
苯基或联苯基桥连双环戊二烯基铼羰基化合物的合成、结构及催化性能
-
关键词:
- 合成
- / 单桥连双环戊二烯
- / Friedel-Crafts酰基化反应
- / 铼羰基配合物
- / 催化
English
Syntheses, Structures and Catalytic Activity of p-Phenylene-or p-Biphenylene-Bridged Biscyclopentadienyl Dinuclear Rhenium Carbonyl Complexes
-
0 Introduction
Much attention has been focused on the synthesis of a series of biscyclopentadienyl carbonyl ruthenium complexes in recent decades, which mainly include non-bridged[1-2], singly bridged[3-8] and doubly bridged[9-10] biscyclopentadienyl complexes. Bridged bis(cyclopentadienyl) ligands have been extensively studied as frameworks for dinuclear metal complexes that are resistant to fragmentation and maintain two metal centers in close proximity even after metal-metal bond cleavage[11-13]. Especially, rhenium carbonyl complexes have been studied as catalysts for many reactions due to their catalytic activity. When compared to mononuclear rhenium complexes, bridged dicyclopentadienyl dirhenium analogues, in which the bridging ligand binds two reactive metal centers, may promote the distinctive chemical reactivity and catalytic properties. However, only a few examples of Friedel-Crafts reactions catalyzed by rhenium carbonyl complexes have been reported up to now[14-16]. Recently, Our group have reported the synthesis and catalytic activity of three monobridged bis(cyclopentadienyl)rhenium carbonyl complexes[17], showing that these rhenium carbonyl complexes have catalytic activity for Friedel-Crafts alkylation reactions. To develop a deeper understanding of the structures and reactivity of bridged bis(cyclopentadienyl)rhenium carbonyl complexes, here in this paper we selected two bridged ligand precursors (C5Me4H)E(C5Me4H) (E=C6H4, (C6H4)2), in which both two bridging groups have planer structure, and expected to see the catalytic reactivity of both p-phenylene-and p-biphenylene-bridged bis(cyclopentadienyl)rhenium carbonyl complexes.
1 Experimental
1.1 General considerations
All procedures were performed under an argon atmosphere by using standard Schlenk techniques. Solvents were distilled from appropriate drying agents under nitrogen atmosphere. Elemental analyses of C and H were performed with a Vario EL Ⅲ elemental analyzer. The IR spectra were recorded as KBr disks on a Thermo Fisher is50 spectrometer. Gas chromatograms were recorded with an Agilent 6820 gas chromatograph. 1H and 13C NMR spectra were recorded on a Bruker AV Ⅲ-500 (or 600) instrument in CDCl3. The ligand precursors (C5Me4H)E(C5Me4H) (E=C6H4, (C6H4)2) were synthesized according to the literature[18-20].
1.2 Synthesis of complex 1
A solution of free ligand (C5Me4H)C6H4(C5Me4H) (0.19 g, 0.6 mmol) and Re2(CO)10 (0.2 g, 0.3 mmol) in mesitylene (15 mL) was refluxed for 48 h. After removal of solvent, the residue was placed on an alumina column. Elution with petroleum ether/CH2Cl2 (2:1, V/V) developed a colorless band, which was collected, and after concentration, afforded (C6H4)[(η5-C5Me4)Re(CO)3]2 (1) as a white solid. Yield: 52.1% (0.134 g). m.p. 316 ℃; Anal. Calcd. for C30H28O6Re2(%): C, 42.05; H, 3.29. Found(%): C, 42.32; H, 3.13. 1H NMR (CDCl3, 500 MHz): δ 2.16 (s, 12H, C5Me4), 2.25 (s, 12H, C5Me4), 7.28 (s, 4H, C6H4). 13C NMR (CDCl3, 125 MHz): δ 10.9, 11.4, 97.7, 102.2, 104.0, 131.8, 132.4, 197.37. IR (KBr, cm-1): 1 900(s), 2 002(s).
1.3 Synthesis of complex 2
Using a procedure similar to that described above, ligand precursor (C5Me4H)(C6H4)2(C5Me4H) was reacted with Re2(CO)10 in refluxing mesitylene for 48 h. After chromatography with petroleum ether/CH2Cl2, (C6H4)2)[(η5-C5Me4)Re(CO)3]2 (2) was obtained as white crystals. Yield: 77.8% (0.217 g). m.p. 313 ℃; Anal. Calcd. for C36H32O6Re2(%): C, 46.34; H, 3.46. Found(%): C, 45.98; H, 3.59. 1H NMR (CDCl3, 500 MHz): δ 2.17 (s, 12H, C5Me4), 2.26 (s, 12H, C5Me4), 7.38 (d, 4H, J=8.0 Hz, C6H4), 7.61 (d, 4H, J=8.5 Hz, C6H4). 13C NMR (CDCl3, 125 MHz): δ 10.9, 11.3, 97.7, 102.1, 104.4, 127.0, 131.4, 133.0, 139.8, 197.4. IR (KBr, cm-1): 1 903(s), 2 012(s).
1.4 Crystallographic analysis
Crystallographic data for complexes 1 and 2 were collected at 298 K on a Bruker AXS SMART 1000 CCD diffractometer with Mo Kα radiation (λ=0.071 073 nm) using the φ-ω scan technique. The crystal structures were solved by direct method and refined on F2 by full-matrix least-squares technique using the SHELXL-97 program package[21]. All non-hydrogen atoms were found from the Fourier difference maps refined anisotropically, hydrogen atoms were included in calculated positions riding on the parent atoms and refined with fixed thermal parameters. Crystallographic data and experimental details for structural analysis of the complexes are summarized in Table 1.

CCDC: 1506755, 1; 1506754, 2.
1.5 General procedure for catalytic tests
The catalytic reactions were carried out under an argon atmosphere with magnetic stirring. The required complexes (0.02 mmol) was mixed with 1, 2-dichloroethane (3.5 mL) in a 25 mL round-bottom flask at room temperature. Aromatic compounds and acylation reagents were added by syringe. The reaction mixture was heated at 80 ℃ for 24 h. After cooling to room temperature, the solid catalyst was separated from the reaction mixture by filtration. The solvent was removed by rotary evaporation, and the residue was purified by Al2O3 column chromatography, eluting with petroleum ether and ethyl acetate to give a white solid.
2 Results and discussion
2.1 Preparation of complexes 1 and 2
Reactions of ligand precursors (C5Me4H)E(C5Me4H) (E=C6H4, (C6H4)2) with Re2(CO)10 in refluxing mesitylene for 48 h afforded the corresponding complexes (E)[(η5-C5Me4)Re(CO)3]2 (E=C6H4 (1), (C6H4)2 (2)) in yield of 52% and 78%, respectively (Scheme 1). The IR spectra of complexes 1 and 2 all exhibited only terminal carbonyl bands (1: 1 900, 2 002 cm-1; 2: 1 903, 2 012 cm-1). The 1H NMR spectra of 1 and 2 all displayed two groups of singlets for the four methyl protons, indicating the symmetrical structure in solution, in addition, complex 1 showed a singlet at δ 7.28 for the phenylene protons and complex 2 showed two doublets at δ 7.38 and 7.61 for the biphenylene protons.
2.2 Crystal structures of complexes 1 and 2
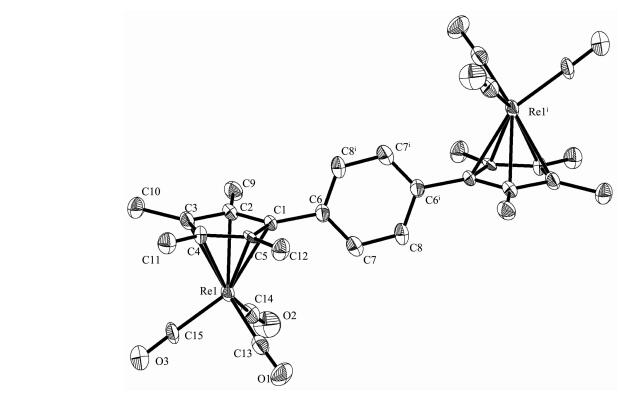
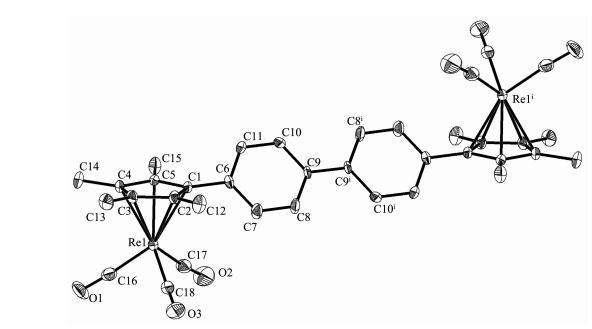
Slow evaporation of the solvent from the complexes in hexane-CH2Cl2 solution gave single crystals 1 and 2 suitable for X-ray diffraction. Selected bond parameters are presented in Table 2 and their structures are depicted in Fig. 1 and 2, respectively.
Single-crystal X-ray diffraction analysis reveals that 1 and 2 crystallize in the triclinic space group P1 Complexes 1 and 2 are monobridged bis(cyclopentadienyl)rhenium carbonyl complexes and similar to each other. Both structures show that the molecule consists of two [(η5-C5Me4)Re(CO)3] moieties linked by a single bridge, with each rhenium atom is η5-coordinated to the cyclopentadienyl ring and three terminal CO ligands. Two Re(CO)3 units are located on the opposite site of the monobridged ligand and anti to each other, the Re atoms exhibit a three-legged piano-stool geometry. Re-Re bonds are not observed in both complexes. Complex 1 has a symmetric structure, the molecule consists of two [(η5-C5Me4)Re(CO)3] moieties linked by a p-phenylene-bridge. Complex 2 also has similar structure, consisting of two [(η5-C5Me4)Re(CO)3] moieties linked by a p-biphenylene-bridge. The dihedral angle between the two Cp ring planes is 0° and the torsion angle Re1-Cp(centroid)…Cp(centroid)-Re1ⅰ is -180°, indicating two Cp rings are parallel to each other. For complexes 1 and 2, the distances of Re1-CEN and Re1ⅰ-CEN (CEN means the centroid of the cyclopentadienyl ring) are equal (Table 2). The Re-CEN distance in 1 is 0.195 6 nm and the Re-CEN distance in 2 is 0.194 6 nm, which compare very well with those values in the analogues [(η5-C5H4)2C(CH2)5][Re(CO)3]2 (0.195 7 and 0.196 0 nm) and [(η5-C5H4)2 Si(CH3)2][Re(CO)3]2 (0.194 6 and 0.195 2 nm)[17]. From above data, we can conclude that the different ligand bridges in these complexes have no obvious effect on their structures.
2.3 Catalysis of Friedel-Crafts acylation reactions
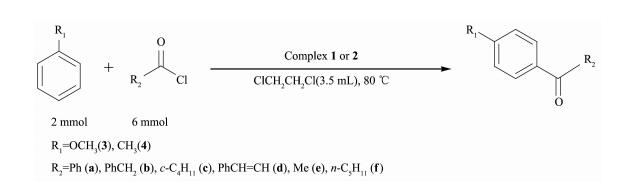
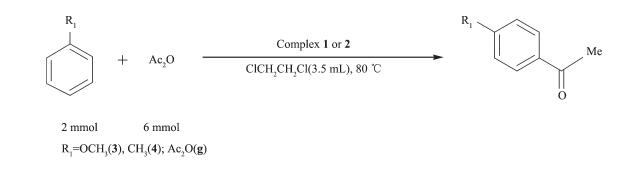
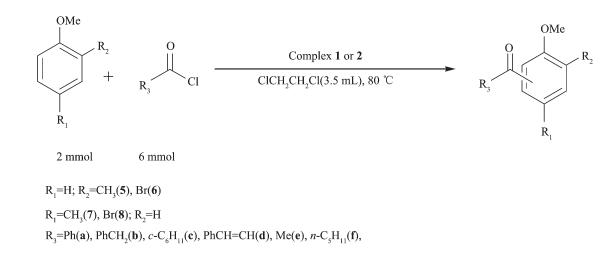
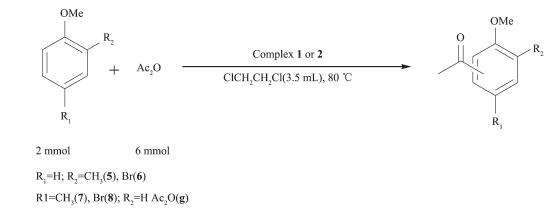
In order to test the catalytic capability in Friedel -Crafts acylation reactions (Scheme 2~6) catalyzed by two complexes, considering factors such as the reaction time, yield, economic considerations, the following experimental conditions were chosen for next work: reaction time 24 h; 1, 2-dichloroethane as solvent; 80 ℃; the molar ratio of aromatic substrates and acylation reagents was 1:3; the amount of catalyst was 1.0%(n/n) (substrate as reference).
With the above studies, the yields(%) were found to vary with aromatic substrates with different acylation reagents, the catalytic results of complex 1 and 2 are shown in Table 3. Friedel-Crafts acylation reactions are electrophilic substitution reactions, however halogen element and carbonyl formed p-π conjugation in acylating agent, thus the acylating agents were difficult to lose halogen element to form carbocation. Benzoyl chloride, phenylacetyl chloride, cinnamoyl chloride and cyclohexanecarboxylic acid chloride could be used as acylation reagents in these reactions and the corresponding products were obtained with high selectivity for the para-products without detection of di-substituted in all cases, suggesting that the catalytic reaction has high regioselectivity. The order of increasing reactivity was found to be: 4-bromoanisole < 4-methyl anisole < methylbenzene < 2-bromoanisole < anisole < 2-methylanisole, which was consistent with the characteristics of the aromatic electrophilic substitution mechanism. Overall, both complexes gave similar results, showing that the different ligand bridges have only a small influence on the catalytic behavior.
 Table 3.
Catalyzed Friedel-Crafts acylation reaction of aromatic substrates with different acylation reagents
Table 3.
Catalyzed Friedel-Crafts acylation reaction of aromatic substrates with different acylation reagents

3 Conclusions
Two new bridged bis(cyclopentadienyl)rhenium carbonyl complexes (E)[(η5-C5Me4)Re(CO)3]2 (E=C6H4 (1), (C6H4)2 (2)) have been synthesized and structurally characterized. Friedel-Crafts reactions of aromatic substrates with acylation reagents showed that two complexes have catalytic activity. Compared with traditional catalysts, these complexes have significant practical advantages, namely lower amounts of catalyst, mild reaction conditions, and high selectivity. Further studies to elucidate the reaction mechanism and expand the synthetic utility of these catalysts are in progress.
Supporting information is available at http://www.wjhxxb.cn
-
-
[1]
Bailey N A, Radford S L, Sanderson J A, et al. J. Organomet. Chem., 1978, 154:343-351 doi: 10.1016/S0022-328X(00)90874-X
-
[2]
Nataro C, Angelici R J. Inog. Chem., 1998, 37:2975-2983 doi: 10.1021/ic971516v
-
[3]
Knox S A R, Macpherson K A, Orper A G, et al. J. Chem. Soc., Dalton Trans., 1989:1807-1813
-
[4]
Burger P. Angew. Chem. Int. Ed., 2001, 40:1917-1919 doi: 10.1002/(ISSN)1521-3773
-
[5]
Zhou X, Zhang Y, Xu S, et al. Inorg. Chim. Acta, 1997, 262:109-112 doi: 10.1016/S0020-1693(97)05505-9
-
[6]
Fox T, Burger P. Eur. J. Inorg. Chem., 2001:795-803
-
[7]
Zhang Y, Wang B, Xu S, et al. Transition Met. Chem., 1999, 24:610-614 doi: 10.1023/A:1006918100619
-
[8]
Zhang Y, Xu S, Zhou X. Organometallics, 1997, 16:6017-6020 doi: 10.1021/om9706424
-
[9]
Ovchinnikov M V, Guzei I A, Angelici R J. Organometallics, 2001, 20:691-696 doi: 10.1021/om000825h
-
[10]
Ovchinnikov M V, Wang X, Schultz A J, et al. Organometallics, 2002, 21:3292-3296 doi: 10.1021/om020167w
-
[11]
Bonifaci C, Ceccon A, Gambaro A, et al. J. Organomet. Chem., 1998, 557:97-109 doi: 10.1016/S0022-328X(97)00737-7
-
[12]
Cuenca T, Royo P. Coord. Chem. Rev., 1999, 193-195:447-498 doi: 10.1016/S0010-8545(99)00139-3
-
[13]
Ceccon A, Santi S, Orian L, et al. Coord. Chem. Rev., 2004, 248:683-724 doi: 10.1016/j.ccr.2004.02.007
-
[14]
Nishiyama Y, Kakushou F, Sonoda N. Bull. Chem. Soc. Jpn., 2000, 73:2779-2782 doi: 10.1246/bcsj.73.2779
-
[15]
Kusama H, Narasaka K. Bull. Chem. Soc. Jpn., 1995, 68:2379-2383 doi: 10.1246/bcsj.68.2379
-
[16]
Kuninobu Y, Matsuki T, Takai K. J. Am. Chem. Soc., 2009, 131:9914-9915 doi: 10.1021/ja904360k
-
[17]
Li Z, Ma Z H, Wang H, et al. Transition Met. Chem., 2016, 41:647-653 doi: 10.1007/s11243-016-0064-1
-
[18]
Meng X, Sabat M, Grimes R. N. J. Am. Chem. Soc., 1993, 115:6143-6151 doi: 10.1021/ja00067a033
-
[19]
Bunel E E, Campos P, Ruz J, et al. Organometallics, 1998, 7:474-476
-
[20]
Yuen H F, Marks T J. Organometallics, 2008, 27:155-158 doi: 10.1021/om7008952
-
[21]
Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997.
-
[1]
-
Table 1. Crystal data and structure refinement parameters for complexes 1 and 2

Table 2. Selected bond distances (nm) and angles (°) for complexes 1 and 2

Table 3. Catalyzed Friedel-Crafts acylation reaction of aromatic substrates with different acylation reagents

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 4
- 文章访问数: 693
- HTML全文浏览量: 167

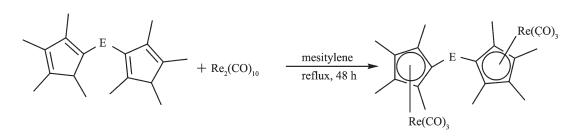


 下载:
下载:







 下载:
下载:

