
含取代基苯基羧酸与柔性含氮配体构筑的三个过渡配位化合物的合成、晶体结构与物理性质
English
Three Transition Metal Coordination Complexes Based on the Substituted Benzenecarboxylic Acid and Flexible N-donor Co-ligands: Syntheses, Crystal Structures, and Physical Properties
-
Key words:
- transition metal complexes
- / magnetic property
- / photocatalytic activity
-
0 Introduction
Over the last few years, coordination complexes have attracted much attention not only because of their fascinating topological structures but also due to their potential applications in many fields, such as gas storage/separation, catalysis, luminescence, ion-exchange, and magnetism[1-6]. But they are less investi-gated for pollutant organic dyes adsorption and removal[7-9]. Recently, a particularly interesting and challenging research in the coordination chemistry is the design and synthesis of photocatalytic coordination complexes due to their high thermal and chemical stabilities[10-13]. On the other hand, coordination compl-exes could involve various kinds of charge transfer between metal ions and organic linkers, which enable coordination complexes to exhibit adsorption band in the visible region. Thus, coordination complexes may undergo photochemical process under visible light irradiation and be good candidate for photocatalytic materials[14-16]. Compared with the commonly employed many coordination complexes, however, limited works focused on the development of such new coordination complexes as photocatalytic materials have been reported. 3,5-dinitrobenzoic acid (3,5-DNBH) is a multifunctional ligand containing two kind of coordination modes, concluding neutral (3,5-DNBH) and in the anionic form (3,5-DNB-)[17-20]. In this work, three transition metal complexes, namely, [M(3,5-DNB)2(1,2-bimb)] [M=Co(Ⅱ) (1), Cd(Ⅱ) (3)] and [Zn(3,5-DNB)2(1,2-bimb)]n (2) have been obtained through using the flexible bis(imidazole) ligand (1,2-bimb) and 3,5-dinitrobenzoic acid (3,5-DNBH) in the presence of transition metal salts. Complexes 1 and 3 are isostru-ctural containing a dinuclear [M2(COO)2] (M=Co(Ⅱ) (1), Cd(Ⅱ) (3)) unit, and complex 2 features a 1D chain structure, which further packs to the supramolecular structure by the π…π interactions between the benzene rings from 1,2-bimb molecules and 3,5-DNB anions. Notably, complex 1 shows good degradation ability over rhodamine B (RhB) under visible light irradiation. The fluorescence properties of 2 and 3 have been investigated in their dispersion methanol solution state at room temperature. In addition, the magnetic properties of 1 have also been studied in detail.
1 Experimental
1.1 Materials and methods
The flexible bis(imidazole) ligand (1,2-bimb) was synthesized according to a previous report[21]. All other chemicals were obtained from commercial sources and used without further purification. C, H, and N elemental analyses were performed on a PerkinElmer 240C elemental analyzer. The infrared spectra (KBr pellets) were recorded on a Nicolet iS10 spectrometer in the range of 500~4 000 cm-1. Powder XRD data were collected on a Rigaku Ultima Ⅳ X-ray diffracto-meter with Cu Kα radiation (λ=0.154 056 nm) over the 2θ range of 5°~50° at room temperature. The XRD accelerating voltage and emission current were 30 kV and 50 mA, respectively. Thermogravimetric analyses (TGA) were performed on a Perkin-Elmer Pyris 1 TGA instrument in the range of 30~800 ℃ under a nitrogen flow at a heating rate of 20 ℃·min-1. Fluorescence spectra of the dispersion methanol solution of comp-lexes 2 and 3 were recorded using a Shimadzu RF-5301PC spectrofluorometer at room temperature. The solid-state diffuse-reflectance UV-Vis spectra for powder samples were recorded on a Shimadzu UV-3700 equipped with an integrating sphere by using BaSO4 as a white standard. The UV-Vis spectra for solution samples were obtained on a Hitachi UV-3900 spectrometer. The dc magnetic susceptibility data of 1 were measured on polycrystalline sample using a Quantum Design MPMS-XL-7 SQUID magnetometer.
1.2 Syntheses of complexes 1~3
1.3 Photocatalytic experiments
The photocatalytic activity of 1 was tested by degradation of RhB, methyl blue (MB), methyl orange (MO) solution under a 500 W xenon arc lamp at ambient temperature (25 ℃). The typical process was as follows: a suspension containing 1 (50 mg) and RhB (20.0 mg·L-1, 50 mL) solution was stirred contin-uously in the dark for about 30 min to establish an adsorption-desorption equilibrium. The samples were withdrawn regularly from the reactor, and dispersed powders were removed by centrifugation. At different time intervals, analytical samples were withdrawn and analyzed by UV-Vis spectroscopy.
1.4 Crystallographic analyses
Single-crystal X-ray diffraction data for compl-exes 1~3 were collected on a Bruker SMART APEX CCD diffractometer with Mo Kα (graphite monochro-mator, λ=0.071 073 nm). The data collected by a CCD diffractometer were processed by SAINT program[22]. Lorentz and polarization effects and empirical absorp-tion corrections were applied. The structure was solved by the direct method using SHELXS-97[23] and refined by a full-matrix least-squares on F2 using the SHELXL -97 program[24]. All non-hydrogen atoms were refined anisotropically and all the hydrogen atoms except those attaching to water molecule were put in calculated positions. All hydrogen atoms were refined isotropically with the isotropic vibration parameters related to the non-hydrogen atoms to which they bonded. The detailed crystallographic data and structure refinement parameters for complexes 1~3 are listed in Table 1. Selected bond lengths and angles are given in Table 2~4.
1 2 3 Formula C28H20CON8O12 C28H20N8O12Zn C56H40Cd2N16O24 Molecular weight 719.45 725.89 1 545.84 Crystal system Monoclinic Monoclinic Monoclinic Space group C2/c P21/c C2/c a/nm 2.916(3) 1.262 6(5) 2.990 5(18) b/nm 1.176 5(12) 1.577 3(7) 1.194 8(7) c/nm 1.753 2(19) 1.644 1(7) 1.810 0(10) β/(°) 109.390(19) 109.721(8) 107.828(10) V/nm3 5.673(10) 3.082(2) 6.157(6) Z 8 4 4 Dc/(g·cm-3) 1.685 1.564 1.668 u/mm-1 0.690 0.875 0.788 F(000) 2 936 1 480 3 104 GOF on F2 1.002 1.006 1.019 R1, wR2a [I > 2σ(I)] 0.064 8, 0.181 7 0.046 3, 0.089 6 0.041 7, 0.077 0 R1, wR2a(all data) 0.106 4, 0.233 3 0.094 0, 0.101 2 0.073 6, 0.085 3 (Δρ)max, (Δρ)min/(e·nm-3) 540, -718 276, -357 564, -870 \begin{document}$ ^{\rm{a}}{\mathit{R}_{\rm{1}}}{\rm{ = }}\sum {\rm{||}}{\mathit{F}_{\rm{o}}}{\rm{| - |}}{\mathit{F}_{\rm{c}}}{\rm{||/}}\sum {\rm{|}}{\mathit{F}_{\rm{o}}}{\rm{|;}}\;\mathit{w}{\mathit{R}_{\rm{2}}}{\rm{ = [}}\sum \mathit{w}{{\rm{(}}\mathit{F}_{\rm{o}}^{\rm{2}}{\rm{-}}\mathit{F}_{\rm{c}}^{\rm{2}}{\rm{)}}^{\rm{2}}}{\rm{/}}\sum \mathit{w}{{\rm{(}}\mathit{F}_{\rm{o}}^{\rm{2}}{\rm{)}}^{\rm{2}}}{{\rm{]}}^{{\rm{1/2}}}}$\end{document} Co(1)-O(1) 0.217 9(4) Co(1)-O(7) 0.203 6(4) Co(1)-N(5) 0.205 3(4) Co(1)-O(2) 0.219 7(4) Co(1)-O(8)A 0.201 6(4) Co(1)-N(8)A 0.205 4(4) O(8)A-Co(1)-O(7) 122.49(15) N(5)-Co(1)-N(8)A 173.83(15) O(8)A-Co(1)-O(2) 86.32(14) O(8)A-Co(1)-N(5) 87.93(17) O(8)A-Co(1)-O(1) 145.02(15) O(7)-Co(1)-O(2) 151.19(13) O(7)-Co(1)-N(5) 88.14(14) O(7)-Co(1)-O(1) 92.49(14) N(5)-Co(1)-O(2) 92.77(14) O(8)A-Co(1)-N(8)A 87.03(18) N(5)-Co(1)-O(1) 93.58(16) N(8)A-Co(1)-O(2) 90.45(14) O(7)-Co(1)-N(8)A 91.58(14) N(8)A-Co(1)-O(1) 92.59(16) O(1)-Co(1)-O(2) 58.70(12) Symmetry codes: A: -x+3/2, -y-1/2, -z+1. Zn(1)-O(1) 0.193 3(2) Zn(1)-N(3) 0.200 4(3) Zn(1)-N(6)B 0.198 7(2) Zn(1)-O(7) 0.194 3(2) O(1)-Zn(1)-O(7) 108.70(9) O(7)-Zn(1)-N(8)B 110.06(10) O(7)-Zn(1)-N(3) 100.79(10) O(1)-Zn(1)-N(8)B 123.52(10) O(1)-Zn(1)-N(3) 106.58(11) N(6)B-Zn(1)-N(3) 104.67(10) Symmetry codes: A: x, -y+3/2, z+1/2; B: x, -y+3/2, z-1/2. Cd(1)-N(5) 0.223 3(3) Cd(1)-O(1) 0.228 2(3) Cd(1)-O(7) 0.236 4(3) Cd(1)-N(8)A 0.223 8(3) Cd(1)-O(2)A 0.232 8(3) Cd(1)-O(8) 0.244 9(3) N(5)-Cd(1)-N(8)A 170.34(11) O(1)-Cd(1)-O(2)A 130.48(12) N(5)-Cd(1)-O(8) 94.11(11) N(5)-Cd(1)-O(1) 88.03(12) N(5)-Cd(1)-O(7) 97.10(11) N(8)A-Cd(1)-O(8) 90.36(11) N(8)A-Cd(1)-O(1) 93.21(12) N(8)A-Cd(1)-O(7) 92.46(11) O(1)-Cd(1)-O(8) 145.01(11) N(5)-Cd(1)-O(2)A 87.80(13) O(1)-Cd(1)-O(7) 91.02(12) O(2)A-Cd(1)-O(8) 84.51(12) N(8)A-Cd(1)-O(2)A 84.11(13) O(2)A-Cd(1)-O(7) 138.44(12) O(7)-Cd(1)-O(8) 54.04(9) ymmetry codes: A: -x+1/2, -y+3/2, -z. CCDC: 1476334, 1; 1476335, 2; 1476336, 3.
1.2.3 Synthesis of [Cd(3,5-DNB)2(1,2-bimb)] (3)
The synthesis method of 3 is similar to that of 1 except for Cd(NO3)2·4H2O (0.030 8 g, 0.1 mmol) instead of Co(NO3)2·6H2O, and a different amount of NaOH (0.002 0 g, 0.05 mmol) was added. Colorless block-like crystals of 3 were obtained in yield 15% based on Cd. Anal. Calcd. for C56H40Cd2N16O24(%): C, 43.51; H, 2.61; N, 14.50. Found(%): C, 43.40; H, 2.65; N, 14.43%. IR (KBr, cm-1): 3 438 (m), 3 104 (m), 1 626 (s), 1 613 (s), 1 576 (m), 1 540 (s), 1 461 (m), 1 390 (m), 1 348 (s), 1 301 (w), 1 235 (w), 1 113 (m), 1 087 (m), 1 074 (w), 1 031 (w), 943 (w), 925 (w), 827 (w), 794 (w), 756 (w), 729 (s), 651 (m), 618 (w).
1.2.2 Synthesis of [Zn(3,5-DNB)2(1,2-bimb)]n (2)
Complex 2 was prepared following a similar synthetic method except that Zn(NO3)2·6H2O (0.029 7 g, 0.1 mmol) was used as the metal sources. Colorless block-like crystals of 2 were collected as a pure phase. Yield: 40% based on Zn. Anal. Calcd. for C28H20N8O12Zn(%): C, 46.33; H, 2.78; N, 15.44. Found(%): C, 46.46; H, 2.85; N, 15.31. IR (KBr, cm-1): 3 438 (m), 3 127 (w), 3 092 (m), 1 637 (s), 1 589 (w), 1 536 (s), 1 457 (m), 1 348 (s), 1 291 (w), 1 238 (m), 1 118 (w), 1 109 (w), 1 094 (m), 955 (m), 923 (m), 835 (m), 792 (m), 769 (m), 730 (s), 687 (w), 655 (m), 631 (w), 559 (w).
1.2.1 Synthesis of [Co(3,5-DNB)2(1,2-bimb)] (1)
A mixture containing Co(NO3)2·6H2O (0.029 1 g, 0.1 mmol), 3,5-dinitrobenzoic acid (3,5-DNBH) (0.042 g, 0.2 mmol), 1,2-bimb (0.023 8 g, 0.1 mmol) and NaOH (0.004 0 g, 0.1 mmol) in 10 mL deionized water was stirred at room temperature for 30 min. Then the mixture was transferred to a Teflon-lined autoclave and heated at 140 ℃ for 72 h. After cooling to room temperature, purple block-like crystals were collected by filtration and washed with deionized water and ethanol several times with a yield of 45% based on Co. Anal. Calcd. for C28H20CoN8O12(%): C, 46.74; H, 2.80; N, 15.58. Found(%): C, 46.58; H, 2.87; N, 15.61. IR (KBr, cm-1): 3 447 (m), 3 113 (m), 1 646 (s), 1 612 (s), 1 574 (m), 1 541 (s), 1 470 (m), 1 398 (m), 1 348 (s), 1 303 (w), 1 234 (w), 1 112 (w), 1 087 (w), 1 074 (w), 1 030 (w), 945 (w), 925 (w), 824 (w), 793 (w), 752 (w), 729 (s), 660 (m), 620 (w).
2 Results and discussion
2.1 Structural analyses of 1~3
2.2 IR spectra, powder-XRD analyses, and thermogravimetric analyses (TGA) of 1~3
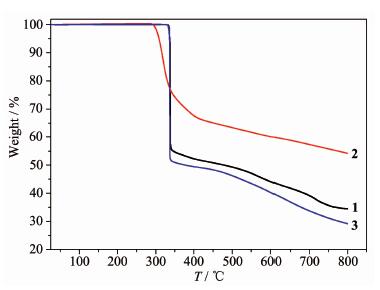
The IR spectra of 1~3 show the typical stretching mode in the 1 646~1 348 cm-1 range (Fig.S1, supporting information). Strong and broad absorption associated with asymmetric (νas) and symmetric (νs) vibrations of the carboxylate groups appear in the 1 646~1 536 cm-1 and 1 470~1 348 cm-1 ranges, respectively, which indicate the presence of deprotonated carboxylate groups coordinate to the metal ions[26]. The experi-mental and simulated X-ray diffraction patterns of 1~3 were shown in Fig.S2~S4. Their peaks are in good agreement with each other, indicating phase purities of these samples. To examine the thermal stabilities of 1~3, TG analyses were carried out. As shown in Fig. 3. Three complexes show relatively high thermal stabilities, which are stable up to ca. 300 ℃. Then, a quick weigh loss are observed and the complexes begin to decompose with the collapse of the structures.
2.3 Fluorescence properties of 2~3
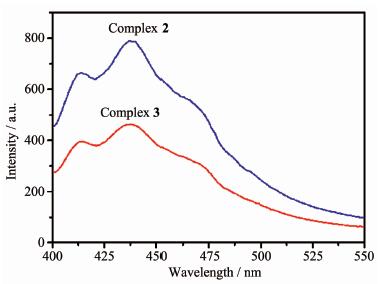
Fluorescence properties of complexes 2 and 3 were studied in the dispersion methanol solution state at room temperature. According to the reported literatures[18, 27], the free 3,5-DNBH and 1,2-bimb exhibit weak broad emission bands at 350 (λex=275 nm), 395 nm (λex=335 nm), respectively. The emission bands of the two free ligands are probably attributable to the π*→n or π*→π transition. As shown in Fig. 4, the excitation wavelength is 372 nm, and the maximum emission band is 437 nm (λex=372 nm) for 2, and 438 nm (λex=372 nm) for 3, respectively. The emission bands of 2~3 display the main emission peaks exhi-biting a red-shift with respect to the free ligands, which are considered to be caused by the ligand-to-metal charge transfer (LMCT), which is observed in other Zn(Ⅱ) and Cd(Ⅱ) coordination complexes, primarily in structures containing benzene dervatives[28].
2.4 Photocatalytic activity of 1
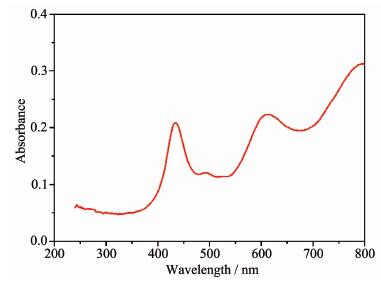
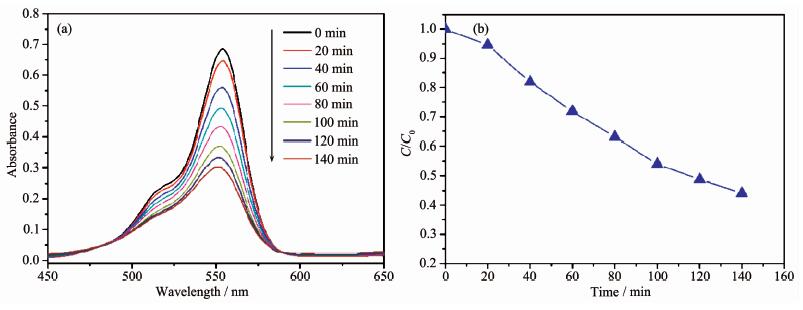
The diffuse-reflectance UV-Vis spectrum of 1 was investigated in the solid state at room temperature. As depicted in Fig. 5, complex 1 consists of additional clear absorption bands in the visible regions at 434 and 615 nm, which probably originated from the d-d spin-allowed transition of Co(Ⅱ) (d7) ions. The presence of visible light region transitions encouraged us to investigate applications of 1 in photocatalytic decolorization of organic dyes. Herein, we selected three organic dyes RhB, MB and MO as the target pollutant for degradation experiments to evaluate the photocatalytic performance of 1. As shown in Fig. 6, the absorption peaks of RhB solution decrease obviously with increasing reaction time for 1. It can be found that decomposition percentage of RhB increases to about 60% within 140 min. For comparison, the photodegradation process of RhB without 1 has also been carried out under the same conditions. About 5% of the RhB decomposes under visible light irradiation without 1 in 140 min. Similar procedures were performed to check the photocatalytic activities of 1 upon degradation of MB and MO, respectively (Fig.S5, Fig.S6). The decomposition percentage of MB and MO increases to about 13% and 25% within 100 min, respectively. The results indicate that 1 is active for the decomposition of RhB in the presence of visible light irradiation.
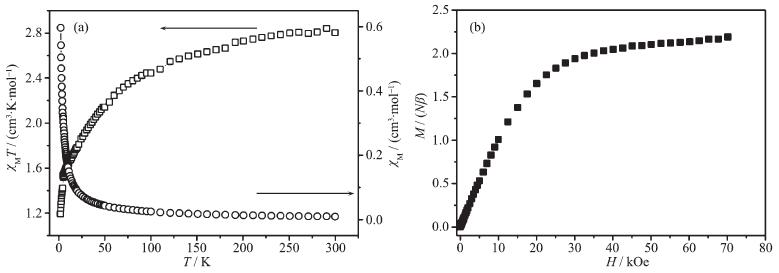
2.5 Magnetic properties of 1
The dc variable-temperature magnetic susceptib-ility measurement was performed on the polycrys-talline sample of 1 at 1 000 Oe in the temperature range of 2~300 K (Fig. 7a). At room temperature, the χMT value of 2.85 cm3·K·mol-1 of 1 is much higher than the expected spin-only value of 1.875 cm3·K·mol-1 for S=3/2 with g=2.0, which can be attributed to the orbital contribution arising from the high-spin octahedral Co(Ⅱ) ions. On cooling down, the χMT value decreases continuously, indicating a dominant antiferromagnetic interaction between the magnetic centers or the spin-orbital coupling of the Co(Ⅱ) ions[29-30]. The magnetic susceptibility of 1 obeys the Curie-Weiss law above 50 K with a Curie constant of 3.03 cm3·K·mol-1 and Weiss constant of θ=-22.08 K. As shown in Fig. 7b, the magnetization at 70 kOe is 2.18Nβ, in agreement with the saturation value for a Co(Ⅱ) ion with an effective spin S'=1/2 and a large g value of 4.3~4.6. As already described, there is one exchange pathway in propagating the magnetic intera-ctions with the dinuclear [Co2(COO)2] unit through the carboxylate group in 1. The carboxylate group adopts the syn-syn coordination mode, by which significant antiferromagnetic interactions between the Co(Ⅱ) ions are mediated[31-32].
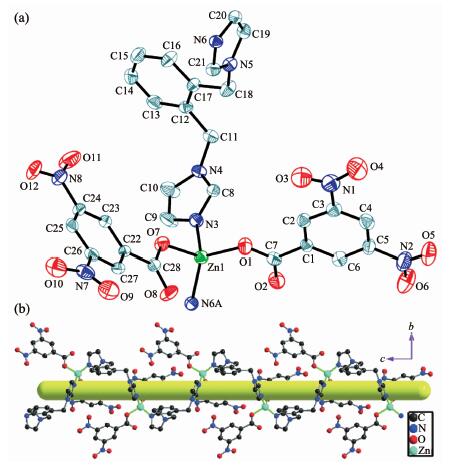
2.1.2 Structural description of 2
Complex 2 crystallizes in the monoclinic space group P21/c. As shown in Fig. 2a, the asymmetric unit is made up of one Zn(Ⅱ) ion, two 3,5-DNB- anions, and one 1,2-bimb molecule. The Zn(Ⅱ) ion is coordinated by two imidazol nitrogen atoms (N3, N6A) from two individual 1,2-bimb molecules and two carboxylate oxygen atoms (O1, O7) from two individual 3,5-DNB-anions to give a {ZnO2N2} tetrahedral geometry. The Zn-O/N bond lengths are in the range of 0.193 3(2)~0.200 4(3) nm. The two 3,5-DNB- anions serve as the same monodentate ligand by using its one carboxylate oxygen atom, which coordinates to an equivalent Zn(Ⅱ) ion. As a result, the {ZnO2N2} tetrahedron are linked by 1,2-bimb molecules to form an infinite chain structure along the c-axis, and the Zn…Zn distance over the 1,2-bimb bridges with the chain is 0.919 2(3) nm. The chains are further extended into a two-dimensional (2D) supramolecular structure in the bc-plane by the π…π interactions between the benzene rings from 3,5-DNB-anions with the centroid-centroid distance of 0.350 0(4) nm[25].
Complex 3 is isostructural to 1. A dinuclear unit [Cd2(COO)2] is again observed where the Cd(Ⅱ) ions are coordinated by four carboxylate oxygen atoms and two nitrogen atoms with a distorted octahedral coordination environment. The Cd-O/N distances are in the range of 0.223 3(3)~0.244 9(3) nm. The Cd…Cd distance over the O-C-O bridge in the unit is 0.403 4 nm.
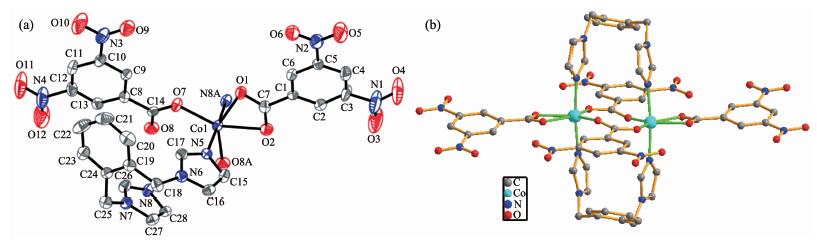
2.1.1 Structural description of 1
Complex 1 crystallizes in a monoclinic space group C2/c. The asymmetric unit contains an indepen-dent Co(Ⅱ) ion, two 3,5-DNB- anions, and one 1,2-bimb molecule. Each Co(Ⅱ) ion is coordinated by four carboxylate oxygen atoms (O1, O2, O7, O8A) from three individual 3,5-DNB-ligands showing (μ-η1:η1; μ2 -η1:η1) type of coordination modes and two nitrogen atoms (N5, N8A) from two individual 1,2-bimb molecules to form a slightly distorted octahedral coordination geometry (Fig. 1a). The Co-O/N bond lengths are in the range of 0.201 6(4)~0.219 7(4) nm, comparable with those observed in the other Co-O/N compounds with octahedral environment. Two crystallographically independent 3,5-DNB- anions are found in the structure. One behaves as a bidentate ligand mode and chelates to one Co(Ⅱ) ion. The other acts as another bidentate ligand mode and bridges two Co(Ⅱ) ions. Therefore, two Co(Ⅱ) ions are linked by two 3,5-DNB anions to give rise to a dinuclear [Co2(COO)2] unit and the Co…Co distance is 0.403 4(3) nm (Fig. 1b). The 1,2-bimb molecule adopts trans-coordination mode to immobilize the two Co(Ⅱ) ions in the dinuclear unit. The dinuclear units are further linked via the O…π weak interactions between the nitro group oxygen atom of 3,5-DNB- anions and the benzene ring of 1,2-bimb molecules, with the (ON)O…π distance of 0.362 5 nm.
3 Conclusions
In conclusion, three coordination complexes of metal carboxylates containing flexible N-donor co-ligands were synthesized via the hydrothermal reaction, namely, [M(3,5-DNB)2(1,2-bimb)] (M=Co(Ⅱ) (1), Cd(Ⅱ) (3)) and [Zn(3,5-DNB)2(1,2-bimb)]n (2). Their structures feature either one-dimensional chains or dinuclear units, which further form the supramolecular structures through weak interactions, depending on the particular co-ligands and metal ions. The results demonstrate that novel transition metal coordination complexes based on the substituted carboxylate may be constructed by suitable flexible co-ligands as auxiliary linkers. Moreover, complex 1 shows good degradation activity under visible light irradiation for the degradation of RhB. Magnetic studies reveal that dominant antiferromagnetic interactions are mediated in 1.
Supporting information is available at http://www.wjhxxb.cn
-
-
[1]
Halder R, Matsuda R, Kitagawa S, et al. Angew. Chem., Int. Ed., 2014, 53:11772-11777 doi: 10.1002/anie.201405619
-
[2]
Cui Y, Yue Y, Chen B L, et al. Chem. Rev., 2012, 112:1126-1162 doi: 10.1021/cr200101d
-
[3]
Wang C, Liu D, Lin W B, et al. J. Am. Chem. Soc., 2013, 135: 13222-13234 doi: 10.1021/ja308229p
-
[4]
Wen T, Zhang D X, Zhang J. Inorg. Chem., 2013, 52:12-14 doi: 10.1021/ic302273h
-
[5]
Zhao S S, Yang J, Ma J F, et al. Inorg. Chem., 2016, 55: 2261-2273 doi: 10.1021/acs.inorgchem.5b02666
-
[6]
Sarma D, Natarajan S, Drillon M, et al. Inorg. Chem., 2012, 51: 4495-4501 doi: 10.1021/ic2020989
-
[7]
Tahmasebi E, Masoomi M Y, Morsali A, et al. Inorg. Chem., 2015, 54:425-433 doi: 10.1021/ic5015384
-
[8]
Khan N A, Jun J W, Jeong J H, et al. Chem. Commun., 2011, 47:1306-1308 doi: 10.1039/C0CC04759G
-
[9]
Lü L L, Yang J, Ma J F. Inorg. Chem., 2015, 54:1744-1755 doi: 10.1021/ic502686b
-
[10]
Gong Y N, He C T, Lu T B, et al. Chem. Sci., 2016, 7:1070-1075 doi: 10.1039/C5SC02679B
-
[11]
Wang C C, Jing H P, Zhang Y Q, et al. Transition Met. Chem., 2015, 40:573-584 doi: 10.1007/s11243-015-9950-1
-
[12]
Zhang T, Lin W B. Chem. Soc. Rev., 2014, 43:5982-5993 doi: 10.1039/C4CS00103F
-
[13]
Wen L L, Zhao J B, Li D F, et al. Cryst. Growth Des., 2012, 12:1603-1612 doi: 10.1021/cg2016512
-
[14]
Wang C C, Li J R, Guo G S, et al. Energy Environ. Sci., 2014, 7:2831-2867 doi: 10.1039/C4EE01299B
-
[15]
Shen L J, Liang S J, Wu W M, et al. Dalton Trans., 2013, 42:13649-13657 doi: 10.1039/c3dt51479j
-
[16]
Shen L J, Wu W M, Liang R W, et al. Nanoscale, 2013, 5: 9374-9382 doi: 10.1039/c3nr03153e
-
[17]
胡春燕, 肖伟, 袁厚群, 等.无机化学学报, 2014, 30(2):257-263 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20140205&journal_id=wjhxxbcnHU Chun-Yan, XIAO Wei, YUAN Hou-Qun, et al. Chinese J. Inorg. Chem., 2014, 30(2):257-263 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20140205&journal_id=wjhxxbcn
-
[18]
聂雪, 陈满生, 庾江喜, 等.无机化学学报, 2013, 29(12):2704-2708 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20131232&journal_id=wjhxxbcnNIE Xue, CHEN Man-Sheng, YU Jiang-Xi, et al. Chinese J. Inorg. Chem., 2013, 29(12):2704-2708 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20131232&journal_id=wjhxxbcn
-
[19]
Jin S W, Huang Y F, Wang D Q, et al. Polyhedron, 2013, 60:10-22 doi: 10.1016/j.poly.2013.04.056
-
[20]
Kaur Jassal A, Sharma S, Hundal G, et al. Cryst. Growth Des., 2015, 15:79-93 doi: 10.1021/cg500883w
-
[21]
Hoskins B F, Robson R, Slizys D A. J. Am. Chem. Soc., 1997, 119:2952-2953 doi: 10.1021/ja9642626
-
[22]
SAINT, Program for Data Extraction and Reduction, Siemens Analytical X-ray Instruments, Madison, WI, 1994-1996.
-
[23]
Sheldrick G M. SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997.
-
[24]
Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997.
-
[25]
Janiak C. J. Chem. Soc., Dalton Trans., 2000:3885-3896
-
[26]
Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. 6th Ed. New Jersey: Wiley, 2009: 64-67
-
[27]
Wang P F, Duan Y, Zheng L M, et al. Dalton Trans., 2010, 39: 4559-4565 doi: 10.1039/b927100g
-
[28]
Allendorf M D, Bauer C A, Houk R J T, et al. Chem. Soc. Rev., 2009, 38:1330-1352 doi: 10.1039/b802352m
-
[29]
Kahn O. Molecular Magnetism. New York: VCH Publishers, 1993:38-39
-
[30]
Carling C L, Translated by WAN Chun-Di(万纯娣), ZANG Yan(臧焰), HU Yong-Zhu(胡永珠), et al. Magnetochemistry(磁化学). Nanjing: Nanjing University Press, 1990: 33-35
-
[31]
Fabelo O, Pasán J, Ruiz-Pérez C, et al. Inorg. Chem., 2009, 48:6086-6095 doi: 10.1021/ic9004483
-
[32]
Díaz-Gallifa P, Fabelo O, Ruiz-Pérez C. Inorg. Chem., 2014, 53:5674-5683 doi: 10.1021/ic500443t
-
[1]
-
Table 1. 1 Crystal data and structure refinement for 1~3
1 2 3 Formula C28H20CON8O12 C28H20N8O12Zn C56H40Cd2N16O24 Molecular weight 719.45 725.89 1 545.84 Crystal system Monoclinic Monoclinic Monoclinic Space group C2/c P21/c C2/c a/nm 2.916(3) 1.262 6(5) 2.990 5(18) b/nm 1.176 5(12) 1.577 3(7) 1.194 8(7) c/nm 1.753 2(19) 1.644 1(7) 1.810 0(10) β/(°) 109.390(19) 109.721(8) 107.828(10) V/nm3 5.673(10) 3.082(2) 6.157(6) Z 8 4 4 Dc/(g·cm-3) 1.685 1.564 1.668 u/mm-1 0.690 0.875 0.788 F(000) 2 936 1 480 3 104 GOF on F2 1.002 1.006 1.019 R1, wR2a [I > 2σ(I)] 0.064 8, 0.181 7 0.046 3, 0.089 6 0.041 7, 0.077 0 R1, wR2a(all data) 0.106 4, 0.233 3 0.094 0, 0.101 2 0.073 6, 0.085 3 (Δρ)max, (Δρ)min/(e·nm-3) 540, -718 276, -357 564, -870 \begin{document}$ ^{\rm{a}}{\mathit{R}_{\rm{1}}}{\rm{ = }}\sum {\rm{||}}{\mathit{F}_{\rm{o}}}{\rm{| - |}}{\mathit{F}_{\rm{c}}}{\rm{||/}}\sum {\rm{|}}{\mathit{F}_{\rm{o}}}{\rm{|;}}\;\mathit{w}{\mathit{R}_{\rm{2}}}{\rm{ = [}}\sum \mathit{w}{{\rm{(}}\mathit{F}_{\rm{o}}^{\rm{2}}{\rm{-}}\mathit{F}_{\rm{c}}^{\rm{2}}{\rm{)}}^{\rm{2}}}{\rm{/}}\sum \mathit{w}{{\rm{(}}\mathit{F}_{\rm{o}}^{\rm{2}}{\rm{)}}^{\rm{2}}}{{\rm{]}}^{{\rm{1/2}}}}$\end{document} Table 2. Selected bond lengths (nm) and angles (°) for 1
Co(1)-O(1) 0.217 9(4) Co(1)-O(7) 0.203 6(4) Co(1)-N(5) 0.205 3(4) Co(1)-O(2) 0.219 7(4) Co(1)-O(8)A 0.201 6(4) Co(1)-N(8)A 0.205 4(4) O(8)A-Co(1)-O(7) 122.49(15) N(5)-Co(1)-N(8)A 173.83(15) O(8)A-Co(1)-O(2) 86.32(14) O(8)A-Co(1)-N(5) 87.93(17) O(8)A-Co(1)-O(1) 145.02(15) O(7)-Co(1)-O(2) 151.19(13) O(7)-Co(1)-N(5) 88.14(14) O(7)-Co(1)-O(1) 92.49(14) N(5)-Co(1)-O(2) 92.77(14) O(8)A-Co(1)-N(8)A 87.03(18) N(5)-Co(1)-O(1) 93.58(16) N(8)A-Co(1)-O(2) 90.45(14) O(7)-Co(1)-N(8)A 91.58(14) N(8)A-Co(1)-O(1) 92.59(16) O(1)-Co(1)-O(2) 58.70(12) Symmetry codes: A: -x+3/2, -y-1/2, -z+1. Table 3. Selected bond lengths (nm) and angles (°) for 2
Zn(1)-O(1) 0.193 3(2) Zn(1)-N(3) 0.200 4(3) Zn(1)-N(6)B 0.198 7(2) Zn(1)-O(7) 0.194 3(2) O(1)-Zn(1)-O(7) 108.70(9) O(7)-Zn(1)-N(8)B 110.06(10) O(7)-Zn(1)-N(3) 100.79(10) O(1)-Zn(1)-N(8)B 123.52(10) O(1)-Zn(1)-N(3) 106.58(11) N(6)B-Zn(1)-N(3) 104.67(10) Symmetry codes: A: x, -y+3/2, z+1/2; B: x, -y+3/2, z-1/2. Table 4. Selected bond lengths (nm) and angles (°) for 3
Cd(1)-N(5) 0.223 3(3) Cd(1)-O(1) 0.228 2(3) Cd(1)-O(7) 0.236 4(3) Cd(1)-N(8)A 0.223 8(3) Cd(1)-O(2)A 0.232 8(3) Cd(1)-O(8) 0.244 9(3) N(5)-Cd(1)-N(8)A 170.34(11) O(1)-Cd(1)-O(2)A 130.48(12) N(5)-Cd(1)-O(8) 94.11(11) N(5)-Cd(1)-O(1) 88.03(12) N(5)-Cd(1)-O(7) 97.10(11) N(8)A-Cd(1)-O(8) 90.36(11) N(8)A-Cd(1)-O(1) 93.21(12) N(8)A-Cd(1)-O(7) 92.46(11) O(1)-Cd(1)-O(8) 145.01(11) N(5)-Cd(1)-O(2)A 87.80(13) O(1)-Cd(1)-O(7) 91.02(12) O(2)A-Cd(1)-O(8) 84.51(12) N(8)A-Cd(1)-O(2)A 84.11(13) O(2)A-Cd(1)-O(7) 138.44(12) O(7)-Cd(1)-O(8) 54.04(9) ymmetry codes: A: -x+1/2, -y+3/2, -z. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 2
- 文章访问数: 860
- HTML全文浏览量: 109


 下载:
下载:







 下载:
下载:

