Q H, Olson D H. Commensurate Adsorption of Hydrocarbons and Alcohols in Microporous Metal Organic Frameworks[J]. Chem Rev, 2012, 112(2): 836-868. doi: 10.1021/cr200216x
-
[2]
Li B Y, Leng K Y, Zhang Y M. Metal Organic Framework Based upon the Synergy of a Brønsted Acid Framework and Lewis Acid Centers as a Highly Efficient Heterogeneous Catalyst for Fixed-Bed Reactions[J]. J Am Chem Soc, 2015, 137(12): 4243-4248. doi: 10.1021/jacs.5b01352
-
[3]
Ye Y X, Zhang L Q, Peng Q F. High Anhydrous Proton Conductivity of Imidazole-Loaded Mesoporous Polyimides over a Wide Range from Subzero to Moderate Temperature[J]. J Am Chem Soc, 2015, 137(2): 913-918. doi: 10.1021/ja511389q
-
[4]
Liu J W, Chen L F, Cui H. Applications of Metal Organic Frameworks in Heterogeneous Supramolecular Catalysis[J]. Chem Soc Rev, 2014, 43(16): 6011-6061. doi: 10.1039/C4CS00094C
-
[5]
Zhang M, Feng G X, Song Z G. Two-Dimensional Metal Organic Framework with Wide Channels and Responsive Turn-On Fluorescence for the Chemical Sensing of Volatile Organic Compounds[J]. J Am Chem Soc, 2014, 136(20): 7241-7242. doi: 10.1021/ja502643p
-
[6]
Gu Z G, Zhan C H, Zhang J. Chiral Chemistry of Metal Camphorate Frameworks[J]. Chem Soc Rev, 2016, 45(11): 3122-3144. doi: 10.1039/C6CS00051G
-
[7]
Liu M, Chen S M, Wen T. Encapsulation of Ln(Ⅲ) Ions/Ag Nanoparticles Within Cd(Ⅱ) Boron Imidazolate Frameworks for Tuning Luminescence Emission[J]. Chem Commun, 2016, 52(55): 8577-8580. doi: 10.1039/C6CC03285K
-
[8]
Banerjeel R, Phanl A, Wang B. Report High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture[J]. Science, 2008, 319(5865): 939-943. doi: 10.1126/science.1152516
-
[9]
Zheng S T, Wu T, Irfanoglu B. Multicomponent Self-Assembly of a Nested Co24@Co48 Metal-Organic Polyhedral Framework[J]. Angew Chem Int Ed, 2011, 50(35): 8034-8037. doi: 10.1002/anie.v50.35
-
[10]
Li B Y, Zhang Y M, Rajamani K. Introduction of π-Complexation into Porous Aromatic Framework for Highly Selective Adsorption of Ethylene over Ethane[J]. J Am Chem Soc, 2014, 136(24): 8654-8660. doi: 10.1021/ja502119z
-
[11]
Liao P Q, Zhang W X, Zhang J P. Efficient Purification of Ethene by an Ethane-Trapping Metal-Organic Framework[J]. Nat Commun, 2015, 6: 8697. doi: 10.1038/ncomms9697
-
[12]
Kang Y, Fang W H, Zhang L. A Structure-Directing Method to Prepare Semiconductive Zeolitic Cluster Organic Frameworks with Cu3I4 Building Units[J]. Chem Commun, 2015, 51(43): 8994-8997. doi: 10.1039/C5CC02598B
-
[13]
Huang X C, Zhang J P, Chen X M. Ligand-Directed Strategy for Zeolite-Type Metal-Organic Frameworks:Zinc(Ⅱ) Imidazolates with Unusual Zeolitic Topologies[J]. Angew Chem Int Ed, 2006, 45(10): 1557-1559. doi: 10.1002/(ISSN)1521-3773
-
[14]
Hayashi H, Côté A P, Furukawa H. Zeolite A Imidazolate Frameworks[J]. Nat Mater, 2007, 6(7): 501-506. doi: 10.1038/nmat1927
-
[15]
Wang B, Côté A, Furukawa H. Colossal Cages in Zeolitic Imidazolate Frameworks as Selective Carbon Dioxide Reservoirs[J]. Nature, 2008, 453(7192): 207-211. doi: 10.1038/nature06900
-
[16]
Tian Y Q, Yao S Y, Gu D. Cadmium Imidazolate Frameworks with Polymorphism, High Thermal Stability, and a Large Surface Area[J]. Chem Eur J, 2010, 16(4): 1137-1141. doi: 10.1002/chem.v16:4
-
[17]
Rahul B, Phan A, Wang B. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture[J]. Science, 2008, 319(5865): 939-943. doi: 10.1126/science.1152516
-
[18]
Phan A, Doonan C J, Uribe-Romo F. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks[J]. Acc Chem Res, 2010, 43(1): 58-67. doi: 10.1021/ar900116g
-
[19]
Zheng S T, Bu J T, Li Y F. Pore Space Partition and Charge Separation in Cage-Within-Cage Indium-Organic Frameworks with High CO2 Uptake[J]. J Am Chem Soc, 2010, 132(48): 17062-17064. doi: 10.1021/ja106903p
-
[20]
Yu Y D, Luo C, Liu B Y. Spontaneous Symmetry Breaking of Co(Ⅱ) Metal Organic Frameworks from Achiral Precursors via Asymmetrical Crystallization[J]. Chem Commun, 2015, 51(77): 14489-14492. doi: 10.1039/C5CC06166K
-
[21]
Zhai Q G, Bai N, Li S N. Design of Pore Size and Functionality in Pillar-Layered Zn-Triazolate-Dicarboxylate Frameworks and Their High CO2/CH4 and C2 Hydrocarbons/CH4 Selectivity[J]. Inorg Chem, 2015, 54(20): 9862-9868. doi: 10.1021/acs.inorgchem.5b01611
-
[22]
Wang F, Tan Y X, Yang H. A New Approach Towards Tetrahedral Imidazolate Frameworks for High and Selective CO2 Uptake[J]. Chem Commun, 2011, 47(20): 5828-5830. doi: 10.1039/c1cc10829h
-
[23]
Wang F, Liu Z S, Yang H. Hybrid Zeolitic Imidazolate Frameworks with Catalytically Active TO4 Building Blocks[J]. Angew Chem Int Ed, 2011, 50(2): 450-453. doi: 10.1002/anie.201005917
-
[24]
Zhang J, Wu T, Zhou C. Zeolitic Boron Imidazolate Frameworks[J]. Angew Chem Int Ed, 2009, 48(14): 2542-2545. doi: 10.1002/anie.v48:14
-
[25]
Panda T, Pachfule P, Chen Y F. Amino Functionalized Zeolitic Tetrazolate Framework(ZTF) with High Capacity for Storage of Carbon Dioxide[J]. Chem Commun, 2011, 47(7): 2011-2013. doi: 10.1039/C0CC04169F
-
[26]
Zhang J P, Zhu A X, Lin R B. Pore Surface Tailored SOD-Type Metal-Organic Zeolites[J]. Adv Mater, 2011, 23(10): 1268-1271. doi: 10.1002/adma.201004028
-
[27]
Wang F, Hou D C, Yang H. Tetrahedral Tetrazolate Frameworks for High CO2 and H2 Uptake[J]. Dalton Trans, 2014, 43(8): 3210-3214. doi: 10.1039/C3DT53269K
-
[28]
Wang F, Fu H R, Kang Y. A New Approach Towards Zeolitic Tetrazolate-Imidazolate Frameworks(ZTIFs) with Uncoordinated N-Heteroatom Sites for High CO2 Uptake[J]. Chem Commun, 2014, 50(81): 12065-12068. doi: 10.1039/C4CC05022C
-
[29]
Tang Y H, Wang F, Liu J X. Diverse Tetrahedral Tetrazolate Frameworks with N-Rich Surface[J]. Chem Commun, 2016, 52(32): 5625-5628. doi: 10.1039/C6CC00589F
-
[30]
Li M Y, Wang F, Zhang J. Zeolitic Tetrazolate-Imidazolate Frameworks with High Chemical Stability for Selective Separation of Small Hydrocarbons[J]. Cryst Growth Des, 2016, 16(6): 3063-3066. doi: 10.1021/acs.cgd.6b00422
-
[1]
-
Figure 4 (A)Photos of the iodine recovery process with 100 mg of crystals of TTF-8 soaked in cyclohexane solution of I2(0.1 mol/L, 1.5 mL); (B)iodine releasing process of TTF-8(10 mg) soaked in 1.5 mL of EtOH; (C)temporal evolution of UV/vis absorption spectra for the I2 releasing from TTF-8 in 9 mL of EtOH
-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 1
- 文章访问数: 806
- HTML全文浏览量: 86


 下载:
下载:




 下载:
下载:

