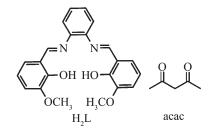
 Figure Scheme 1.
Representation of hexadentate salen-type and acac ligand
Figure Scheme 1.
Representation of hexadentate salen-type and acac ligand

类Salen和β-二酮稀土配合物的晶体结构和荧光性质
-
关键词:
- 1,3-苯二胺缩邻香兰素
- / β-二酮
- / 镧系配合物
- / 配位
English
Crystal Structures and Luminescent Properties of Salen-Type and β-Diketonate Lanthanide Complexes
-
0 Introduction
Lanthanide complexes constructed from multide-ntate ligands are of considerable interest because of their unusual luminescence and magnetism[1-13]. The well-known multidentate salen-type ligands are able to stabilize different metals in various coordination environments although their structures are often influenced by a variety of factors such as radii of lanthanide ions, structures of the ligands and counter ions[14-19]. In recent years, it is known that the β-diketone ligand are perfect sensitizers for Ln(Ⅲ) ion luminescence due to their effective sensitization ability to the metal[20]. In view of the recent important progress on the structure, luminescence and magnetism of salen-type and β-diketones lanthanide complexes as well as our long-standing research on this domain[21-25], the rigid hexadentate salen-type ligand of N, N'-bis(2-oxy-3-methoxybenzylidene)-1,2-phenylenediamine and acetylacetonate were employed to develop salen-type and β-diketonate lanthanide complexes. As a result, two salen-type and β-diketonate dinuclear lanthanide complexes, namely, [Ln2L(acac)4(CH3OH)]2·2CH2Cl2(Ln=Ce (1), Eu (2); H2L=N, N'-bis(2-oxy-3-methoxy-benzylidene)-1,2-phenylenediamine; acac=acetylaceto-nate) have been synthesized, and their crystal stru-ctures have been determined.
1 Experimental
1.1 Materials and instruments
All chemicals and solvents except Ln(acac)3·H2O and H2L were obtained from commercial sources and used without further purification. The salen-type ligand H2L (Scheme 1) was prepared according to the literature and lanthanide precursors[26]. Ln(acac)3·H2O were prepared according to a literature procedure previously described[27]. Elemental (C, H and N) analyses were performed on a Perkin-Elmer 2400 analyzer. FT-IR data were collected on a Perkin-Elmer 100 spectrophotometer by using KBr disks in the range of 4 000~500 cm-1. UV spectra (in methanol) were recorded on a Perkin-Elmer 35 spectrophotometer. Thermal analyses were carried out on a STA-6000 with a heating rate of 10 ℃·min-1 in a temperature range from 30 to 800 ℃ in atmosphere. The Powder X-ray diffraction (PXRD) patterns were recorded on a Rigaku D/Max-3B X-ray diffractometer with Cu Kα radiation (λ=0.154 06 nm) under current of 40 mA and voltage of 200 kV, and the scanning rate is 4°·s-1 with 2θ ranging from 5°~40°.
1.2 Synthesis of complexes 1 and 2
A solution of Ln(acac)3·H2O (Ln=Ce, Eu) (1.0 mmol) in CH3OH (10 mL) were added to a solution of H2L (0.5 mmol) in CH2Cl2 (25 mL). The mixed solution was stirred for 4 h at room temperature, and the filtrate was stored in the refrigerator to crystallize at low temperature (278 K). Yellow crystals suitable for single-crystal X-ray diffraction analysis were obtained after 2 days.
[Ce2L(acac)4(CH3OH)]2·CH2Cl2 (1) Yield: 0.632 g (50.5%); Elemental analysis Calcd. for C45H54Ce2Cl4N2O13(%): C, 43.14; H, 4.34; N, 2.24; Found(%): C, 43.40; H, 4.20; N, 2.30; IR (KBr, cm-1): 3 431(s), 2 947(w), 1 651(s), 1 645(s), 1 620(s), 1 529(s), 1 476(m), 1 430(m), 1 199(w), 754(w); UV-Vis (MeOH, λ): 232, 265, 339 nm.
[Eu2L(acac)4(CH3OH)]2·CH2Cl2 (2) Yield: 0.432 g (73.7%); Elemental analysis Calcd. for C45H54Eu2Cl4N2O13(%): C, 42.34; H, 4.26; N, 2.19; Found(%): C, 42.40; H, 4.20; N, 2.20; IR (KBr, cm-1): 3 421(s), 2 957(w), 1 654(s), 1 648(s), 1 616(s), 1 521(s), 1 471(m), 1 439(m), 1 198(w), 756(w); UV-Vis (MeOH, λ): 236, 261, 337 nm.
1.3 Crystallography
Single-crystal X-ray data of complexes 1 and 2 were collected on a Rigaku R-AXIS RAPID imaging plate diffractometer with graphite-monochromated Mo Kα(λ=0.071 073 nm) at 293 K. Empirical absor-ption corrections based on equivalent reflections were applied. The structures of complexes 1 and 2 were solved by direct methods and refined by full-matrix least-squares methods on F2 using SHELXS-97 crysta-llographic software package[28]. The larger Ueq values of the dichloromethane molecules might be ascribed to the larger thermal motions of the guest species. All non-hydrogen atoms were anisotropically refined. Selected crystal data and structure refinement details for complexes 1 and 2 were summarized in Table 1.
Complex 1 2 Formula C45H54Ce2Cl4N2O13 C45H54Eu2Cl4N2O13 Formula weight 1 252.94 1 276.62 Crystal system Triclinic Triclinic Space group P1 P1 a/nm 1.467 0(4) 1.469 4(5) b/nm 1.598 8(4) 1.604 0(5) c/nm 2.331 5(7) 2.329 9(5) α/(°) 101.520(2) 101.517(5) β/(°) 104.172(2) 104.143(5) γ/(°) 91.254(2) 91.298(5) V/nm3 5 180(3) 5 203(3) Z 4 4 Dc/(g·cm-3) 1.606 1.630 μ/mm-1 2.002 2.654 F(000) 2 504 2 544 R1[I > 2σ(I)] 0.065 6 0.052 9 wR2[I > 2σ(I)] 0.169 3 0.123 6 R1(all data) 0.087 2 0.072 2 wR2(all data) 0.185 1 0.137 7 GOF on F2 1.099 1.065 CCDC: 1482355, 1; 1449142, 2.
2 Results and discussion
2.1 Spectral analysis
Infrared spectra of the ligand, complexes 1 and 2 are showed in Fig.S1. In a typical spectrum of complex 1, the broad weak O-H stretching vibration at 3 414 cm-1 disappeared, while a weak and broad band at about 3 423 cm-1 is newly generated from the N-H vibration. The strong ν(C=N) bands occurring in the range of 1 647~1 656 cm-1 for complexes 1 and 2 shifts to higher wavenumber in comparison with that for free H2L (1 635 cm-1), due to the coordination of C=N groups, which reduces the strengthening of C=N groups. The UV-Vis spectra of the ligand, complexes 1 and 2 are recorded in MeOH solution (Fig.S1 right). For ligand, the typical absorptions at 215, 240 and 309 nm are attributed to the π-π* transition of the aromatic ring and azomethine chromophore. In a typical spectrum of complex 1, the similar ligand-centered solution absorption bands (236, 261, 337 nm) are observed and red-shifted as compared to those (214, 241 and 310 nm) for ligand resulting from the changes in the energy levels of the ligand orbitals upon the coordination of the Ln(Ⅲ) ions.
2.2 TG-DSC analysis
TG-DSC analysis of complexes 1 and 2 are showed in Fig.S2. Complexes 1 and 2 exhibit a gradual weight loss of 11.90% and 12.10% in the range of 33~217 ℃, respectively, which corresponds to the loss of two dichloromethane molecules (Calcd. 13.60% and 13.30%, respectively). TG-DSC data confirm that two crystalline dichloromethane exist in complexes 1 and 2.
2.3 PXRD analysis
Powder X-ray diffraction (PXRD) patterns of complexes 1 and 2 are in agreement with the simulated ones (Fig.S3). PXRD analysis further demonstrates that the crystal structure of complexes 1 and 2 is truly representative of the bulk materials. The differences in intensity are due to the preferred orientation of the powder samples.
2.4 Structural descriptions of complexes 1 and 2
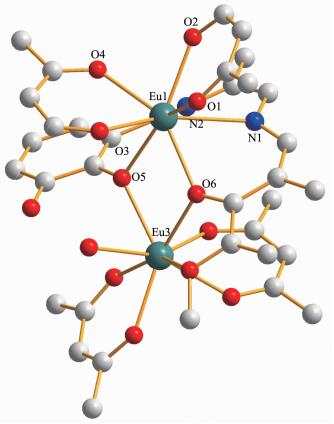
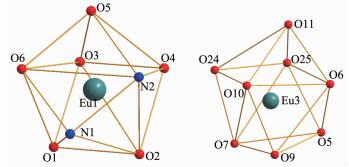
X-ray crystallographic analysis reveals that comp-lexes 1 and 2 are isomorphic. In a typical structure of complex 2 shows a dinuclear core structure in which the positive charges of two Eu(Ⅲ) cations are balanced by one L2- and four acac-. Complex 2 crystallizes in the triclinic space group P1 and as shown in Fig. 1, complex 2 consists of two types of dinuclear lanthanide clusters. The Eu1(Ⅲ) ion displays an eight-coordination and is bonded to six oxygen atoms (four from the two top acac ligands and two from the phenolic oxygen of the salen-type ligand) and two nitrogen atoms from the salen-type ligand to form a square antiprism geometry. The Eu3(Ⅲ) ion displays also an eight-coordination and is bonded to eight oxygen atoms (two oxygen atoms from the phenolic oxygen of the salen-type ligand, four oxygen atoms from the two bottom acac ligands, and two oxygen atom from two methanol molecule) to form a square antiprism geometry as well (Fig. 2). The Eu1(Ⅲ) and Eu3(Ⅲ) ions are bridged by the phenolic O5 and O6 atoms forming a rhombus { Eu1O5Eu3O6} core.
2.5 Luminescent property
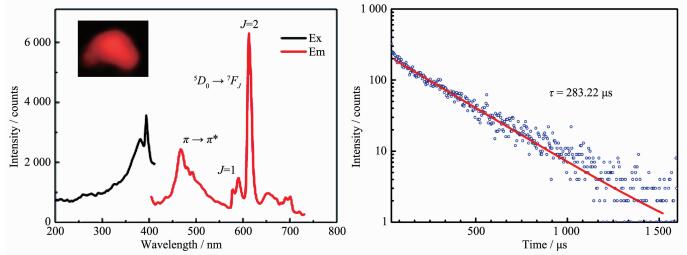
The fluorescence spectrum of complex 2 is recorded in MeOH solution at room temperature (Fig. 3a). The emission spectrum of complex 2 exhibits a weak broad emission band at 450~550 nm with an emission maximum at approximately 510 nm, which can be assigned to the π-π* electronic transition of the ligand. Moreover, the emission spectrum exhibits an intense peak at 614 nm assigned to 5D0→7F2 transition of the Eu(Ⅲ) ion[29]. The emission spectrum suggests that the ligand can sensitize the lumine-scence of Eu(Ⅲ) ion but both the ligand and the Eu(Ⅲ) ions are co-luminescence in complex 2 (Fig. 3a). Furthermore, complex 2 shows bright red emission under UV illumination. The lifetime for complex 2 is found to be 283.22 μs, which is the longest among the salen-type homo-nuclear lanthanide complexes (Fig. 3b).
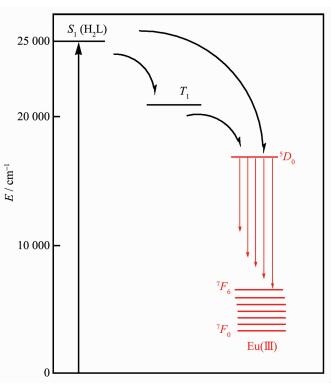
In general, the widely accepted energy transfer mechanism of the luminescence lanthanide complexes is proposed by Crosby[30]. In order to make energy transfer effective, the energy-level match between the lowest triplet energy level (T1) of the ligand and the lowest excited state level of Ln(Ⅲ) ion becomes one of the most important factors dominating the luminescence properties of the complexes. On account of the difficulty in observing the phosphorescence spectra of the ligands, the emission spectrum of the complex [Gd2L(acac)4(CH3OH)]4·2CH2Cl2[31] at 77 K used to estimate the triplet state energy level of the ligand. The single state energy (1ππ*) level of H2L is estimated by referencing its absorbance edge, which is 25 000 cm-1 (400 nm). The triplet (T1) energy level is calculated by referring to the lower wavelength emission peaks of the corresponding phosphorescence spectrum of Gd(Ⅲ) complex, which is 21 505 cm-1 (465 nm). It is known that the gap ΔE(T1-Ln(Ⅲ)) should be intermediate for maximum energy transfer, too big or too small would decrease the efficiency of energy transfer. According to Latva′s empirical rule, an optimal ligand-to-metal energy transfer process for Eu(Ⅲ) needs the energy gap ΔE(3ππ*-5D0) > 2 500 cm-1 [32]. Therefore, the energy gaps between the triplet state of H2L and the resonance energy level of Eu(Ⅲ) are calculated. For complex 2, the energy gap ΔE(3 495 cm-1) is higher than the value of 2 500 cm-1 (Fig. 4). In conclusion, the effective inter-system crossing and ligand to metal energy transfer processes can be found in the complex, which demonstrated that the ligand is suitable for sensitizing the Eu(Ⅲ) ion luminescence.
3 Conclusions
Isolation of complexes 1 and 2 demonstrates that the synthesis of salen-type dinuclear complex with rigid salen-type and β-diketonate ligands are possible, and the structure of the salen-type ligand dominate the structures of the complexes and the coordination geometries of the Ce(Ⅲ) and Eu(Ⅲ) ions. The energy gap analysis suggests that the co-luminescence of Eu(Ⅲ) ion and ligand in complex 2 in MeOH solution is dominated by the good energy match between the triplet state of H2L and resonance energy level of the corresponding Eu(Ⅲ) ion. The lifetime for 2 is found to be 283.22 μs, which is the longest among the salen-type homo-nuclear lanthanide complexes.
Supporting information is available at http://www.wjhxxb.cn
-
-
[1]
Bogani L, Wernsdorfer W. Nature, 2008, 7:179-186 doi: 10.1038/nmat2133
-
[2]
Sorace L, Benelli C, Gatteschi D. Chem. Soc. Rev., 2011, 40: 3092-3104 doi: 10.1039/c0cs00185f
-
[3]
Woodruff D N, Winpenny R E, Layfield R A. Chem. Rev., 2013, 113:5110-5148 doi: 10.1021/cr400018q
-
[4]
Yamanouchi M, Chiba D, Matsukura F. Nature, 2004, 428: 539-542 doi: 10.1038/nature02441
-
[5]
Liu T Q, Yan P F, Luan F, et al. Inorg. Chem., 2015, 54:221 -228 doi: 10.1021/ic502194d
-
[6]
Ishikawa N, Sugita M, Ishikawa T, et al. J. Am. Chem. Soc., 2003, 125:8694-8695 doi: 10.1021/ja029629n
-
[7]
Zhao L, Wu J, Ke H, et al. CrystEngComm, 2013, 15:5301-5309 doi: 10.1039/c3ce40153g
-
[8]
AlDamen M A, Clemente J M, Coronado E. J. Am. Chem. Soc., 2008, 130:8874-8875 doi: 10.1021/ja801659m
-
[9]
AlDamen M A, Cardona S, Clemente J M. Inorg. Chem., 2009, 48:3467-3479 doi: 10.1021/ic801630z
-
[10]
Guo Y N, Chen X H, Xue S. Inorg. Chem., 2011, 50:9705-9713 doi: 10.1021/ic2014978
-
[11]
Liu J, Chen Y C, Liu J L, et al. J. Am. Chem. Soc., 2016, 138:5441-5450 doi: 10.1021/jacs.6b02638
-
[12]
Chen Y C, Liu J L, Ungur L, et al. J. Am. Chem. Soc., 2016, 138:2829-2837 doi: 10.1021/jacs.5b13584
-
[13]
邹晓艳, 闫鹏飞, 张凤鸣, 等.无机化学学报, 2013, 29(8):1680-1686 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20130816&journal_id=wjhxxbcnZOU Xiao-Yan, YAN Peng-Fei, ZHANG Feng-Ming, et al. Chinese J. Inorg. Chem., 2013, 29(8):1680-1686 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20130816&journal_id=wjhxxbcn
-
[14]
Jiang S D, Wang B W, Su G A. Angew. Chem. Int. Ed., 2010, 49:7448-7451 doi: 10.1002/anie.v49:41
-
[15]
Long J, Habib F, Lin P H, et al. J. Am. Chem. Soc., 2011, 133: 5319-5328 doi: 10.1021/ja109706y
-
[16]
Zou H H, Wang R Z, Chen L, et al. Dalton Trans., 2014, 43: 2581-2587 doi: 10.1039/C3DT52316K
-
[17]
Zou X Y, Yan P F, Dong Y P, et al. RSC Adv., 2015, 5: 96573-96579 doi: 10.1039/C5RA18244A
-
[18]
Yue Y M, Yan P F, Sun J W, et al. Polyhedron, 2015, 94: 90-95 doi: 10.1016/j.poly.2015.04.018
-
[19]
Chien Y L, Chang M W, Tsai Y C, et al. Polyhedron, 2015, 102:8-15 doi: 10.1016/j.poly.2015.07.048
-
[20]
Zucchi G, Murugesan V, Tondelier D, et al. Inorg. Chem., 2011, 50:4851-4856 doi: 10.1021/ic2000415
-
[21]
Li B, Li H F, Chen P, et al. Phys. Chem. Chem. Phys., 2015, 17:30510-30517 doi: 10.1039/C5CP05888K
-
[22]
Zhang J W, Li H F, Chen P, et al. J. Mater. Chem. C, 2015, 3:1799-1806 doi: 10.1039/C4TC02512A
-
[23]
Leng J Q, Li H F, Chen P, et al. Dalton Trans., 2014, 43: 12228-12235 doi: 10.1039/C4DT00820K
-
[24]
Lin P H, Sun W B, Yu M F, et al. Chem. Commun., 2011, 47: 10993-10995 doi: 10.1039/c1cc14223b
-
[25]
Sun W B, Han B L, Lin, P H, et al. Dalton Trans., 2013, 42:13397-13403 doi: 10.1039/c3dt51227d
-
[26]
Koner R, Lee G H, Wang Y, et al. Eur. J. Inorg. Chem., 2005, 8:1500-1505 doi: 10.1002/ejic.200400858/pdf
-
[27]
Stites J G, McCarty C, Quill L L. J. Am. Chem. Soc., 1948, 70: 3142-3143 doi: 10.1021/ja01189a509
-
[28]
Sheldrick G M. Acta Crystallogr., Sect. A, 2007, 64:112-122
-
[29]
Zhang H J, Gou R H, Yan L, et al. Spectrochim. Acta Part A, 2007, 66:289-294 doi: 10.1016/j.saa.2006.02.054
-
[30]
Crosby G R, Whan R E, Alire R M. J. Chem. Phys., 1961, 34:743 doi: 10.1063/1.1731670
-
[31]
邹晓艳, 闫鹏飞, 董艳萍, 等.黑龙江大学工程学报, 2015, 6(4): 27-31 http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hljz201504006&dbname=CJFD&dbcode=CJFQZOU Xiao-Yan, YAN Peng-Fei, DONG Yan-Ping, et al. J. Eng. Heilongjaing Univ., 2015, 6(4): 27-31 http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hljz201504006&dbname=CJFD&dbcode=CJFQ
-
[1]
-
Table 1. Crystal data and structures refinement for complexes 1 and 2
Complex 1 2 Formula C45H54Ce2Cl4N2O13 C45H54Eu2Cl4N2O13 Formula weight 1 252.94 1 276.62 Crystal system Triclinic Triclinic Space group P1 P1 a/nm 1.467 0(4) 1.469 4(5) b/nm 1.598 8(4) 1.604 0(5) c/nm 2.331 5(7) 2.329 9(5) α/(°) 101.520(2) 101.517(5) β/(°) 104.172(2) 104.143(5) γ/(°) 91.254(2) 91.298(5) V/nm3 5 180(3) 5 203(3) Z 4 4 Dc/(g·cm-3) 1.606 1.630 μ/mm-1 2.002 2.654 F(000) 2 504 2 544 R1[I > 2σ(I)] 0.065 6 0.052 9 wR2[I > 2σ(I)] 0.169 3 0.123 6 R1(all data) 0.087 2 0.072 2 wR2(all data) 0.185 1 0.137 7 GOF on F2 1.099 1.065 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 8
- 文章访问数: 973
- HTML全文浏览量: 146


 下载:
下载:




 下载:
下载:

