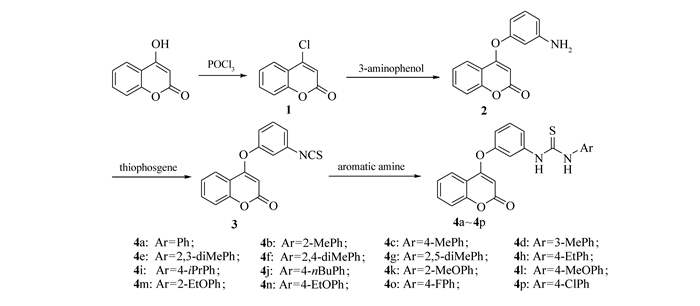
 Scheme 1.
Synthetic routes of the title compounds (4a~4p)
Scheme 1.
Synthetic routes of the title compounds (4a~4p)

Citation: CHEN Meihang, ZHANG Xun, WANG Xiaobin, CHEN Shixue, SHU Hua. Synthesis and Antibacterial Activity of 1-Aryl-3-(3-((2-oxo-2H-Chromen-4-yl)oxy)phenyl)thiourea Derivatives[J]. Chinese Journal of Applied Chemistry, 2017, 34(7): 774-782. doi: 10.11944/j.issn.1000-0518.2017.07.160427

1-芳基-3-(3-(4-氧香豆素基)苯基)硫脲衍生物的合成及抑菌活性
English
Synthesis and Antibacterial Activity of 1-Aryl-3-(3-((2-oxo-2H-Chromen-4-yl)oxy)phenyl)thiourea Derivatives
-
Key words:
- coumarin
- / thiourea derivatives
- / synthesis
- / antibacterial activities
-
近年来,农作物细菌性病害已成为严重影响农业生产的主要病害之一,严重影响农作物的产量和品质,给农户造成重大经济损失。细菌性病害是由细菌病原菌侵染所致的病害,如软腐病、溃疡病、青枯病等,这些侵害植物的细菌可通过自然孔口(气孔、皮孔、水孔等)和伤口侵入,借流水、雨水、昆虫等传播,在病残体、种子、土壤中过冬,在高温、高湿条件下容易发病[1]。目前,防治农作物细菌性病害的农药种类还比较少,现有农药品种如农用链霉素、叶青双和铜制剂(噬菌铜、硫酸铜、氧氯化铜)等药剂[2]。硫脲类衍生物具有抑菌[3-4]、抗病毒[5]、抗肿瘤[6]、抗寄生虫[7]等生物活性。近年来,有大量的文献报道了具有抗菌活性的硫脲类衍生物[8-10]。此外,4-羟基香豆素及其衍生物具有抗菌[11]、除草[12]、抗癌[13]、抗HIV[14]等生物活性,同时也是合成医药、农药、染料的重要中间体[15]。研究发现大多数4-羟基香豆素衍生物具有优良的抑菌活性,如:徐翠莲等[16]报道了含有香豆素骨架的缩氨基硫脲化合物对革兰氏阳性菌和阴性菌均有比较显著的抑菌活性。我们课题组[17]前期发现了具有抑制水稻白叶枯菌和柑橘溃疡菌活性含香豆素的席夫碱类衍生物。为了筛选出更高抗菌活性的含香豆素类衍生物,以4-羟基香豆素为原料,经氯化、醚化异硫氰酸化和加成等反应合成了一系列1-芳基-3-(3-(4-氧香豆素基)苯基)硫脲衍生物,并对目标化合物进行抑制水稻白叶枯菌和柑橘溃疡病菌活性测试。目标化合物(4a~4p)的合成路线如Scheme 1所示。
1 实验部分
1.1 仪器和试剂
IR Affinity-1S型傅里叶变换分光光度计(日本岛津公司);JEOL-ECX500型500 MHz核磁共振仪(日本电子株式会社);X-4型数字显微熔点测定仪(北京泰克仪器有限公司);RE-52AA型旋转蒸发仪(上海亚荣生化有限公司);IKA RCT型基本型磁力搅拌器(广州仪科实验技术有限公司);QY-20型三用紫外分析仪(上海市安亭电子仪器厂)。
4-羟基香豆素(≥98%,天津希恩思生化科技有限公司);对氨基酚(≥98%,Aladdin-上海阿拉丁生化科技股份有限公司);硫光气(≥95%,天津希恩思生化科技有限公司)。其余试剂均为分析纯。
1.2 实验方法
1.3 抑菌生物活性测试
1.2.4 1-芳基-3-(3-(4-氧香豆素基)苯基)硫脲(4a~4p)的合成
将0.295 g(1.0 mmol)4-(4-异硫氰酸酯苯氧基)香豆素(3)和1.1 mmol芳香胺溶于10 mL四氢呋喃中,室温反应,反应1~3 h后停止搅拌,减压除去溶剂,再用甲醇重结晶,得到目标化合物1-芳基-3-(3-(4-氧香豆素基)苯基)硫脲衍生物(4a~4p)。
1-苯基-3-(3-(4-氧香豆素基)苯基)硫脲(4a):白色固体, mp 163~165 ℃, 产率80%;1H NMR(500 MHz, DMSO-d6), δ:5.26(s, 1H, coumarin-3-H), 6.69(d, J=7.5 Hz, 1H, Ar—H), 7.12(d, J=7.0 Hz, 1H, Ar—H), 7.30~7.48(m, 7H, coumarin-6, 8-H, Ar—H), 7.60(d, J=8.0 Hz, 2H, Ar—H), 7.74(t, J=8.5 Hz, 1H, coumarin-7-H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.81(s, 1H, OPhN—H), 9.90(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:93.34, 115.26, 117.07, 121.81, 123.47, 124.36, 125.09, 126.09, 128.93, 133.87, 138.37, 139.83, 148.92, 153.56, 161.65, 166.46, 181.29;IR(KBr), σ/cm-1:3323, 3159, 1716, 1606, 1541, 1388, 1224, 1145;MS(ESI)计算值(C22H16N2O3S)[M+Na]+:403.0, 测量值:403.9。
1-(2-甲基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4b):白色固体, mp 167~169 ℃, 产率78%;1H NMR(500 MHz, DMSO-d6), δ:2.25(s, 3H, CH3), 5.26(s, 1H, coumarin-3-H), 6.69(d, J=7.0 Hz, 1H, Ar—H), 7.12(d, J=7.5 Hz, 1H, Ar—H), 7.30~7.49(m, 6H, coumarin-6, 8-H, Ar—H), 7.61(d, J=7.5 Hz, 2H, Ar—H), 7.75(t, J=8.0 Hz, 1H, coumarin-7-H), 8.05(d, J=8.0 Hz, 1H, coumarin-5-H), 9.76(s, 1H, OPhN—H), 9.98(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:21.36, 93.17, 115.10, 116.94, 121.44, 121.67, 123.35, 124.75, 124.86, 125.71, 126.01, 128.67, 133.75, 137.75, 138.26, 139.52, 148.75, 153.42, 161.54, 166.34, 180.05;IR(KBr), σ/cm-1):3334, 3153, 1707, 1606, 1560, 1386, 1276, 1145;MS(ESI)计算值(C23H18N2O3S)[M+Na]+:425.5, 测量值: 425.7。
1-(4-甲基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4c):白色固体, mp 167~169 ℃, 产率83%;1H NMR(500 MHz, DMSO-d6), δ:2.21(s, 3H, CH3), 5.26(s, 1H, coumarin-3-H), 6.68(d, J=7.5 Hz, 1H, Ar—H), 7.12(d, J=7.0 Hz, 1H, Ar—H), 7.30~7.48(m, 6H, coumarin-6, 8-H, Ar—H), 7.60(d, J=8.0 Hz, 2H, Ar—H), 7.74(t, J=7.5 Hz, 1H, coumarin-7-H), 8.03(d, J=7.0 Hz, 1H, coumarin-5-H), 9.36(s, 1H, OPhN—H), 9.75(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:19.02, 94.02, 115.93, 117.74, 122.52, 124.16, 125.66, 127.07, 127.27, 127.74, 129.24, 131.48, 134.53, 136.09, 139.99, 149.69, 154.24, 162.34, 167.15, 181.72;IR(KBr), σ/cm-1:3323, 3159, 1716, 1606, 1541, 1388, 1224, 1145;MS(ESI)计算值(C23H18N2O3S)[M+Na]+:425.1, 测量值:425.5。
1-(3-甲基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4d):白色固体, mp 169~171 ℃, 产率86%;1H NMR(500 MHz, DMSO-d6), δ:2.25(s, 3H, CH3), 5.26(s, 1H, coumarin-3-H), 6.69(d, J=7.0 Hz, 1H, Ar—H), 7.11(d, J=7.5 Hz, 1H, Ar—H), 7.33~7.48(m, 6H, coumarin-6, 8-H, Ar—H), 7.60(d, J=7.5 Hz, 2H, Ar—H), 7.74(t, J=7.5 Hz, 2H, coumarin-7-H, Ar—H), 8.03(d, J=7.5 Hz, 1H, coumarin-5-H), 9.38(s, 1H, OPhN—H), 9.96(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:18.34, 93.80, 115.69, 117.51, 121.98, 122.36, 123.92, 125.42, 126.98, 127.22, 128.67, 130.67, 132.66, 134.30, 134.90, 138.72, 140.18, 149.64, 154.00, 162.14, 166.91, 181.41;IR(KBr), σ/cm-1:3322, 3132, 1714, 1606, 1543, 1386, 1226, 1147;MS(ESI)计算值(C23H18N2O3S)[M+Na]+:425.1, 测量值:425.1。
1-(2, 3-二甲基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4e):白色固体, mp 175~176 ℃, 产率74%;1H NMR(500 MHz, DMSO-d6), δ:2.15(s, 3H, CH3), 2.26(s, 3H, CH3), 5.26(s, 1H, coumarin-3-H), 6.69(d, J=7.0 Hz, 1H, Ar—H), 7.08~7.30(m, 4H, Ar—H), 7.45~7.49(m, 2H, coumarin-6, 8-H), 7.60(d, J=9.0 Hz, 2H, Ar—H), 7.73(t, J=8.0 Hz, 1H, coumarin-7-H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.41 (s, 1H, OPh—NH), 9.75(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:15.07, 20.87, 93.67, 115.62, 117.42, 122.09, 123.84, 125.34, 126.25, 126.82, 128.93, 134.22, 134.61, 137.98, 138.36, 149.33, 153.93, 162.01, 166.84, 181.52;IR(KBr), σ/cm-1:3325, 3163, 1712, 1606, 1533, 1388, 1224, 1145;MS(ESI)计算值(C24H20N2O3S)[M+Na]+:439.3, 测量值:439.5。
1-(2, 4-二甲基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4f):白色固体, mp 176~178 ℃, 产率72%;1H NMR(500 MHz, DMSO-d6), δ:2.23(s, 3H, CH3), 2.29(s, 3H, CH3), 5.26(s, 1H, coumarin-3-H), 7.00~7.31(m, 5H, Ar—H), 7.44~7.48(m, 2H, coumarin-6, 8-H), 7.61(d, J=8.0 Hz, 2H, Ar—H), 7.73(t, J=8.0 Hz, 1H, coumarin-7-H), 8.03(d, J=8.5 Hz, 1H, coumarin-5-H), 9.48(s, 1H, OPh—NH), 9.86(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:18.04, 20.80, 93.02, 114.96, 116.81, 121.59, 123.23, 124.75, 126.19, 126.93, 128.01, 128.18, 131.13, 133.63, 134.96, 136.01, 138.07, 148.68, 153.28, 161.44, 166.25, 180.77;IR(KBr), σ/cm-1:3324, 3155, 1707, 1606, 1560, 1386, 1276, 1145;MS(ESI)计算值(C24H20N2O3S)[M+Na]+:439.3, 测量值:439.5。
1-(2, 5-二甲基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4g):白色固体, mp 177~179 ℃, 产率70%;1H NMR(500 MHz, DMSO-d6), δ:2.20(s, 3H, CH3), 2.26(s, 3H, CH3), 5.26(s, 1H, coumarin-3-H), 6.98(d, J=7.5 Hz, 1H, Ar—H), 7.11(d, J=7.5 Hz, 1H, Ar—H), 7.31~7.49(m, 6H, coumarin-6, 8-H, Ar—H), 7.61(d, J=7.5 Hz, 2H, Ar—H), 7.73(t, J=8.5 Hz, 1H, coumarin-7-H), 8.03(d, J=7.5 Hz, 1H, coumarin-5-H), 9.45(s, 1H, OPhN—H), 9.89(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:17.89, 20.87, 93.26, 115.19, 117.02, 121.78, 123.43, 126.40, 126.40, 127.73, 128.87, 130.60, 132.18, 133.82, 135.61, 138.28, 148.94, 153.50, 161.63, 166.44, 180.87;IR(KBr), σ/cm-1:3327, 3149, 1703, 1605, 1560, 1392, 1219, 1184;MS(ESI)计算值(C24H20N2O3S)[M+Na]+:439.3, 测量值:439.6。
1-(4-乙基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4h):白色固体, mp 172~174 ℃, 产率87%;1H NMR(500 MHz, DMSO-d6), δ:1.18(t, J=7.5 Hz, 3H, CH3), 2.51~1.54(m, 2H, CH2), 5.26(s, 1H, coumarin-3-H), 6.69(d, J=7.5 Hz, 1H, Ar—H), 7.16(d, J=8.0 Hz, 2H, Ar—H), , 7.31~7.49(m, 6H, coumarin-6, 8-H, Ar—H), 7.60(d, J=8.0 Hz, 2H, Ar—H), 7.61(d, J=8.5 Hz, 2H, Ar—H), 7.74(t, J=8.0 Hz, 1H, coumarin-7-H), 8.03(d, J=7.5 Hz, 1H, coumarin-5-H), 9.87(s, 1H, OPhN—H), 9.95(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:16.51, 28.55, 93.74, 115.68, 117.17, 122.17, 123.89, 124.99, 125.41, 126.49, 128.64, 134.29, 137.81, 138.89, 141.15, 149.26, 153.98, 162.07, 166.88, 180.68;IR(KBr), σ/cm-1:3329, 3174, 1685, 1606, 1541, 1384, 1222, 1145;MS(ESI)计算值(C24H20N2O3S)[M+Na]+:439.1, 测量值:439.9。
1-(4-异丙基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4i):白色固体, mp 186~188 ℃, 产率77%;1H NMR(500 MHz, DMSO-d6), δ:1.20(s, 6H, CH3), 2.88~2.30 (m, 1H, CH), 5.26(s, 1H, coumarin-3-H), 6.69(d, J=7.5 Hz, 1H, Ar—H), 7.12(d, J=7.5 Hz, 2H, Ar—H), 7.21~7.46(m, 6H, coumarin-6, 8-H, Ar—H), 7.62(d, J=8.5 Hz, 2H, Ar—H), 7.73(t, J=8.0 Hz, 1H, coumarin-7-H), 8.03(d, J=8.5 Hz, 1H, coumarin-5-H), 9.75(s, 1H, OPhN—H), 9.94(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:24.30, 33.33, 93.17, 115.11, 116.98, 121.69, 123.37, 124.43, 124.91, 125.93, 126.66, 133. 79, 137.35, 138.35, 145.23, 148.71, 153.44, 161.57, 166.38, 180.07;IR(KBr), σ/cm-1:3325, 3116, 1701, 1606, 1541, 1386, 1219, 1186;MS(ESI)计算值(C25H22N2O3S)[M+Na]+:453.1, 测量值:453.2。
1-(4-丁基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4j):白色固体, mp 196~198 ℃, 产率71%;1H NMR(500 MHz, DMSO-d6), δ:1.14(t, J=7.0 Hz, 3H, CH3), 1.56~1.59(m, 2H, CH2), 2.55(t, J=7.5 Hz, 2H, CH2), 5.26(s, 1H, coumarin-3-H), 6.68(d, J=7.0 Hz, 1H, Ar—H), 7.14(d, J=8.0 Hz, 2H, Ar—H), 7.31~7.46(m, 6H, coumarin-6, 8-H, Ar—H), 7.61(d, J=9.0 Hz, 2H, Ar—H), 7.73(t, J=7.0 Hz, 1H, coumarin-7-H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.68(s, 1H, OPhN—H), 9.98(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:14.19, 22.15, 33.56, 34.72, 93.27, 115.21, 117.02, 121.71, 123.42, 124.42, 124.94, 126.03, 128.69, 133.82, 137.32, 138.40, 139.26, 148.80, 153.51, 161.60, 166.41, 180.17;IR(KBr), σ/cm-1:3327, 3124, 1715, 1606, 1547, 1384, 1225, 1145;MS(ESI)计算值(C26H24N2O3S)[M+Na]+:467.1, 测量值:467.3。
1-(2-甲氧基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4k):白色固体, mp 175~176 ℃, 产率80%;1H NMR(500 MHz, DMSO-d6), δ:3.89(s, 3H, OCH3), 5.26(s, 1H, coumarin-3-H), 6.98(d, J=7.5 Hz, 1H, Ar—H), 7.20(d, J=8.0 Hz, 1H, Ar—H), 7.31~7.49(m, 4H, coumarin-6, 8-H, Ar—H), 7.61(d, J=8.0 Hz, 2H, Ar—H), 7.74(t, J=7.5 Hz, 1H, coumarin-7-H), 7.80(d, J=8.0 Hz, 2H, Ar—H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.41(s, 1H, OPhN—H), 9.96(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:56.51, 93.66, 112.36, 115.60, 117.41, 120.66, 122.08, 123.82, 125.33, 126.38, 126.97, 128.39, 134.22, 138.68, 149.21, 152.99, 153.92, 161.98, 166.79, 180.48;IR(KBr), σ/cm-1:3331, 3136, 1708, 1606, 1538, 1388, 1219, 1145;MS(ESI)计算值(C23H18N2O4S)[M+Na]+:431.2, 测量值:431.5。
1-(4-甲氧基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4l):白色固体, mp 178~179 ℃, 产率83%;1H NMR(500 MHz, DMSO-d6), δ:3.75(s, 3H, OCH3), 5.26(s, 1H, coumarin-3-H), 6.96(d, J=8.5 Hz, 1H, Ar—H), 7.43~7.48(m, 6H, coumarin-6, 8-H, Ar—H), 7.61(d, J=8.0 Hz, 2H, Ar—H), 7.73(t, J=8.0 Hz, 1H, coumarin-7-H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.56(s, 1H, OPhN—H), 9.88(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:55.88, 93.49, 114.34, 115.43, 117.23, 121.92, 123.63, 125.15, 126.33, 126.86, 132.70, 134.03, 138.62, 149.02, 153.72, 157.34, 161.82, 166.63, 180.73;IR(KBr), σ/cm-1:3337, 3116, 1710, 1606, 1541, 1386, 1222, 1145;MS(ESI)计算值(C23H18N2O4S)[M+Na]+:431.2, 测量值:431.3。
1-(2-乙氧基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4m):白色固体, mp 174~176 ℃, 产率73%;1H NMR(500 MHz, DMSO-d6), δ :1.41(t, J=6.5 Hz, 3H, CH3), 4.14~4.18(m, 2H, OCH2), 5.26(s, 1H, coumarin-3-H), 6.98(d, J=8.0 Hz, 1H, Ar—H), 7.10(d, J=7.0 Hz, 1H, Ar—H), 7.16~7.48(m, 6H, coumarin-6, 8-H, Ar—H), 7.61(d, J=8.5 Hz, 2H, Ar—H), 7.73(t, J=8.5 Hz, 1H, coumarin-7-H), 7.80(d, J=8.0 Hz, 2H, Ar—H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.31(s, 1H, OPhN—H), 9.85(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:15.03, 64.21, 93.18, 112.82, 115.09, 116.91, 120.09, 121.68, 123.32, 124.83, 125.98, 126.37, 128.39, 129.33, 132.26, 133.75, 138.04, 148.81, 151.69, 153.41, 161.47, 166.28, 179.76;IR(KBr), σ/cm-1:3332, 3119, 1705, 1606, 1548, 1387, 1219, 1145;MS(ESI)计算值(C24H20N2O4S)[M+Na]+:455.2, 测量值:455.4。
1-(4-乙氧基苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4n):白色固体, mp 172~174 ℃, 产率84%;1H NMR(500 MHz, DMSO-d6), δ:1.38(t, J=8.0 Hz, 3H, CH3), 4.02~4.05(m, 2H, OCH2), 5.26(s, 1H, coumarin-3-H), 6.96(d, J=9.5 Hz, 1H, Ar—H), 7.31(d, J=8.0 Hz, 4H, Ar—H), 7.44~7.47(m, 2H, coumarin-6, 8-H), 7.61(d, J=8.0 Hz, 2H, Ar—H), 7.74(t, J=7.0 Hz, 1H, coumarin-7-H), 8.04(d, J=7.0 Hz, 1H, coumarin-5-H), 9.43(s, 1H, OPhN—H), 9.82(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:15.15, 63.59, 93.27, 114.60, 115.21, 117.07, 121.80, 123.47, 125.00, 126.19, 126.68, 132.36, 133.88, 138.42, 148.81, 153.52, 156.40, 161.68, 166.49, 180.43;IR(KBr), σ/cm-1:3329, 3117, 1701, 1606, 1537, 1389, 1226, 1145;MS(ESI)计算值(C24H20N2O4S)[M+Na]+:455.1, 测量值:455.3。
1-(4-氟苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4o):白色固体, mp 177~179 ℃, 产率65%;1H NMR(500 MHz, DMSO-d6), δ:5.26(s, 1H, coumarin-3-H), 7.12(d, J=8.0 Hz, 2H, Ar—H), 7.31(d, J=6.5 Hz, 2H, Ar—H), 7.44~7.49(m, 4H, coumarin-6, 8-H, Ar—H), 7.61(d, J=7.5 Hz, 2H, Ar—H), 7.74(t, J=8.5 Hz, 1H, coumarin-7-H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.68(s, 1H, OPhN—H), 9.89(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6),δ:93.61, 115.51, 115.92, 117.36, 122.17, 123.76, 125.28, 126.52, 127.22, 127.28, 134.16, 136.41, 138.52, 149.28, 153.83, 161.95, 166.74, 180.92;IR(KBr), σ/cm-1:3335, 3125, 1707, 1606, 1541, 1376, 1218, 1145;MS(ESI)计算值(C22H15FN2O3S)[M+Na]+:429.0, 测量值:429.2。
1-(4-氯苯基)-3-(3-(4-氧香豆素基)苯基)硫脲(4p):白色固体, mp 183~185 ℃, 产率61%;1H NMR(500 MHz, DMSO-d6), δ:5.26(s, 1H, coumarin-3-H), 7.33~7.48(m, 7H, coumarin-6, 8-H, Ar—H), 7.61(d, J=8.0 Hz, 2H, Ar—H), 7.73(t, J=8.0 Hz, 2H, coumarin-7-H, Ar—H), 8.03(d, J=8.0 Hz, 1H, coumarin-5-H), 9.45(s, 1H, OPhN—H), 9.87(s, 1H, ArN—H); 13C NMR(125 MHz, DMSO-d6), δ:93.78, 115.68, 117.50, 122.32, 123.59, 123.90, 124.92, 125.42, 126.67, 131.71, 132.00, 133.36, 133.95, 134.30, 138.59, 139.41, 149.49, 153.99, 162.08, 166.86, 180.76;IR(KBr), σ/cm-1:3336, 3151, 1730, 1606, 1539, 1382, 1220, 1176;MS(ESI)计算值(C22H15ClN2O3S)[M+Na]+:445.1, 测量值:445.4。
1.2.2 中间体2的合成
将9.0 g(0.05 mol)中间体1、5.45 g(0.05 mol)间氨基酚和13.8 g(0.1 mol)无水碳酸钾溶于250 mL乙腈中,在80 ℃下,回流反应6 h,冷却至室温,过滤除去不溶性盐,减压蒸去溶剂,剩余物中加无水乙醇溶解并重结晶,得到白色固体4-(3-氨基苯氧基)-香豆素(2),产率:81%,mp 124~126 ℃。1H NMR(500 MHz, DMSO-d6), δ:8.17(d, J=7.0 Hz, 1H, coumarin-5-H), 7.60(t, J=5.0 Hz, 1H, coumarin-7-H), 7.34~7.30(m, 2H, coumarin-6, 8-H); 7.18(d, J=8.5 Hz, 2H, Ar—H), 6.95(d, J=8.5 Hz, 2H, Ar—H), 5.26(s, 1H, coumarin-3-H); 13C NMR(125 MHz, DMSO-d6), δ:166.37, 161.83, 153.64, 151.52, 133.86, 131.13, 125.04, 123.49, 117.14, 112.72, 107.86, 106.08, 93.06。
1.2.3 中间体3的合成
将2.53 g(10 mmol)4-(3-氨基苯氧基)-香豆素(2)溶于50.0 mL氯苯中,加入1.0 mL三乙胺,加热,当温度为升高到35 ℃时,缓慢滴加1.02 mL(12 mmol)硫光气和50.0 mL氯苯的混合溶液,并保持35 ℃,搅拌8 h。反应结束后,冷却至室温,减压除去溶剂,加入200 mL水,过滤,干燥,再用无水乙醇重结晶,得到白色固体4-(4-异硫氰酸酯苯氧基)香豆素(3),产率:86%,mp 147~148 ℃。1H NMR(500 MHz,DMSO-d6), δ:8.03(d, J=8.5 Hz, 1H, coumarin-5-H), 7.74(t, J=8.0 Hz, 1H, coumarin-7-H), 7.48~7.43(m, 2H, coumarin-6, 8-H), 7.16(d, J=8.0 Hz, 2H, Ar—H), 6.98(d, J=8.0 Hz, 2H, Ar—H), 5.26(s, 1H, coumarin-3-H); 13C NMR(125 MHz, DMSO-d6), δ:165.93, 161.35, 154.16, 151.52, 136.69, 133.35, 131.16, 128.22, 125.10, 123.48, 122.31, 117.25, 115.31, 93.21。
1.2.1 中间体1的合成
中间体1的制备参考文献[18]的合成方法。
1.3.1 目标化合物的初步抑菌生物活性测试
采用浑浊度法[19],在药剂质量浓度为200和100 mg/L时,以水稻白叶枯菌和柑橘溃疡菌为供试菌株,对目标化合物4a~4p进行了抑菌活性的测定,以商品药剂噻唑酮为对照药剂。
1.3.2 部分目标化合物的抑菌活性EC50值测定
采用质量浓度倍减法将部分供试化合物用溶剂配制成5个质量浓度(200、100、50、25和12.5 mg/L),采用浊度法测定各个质量浓度抑制率,每处理重复3次,计算药剂对病原菌的有效抑制中浓度(EC50)值,结果见表 2所示。
2 结果与讨论
2.1 目标化合物的合成
以关键中间体3的合成为例,探讨了不同溶剂、温度和时间对其收率的影响,结果见表 1。
由表 1可以看出,当溶剂为氯苯,室温下反应8 h,收率最高为70%。然后,用氯苯为溶剂,反应8 h,反应温度升高至35 ℃,收率增加至85%。最后,用氯苯为溶剂,反应温度为35 ℃,分别缩短或增加反应时间(4、6、10 h),其收率分别为48%、74%和83%。因此,合成4-(4-异硫氰酸酯苯氧基)香豆(3)的最佳反应条件为:氯苯作溶剂、反应温度为35 ℃,反应时间为8 h。
2.2 目标化合物的图谱解析
所有化合物的结构均经过IR、1H NMR、13C NMR和MS等手段进行了表征。在IR谱图中,3116~3334 cm-1是─NH─C═S─NH─中NH的伸缩振动引起的;官能团─O─C═O─和─NH─C═S─N─H─中C═S伸缩振动吸收峰则出现在1701~1730和1145~1186 cm-1处。在1H NMR谱图中,硫脲基团的质子的化学位移在9.31~9.98处;δ 5.6是香豆素环上3位的质子的化学位移。在13C NMR谱图中,δ 179.76~181.72是─NH─C═S─NH─中碳的化学位移。
2.3 目标化合物的抑菌活性
采用浑浊度法,在药剂质量浓度为200和100 mg/L时,以水稻白叶枯菌和柑橘溃疡病菌为供试菌株,测定目标化合物4a~4p的抑菌活性,其结果如表 2所示。
由表 2可以看出,目标化合物具有以水稻白叶枯菌和柑橘溃疡病菌活性。当药剂质量浓度为200 mg/L时,化合物4d、4f、4g、4h、4i、4k、4l、4m和4n对水稻白叶枯菌的抑制率分别为80.4%、82.7%、80.5%、80.3%、82.5%、90.1%、94.1%、87.3%和92.2%,优于对照药剂噻菌铜(66.3%);当药剂质量浓度为100 mg/L,化合物4d、4e、4h、4k、4l、4m和4n对烟草青枯菌的抑制率分别为47.2%、46.4%、49.6%、48.2%、55.1%、52.7%和57.9%,均优于对照药剂噻菌铜(40.6%)。此外,当质量浓度为200 mg/L时,化合物4d、4e、4f、4h、4k、4l、4m和4n对柑橘溃疡病菌的抑制率分别为80.1%、83.8%、85.6%、84.1%、93.1%、96.3%、90.7%和94.5%,优于对照药剂噻菌铜的活性(76.4%)。当质量浓度为100 mg/L时,化合物4d、4e、4f、4h、4k、4l、4m和4n对柑橘溃疡病菌的抑制率分别为48.1%、50.1%、55.3%、55.3%、50.2%、58.7%、51.3%和53.9%,优于对照药剂噻菌铜的活性(46.8%)。
选择抑菌活性高的4个目标化合物4k、4l、4m和4n测定抑制水稻白叶枯菌和柑橘溃疡病菌活性EC50值,结果见表 3。
从表 3可以看出,目标化合物衍生物对水稻白叶枯菌和柑橘溃疡病菌均具有较好的抑制活性。其中化合物4k、4l、4m和4n抑制烟草青枯菌活性EC50值分别为137.42、131.05、129.23和117.43 mg/L,优于对照药剂噻菌铜(195.24 mg/L);化合物4k、4l、4m和4n抑制番茄青枯菌活性EC50值和97.02、94.31、102.28和90.52 mg/L,优于对照药剂噻菌铜的活性(120.25 mg/L)。初步构效关系表明:当目标化合物中的Ar为2-甲氧基苯基(2-MeOPh)、4-甲氧基苯基(4-MeOPh)、2-乙氧基苯基(2-EtOPh)和4-乙氧基苯基(4-EtOPh)时,相对应的目标化合物对水稻白叶枯菌和柑橘溃疡病菌具有较优的抑制活性,均优于对照药剂噻菌铜的活性。此外,大多数目标化合物抑制柑橘溃疡病菌的活性优于抑制水稻白叶枯菌的活性。
3 结论
以4-羟基香豆素为先导,采用结构多样性衍生,设计合成了16个1-芳基-3-(3-(4-氧香豆素基)苯基)硫脲衍生物。采用浑浊度法,以水稻白叶枯菌和柑橘溃疡病菌为供试菌株,测定目标化合物的抑菌活性。测试结果表明:大多数目标化合物具有较好的抑菌活性。其中化合物4k、4l、4m和4n对水稻白叶枯菌和柑橘溃疡病菌具有较优的抑制活性,均优于对照药剂噻菌铜的活性。对其结构进一步修饰和改造,有可能得到活性更高的化合物,该研究工作正在进行中。
-
-
[1]
陈亮, 刘君丽. 农作物细菌性病害发生的新趋势[J]. 农药市场信息, 2010,20,(31): 48-50. CHEN Liang, LIU Junli. The New Tendency of Crops Bacterial Diseases Occur[J]. Pestic Mark News, 2010, 20(31): 48-50.
-
[2]
左经龙, 姜桂霞. 细菌性病害主要化学防治药剂[J]. 吉林蔬菜, 2016,3,21-22. ZUO Jinglong, JIANG Guixia. Bacterial Disease Prevention and Control of Major Chemical Reagents[J]. Jilin Veget, 2016, 3: 21-22.
-
[3]
周毓萍, 杨正银, 于红娟. 1-苯基-3-甲基-4-苯甲酞基吡唑酮-5-缩氨基硫腮稀土配合物的合成、表征及抑菌活性[J]. 应用化学, 1999,16,(6): 37-41. ZHOU Yuping, YANG Zhengyin, YU Hongjuan. Syntheses, Characterization and Bacteriostatic Activities of 1-Phenyl-3-methyl-4-benzoylpyrazolone-5-thiosemicarbazone and Its Rare Earth Complexes[J]. Chinese J Appl Chem, 1999, 16(6): 37-41.
-
[4]
苏桂发, 霍丽妮, 覃江克. 1-脱氢松香酰基-3-芳酰胺基硫脲及其1, 3, 4-噻二唑类衍生物的合成及抑菌活性[J]. 应用化学, 2008,25,(7): 803-807. SU Guifa, HE Lini, QIN Jiangke. Synthesis and Antibacterial Activity of 1-Dehyoabicticacy-3-Aroylthiosem-Icarbazides Compounds and Their 1, 3, 4-Thiadiazole Derivatives[J]. Chinese J Appl Chem, 2008, 25(7): 803-807.
-
[5]
Lu A D, Wang Z W, Zhou Z H. Application of "Hydrogen Bonding Interaction" in New Drug Development: Design, Synthesis, Antiviral Activity, and SARs of Thiourea Derivatives[J]. J Agric Food Chem, 2015, 63(5): 1378-1384. doi: 10.1021/jf505355r
-
[6]
Ghorab M M, Alsaid M S, El-Gaby M S A. Biological Evaluation of Some New N-(2, 6-Dimethoxypyrimidinyl) Thioureido Benzenesulfonamide Derivatives as Potential Antimicrobial and Anticancer Agents[J]. Eur J Med Chem, 2016, 124: 299-310. doi: 10.1016/j.ejmech.2016.08.060
-
[7]
Yapati H, Devineni S R, Chirumamilla S. Synthesis, Characterization and Studies on Antioxidant and Molecular Docking of Metal Complexes of 1-(Benzo[d]thiazol-2-yl)thiourea[J]. J Chem Sci, 2016, 128(1): 43-51.
-
[8]
Chetana P R, Srinatha B S, Somashekar M N. Synthesis, Spectroscopic Characterisation, Thermal Analysis, DNA Interaction and Antibacterial Activity of Copper(Ⅰ) Complexes with N, N'-Disubstituted Thiourea[J]. J Mol Struct, 2016, 1106: 352-365. doi: 10.1016/j.molstruc.2015.10.010
-
[9]
Kadir M A, Ramli R, Yusof M S M. Synthesis, Spectroscopic Studies and Antibacterial Activity of New Lauroyl Thiourea Amino Acid Derivatives[J]. Asian J Chem, 2016, 28(3): 596-600. doi: 10.14233/ajchem
-
[10]
Khare R, Sharma J, Sharma A. Synthesis, Characterization, and Antibacterial Activity of Some Thiazoles Derived from Allylthioureas[J]. Russ J Gen Chem, 2016, 86(3): 702-707. doi: 10.1134/S1070363216030312
-
[11]
王诚, 江润生, 冯锋. 4-羟基香豆素Schiff碱的合成及其抑菌活性[J]. 化学试剂, 2008,30,(12): 935-936. doi: 10.3969/j.issn.0258-3283.2008.12.018WANG Cheng, WANG Runsheng, FENG Feng. Synthesis of a New Schiff Base Containing 4-Hydroxy Coumarin and Characterization of Its Antibacterial Activity[J]. Chem Reag, 2008, 30(12): 935-936. doi: 10.3969/j.issn.0258-3283.2008.12.018
-
[12]
刘斌, 谢龙观, 徐效华. 3-苯甲酰基-4-羟基香豆素衍生物的合成, 晶体结构及其除草活性研究[J]. 有机化学, 2011,31,(12): 2067-2073. LIU Bin, XIE Longguan, XU Xiaohua. Synthesis, Crystal Structure and Herbicidal Activity of 3-Benzoyl-4-hydroxycoumarin Derivative[J]. Chinese J Org Chem, 2011, 31(12): 2067-2073.
-
[13]
Cao D, Liu Y B, Yan W. Design, Synthesis, and Evaluation of in Vitro and in Vivo Anticancer Activity of 4-Substituted Coumarins:A Novel Class of Potent Tubulin Polymerization Inhibitors[J]. J Med Chem, 2016, 59(12): 5721-5739. doi: 10.1021/acs.jmedchem.6b00158
-
[14]
Aws M, Hamdy A M, Khaddour Z. Synthesis of Arylated Coumarins by Suzuki-Miyaura Cross-Coupling. Reactions and Anti-HIV Activity[J]. Bioorg Med Chem, 2016, 24(21): 5115-5126. doi: 10.1016/j.bmc.2016.08.029
-
[15]
Buzad C, Doherty M F. Design of Three-Component Kioetically Controlled Reactive Distillation Columus Using Fixed-Point Methods[J]. Chem Eng Sci, 1994, 49(12): 1947-1963. doi: 10.1016/0009-2509(94)80079-0
-
[16]
徐翠莲, 陈钢, 夏百根. 含香豆素骨架的缩氨基硫脲化合物的合成和抑菌活性研究[J]. 化学通报, 2009,9,815-819. XU Cuilian, CHEN Gang, XIA Baigen. Synthesis and Antibacterial Activity of Thiosemicarbazone Compounds Containing Coumarin-Skeleton[J]. Chem Bull, 2009, 9: 815-819.
-
[17]
Chen M H, Tang B C, Zhang X. Synthesis and Antibacterial Activity Evaluation of Novel (E)-4-(4-((arylid-ene)amino)phenoxy)coumarin Derivatives[J]. J Heterocycl Chem, 2016, : .
-
[18]
Rao M L N, Kumar A. Pd-Catalyzed Chemo-Selective Mono-Arylations and Bis-Arylations of Functionalized 4-Chlorocoumarins with Triarylbismuths as Threefold Arylating Reagents[J]. Tetrahedron, 2014, 70(39): 6995-7005. doi: 10.1016/j.tet.2014.07.059
-
[19]
Xu W M, Han F F, He M. Inhibition of Tobacco Bacterial Wilt with Sulfone Derivatives Containing an 1, 3, 4-Oxadiazole Moiety[J]. J Agric Food Chem, 2012, 60(4): 1036-1041. doi: 10.1021/jf203772d
-
[1]
-
表 2 标化合物4a~4p的抑菌活性
Table 2. Antibacterial activities of the title compounds 4a~4p

表 1 优化中间体3的合成条件
Table 1. Optimization of the synthesis conditions of intermediate 3

表 3 部分化合物的抗菌活性EC50值
Table 3. EC50 values of Antibacterial activities of the part of title compounds

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 327
- HTML全文浏览量: 19


 下载:
下载:
 下载:
下载:

