
一例对二硫化碳具有荧光传感性能的Mg-金属有机框架化合物
English
A Fluorescent Magnesium-Based Metal-Organic Framework with a Sensitive Sensing Property for Carbon Disulfide
-
Key words:
- magnesium
- / metal-organic framework
- / fluorescence sensor
- / carbon disulfide
-
With growing concerns over the major ecosystem and health risk, the detection of harmful volatile organic compounds(VOCs) has attracted tremendous attention over the decades. Acting as a typically toxic molecule of VOCs, carbon disulfide(CS2) can trigger severe multiorgan diseases when people are continuously exposed to it even in extremely low concentrations[1-2]. Currently, well-trained canines, sophisticated analytical instruments, chemiluminescence-based methods and nanoprobes are used for CS2 sensing[3-6]. However, the above detection methods have different degrees of weakness, such as low sensitivity, high costs and high complexity. Hence, it is a significant but challenging task to explore new sensing materials for rapid and selective detection of the CS2.
Fluorescent metal-organic-frameworks(MOFs) have received much attention due to their potential application in sensitive and selective detection of hazardous substances[7-11]. As a new type of chemosensor, the fluorescent detection based on MOFs can be conveniently monitored by using changes in fluorescent properties caused by host-guest interactions as interpretable signals[12]. Magnesium as a candidate metal ion for the construction of MOFs has caused the climax of researches over the past few years because of its low-cost, nontoxicity and especially the unique 3d0 electron configuration favoring ligand-centered emission[13-15]. Some Mg-MOFs have been explored as fluorescence sensors for typical VOCs and water, such as [Mg2(BINDI)2(DMF)2] H2O(H4BINDI=N, N′-bis(5-isophthalic acid) naphthalenediimide)[14], [NH2(CH3)2][Mg3(NDC)2.5(HCO2)2(DMF)0.75(H2O)0.25]·1.25DMF·0.75H2O(H2NDC=1, 4-naphthalene dicarboxylic acid)[16], Mg5(OH)2(BTEC)2(H2O)4·11H2O(H4BTEC=1, 2, 4, 5-benzenetetracarboxylic acid)[17] and [Mg(H2dhtp)(H2O)2]·DMA(H4dhtp=2, 5-dihydroxy-terepthalic acid)[18]. Herein, we present the solvothermal synthesis, crystal structure and characterizations of a new fluorescent Mg-MOF named as [Mg4(1, 4-NDC)4(DMA)2(CH3OH)2(H2O)2]·DMA·CH3OH(1). Fluorescence measurements demonstrated that the title compound displayed a purple light emission(λem=385 nm) and further fluorescent study indicated that compound 1 exhibited a selective and sensitive sensing property for CS2 with a low concentration.
1 Experimental
1.1 Reagent and Instrument
All reagents and chemicals were purchased from commercial sources and used without further purification. (Mg(NO3)2·6H2O(≥99%, Tianjin BoDi Chemical Co., Ltd.); 1, 4-H2NDC(≥95%, Beijing HWRK Chem Co., Ltd.); DMA(≥99%, Shanghai Titan Chemical Co., Ltd.); methanol anhydrous(≥99.5%, Shanghai Titan Chemical Co., Ltd.). Powder X-ray diffraction(PXRD) patterns were recorded on a Rigaku Miniflex Ⅱ diffractometer using CuKα radiation(λ=0.154178 nm). Elemental analyses(EA) of C, N, H were performed on a German Elementary Vario Ⅲ instrument. Thermogravimetric analysis(TGA) was carried out with a NETZACH STA 449F3 unit at a heating rate of 10 ℃/min under a nitrogen atmosphere. Emission and excitation spectra of compound 1 in the solid state and at a suspension system were recorded on a Perkin-Elmer LS55 luminescence spectrometer at room temperature.
1.2 Synthesis of compound 1
A mixture of 1 mmol Mg(NO3)2·6H2O(0.256 g) and 1 mmol 1, 4-H2NDC(0.216 g) in 4 mL DMA(N, N-dimethylacetamide) and 1 mL anhydrous methanol was sealed into a stainless steel reactor with a 20 mL teflon-lined bomb. The mixture was heated at 130 ℃ for 3 days and then was slowly cooled to room temperature. The colorless block-shaped crystals were obtained after being filtered and air-dried. Yield, 84.3%(0.284 g) based on Mg. Anal.(calc.) for compound 1:C 55.90%(56.16%), H 4.82%(5.01%), N 3.08%(3.12%).
1.3 Determination of crystal structure
A suitable single crystal of compound 1 was carefully selected under an optical microscope and glued to a thin glass fiber. The intensity data were collected on a SuperNova CCD diffractometer with MoKα radiation(λ=0.071073 nm) at 100(2) K. The structure was solved by direct methods and refined by full-matrix least-squares on F2 using the SHELX-2016 program package[19]. All the non-hydrogen atoms were refined anisotropically, and the hydrogen atoms bonded to carbon were located by geometrical calculations, while those for O atoms were located from difference-Fourier maps and refined with restrained O-H distances. The empirical formula of compound 1 was further confirmed by the EA and TGA results. The crystallographic data and details of structural refinements for compound 1 are listed in Table 1.
Empirical formula C63H67Mg4N3O24 Formula mass 1 347.431 00(2) T/K 100(2) Crystal system Monoclinic Space group P21/c a/nm 2.060 90(12) b/nm 2.210 14(13) c/nm 1.503 85(10) α/(°) 90 β/(°) 111.399(3) γ/(°) 90 V/nm3 6.377 6(7) Z 4 Dcalc./(g·cm-3) 1.403 λ/nm 0.071 073 μ/mm-1 0.142 F(000) 2 824 Reflections measured 28 773 Independent reflections 12 199 No. of parameters 878 GOF on F2 1.033 R1[I > 2σ(I)]a 0.059 6 wR2[I > 2σ(I)]a 0.122 5 R1[all data] 0.089 9 wR2[all data] 0.146 1 CCDC 1552691 a.R1=Σ‖Fo|-|Fc‖/Σ|Fo|, wR2={Σw[(Fo)2-(Fc)2]2/Σw[(Fo)2]2}1/2. 1.4 Fluorescence detection measurements
The fluorescence properties of compound 1 and the 1, 4-H2NDC ligand were investigated in the solid state at room temperature and the fluorescent spectra were recorded. Then 2 mg of compound 1(the as-made crystalline sample of compound 1 was loaded into an agate mortar and was manually ground with the pestle to afford a fine powder) was dispersed in 2 mL of CS2, CH3OH, acetone, DMA and acetonitrile, respectively. After ultrasonic treatment for a few seconds, the suspension was placed in a quartz cell of 1 cm width for fluorescence detection. Detailed detections were carried out by gradually adding the CS2 as quenchers in an incremental fashion with a pipette. The corresponding fluorescent spectra were recorded at 298 K. For all the measurements, the dispersed suspensions of compound 1 were excited at λex=345 nm(λem=385 nm) and the corresponding emission wavelengths were monitored from 360 nm to 650 nm.
2 Results and discussion
2.1 Crystal structure descriptions
Single-crystal X-ray diffraction analysis reveals that compound 1 crystallizes in the monoclinic space group P21/c. The crystallographic asymmetric unit contains one formula unit. All the Mg2+ ions are six-coordinated except that there are some differences in the coordinated modes. The Mg(1) and Mg(3) atoms have similar coordination modes that are coordinated by five carboxylic O atoms from four 1, 4-NDC2- ligands(one carboxylic group adopts a chelating coordination mode) and one O atom from a water molecule(Fig. 1A); while the Mg(2) and Mg(4) are both coordinated by four carboxylic O atoms from four 1, 4-NDC2- ligands in a monodentate way and one O atom from a methanol molecule and one O atom from a DMA molecule(Fig. 1A). The Mg—O bond lengths range from 0.1991(3) nm to 0.2206(3) nm, which are comparable to those in the reported magnesium-carboxylate compounds[20-23]. As shown in Fig. 1B, the 1, 4-NDC ligands(L1, L2, L3, L4) adopt different coordination modes which can be depicted as (k1-k1-μ2)-(k1-k1-μ2)-μ4(L1 and L3) and (k1-k1-μ2)-(k1-k2-μ2)-μ4(L2 and L4), respectively.
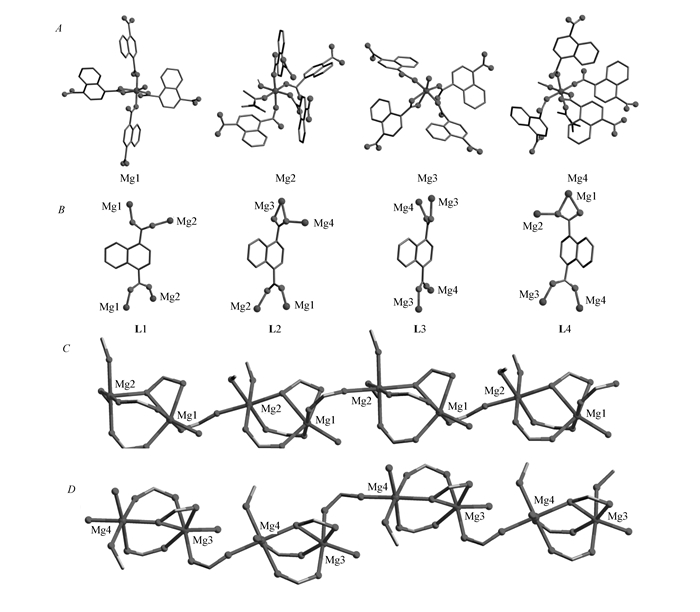 图1
The coordination environments of Mg atoms(A), coordination modes of the four crystallographically independent 1, 4-NDC2- ligands(B), and the 1D chains in compound 1 extended along the c axis(C, D)
Figure1.
The coordination environments of Mg atoms(A), coordination modes of the four crystallographically independent 1, 4-NDC2- ligands(B), and the 1D chains in compound 1 extended along the c axis(C, D)
图1
The coordination environments of Mg atoms(A), coordination modes of the four crystallographically independent 1, 4-NDC2- ligands(B), and the 1D chains in compound 1 extended along the c axis(C, D)
Figure1.
The coordination environments of Mg atoms(A), coordination modes of the four crystallographically independent 1, 4-NDC2- ligands(B), and the 1D chains in compound 1 extended along the c axis(C, D)
As shown in Fig. 1C and Fig. 1D, there exist corner-shared coordination polyhedra of dinuclear [Mg1Mg2] and [Mg3Mg4] units in the structure which can be viewed as the secondary building units(SBU) for compound 1. Then, the adjacent [Mg1Mg2] and [Mg3Mg4] units are, respectively, interconnected by the COO- groups of ligands L1 and L3 to form one-dimensional(1D) infinite chains of [—Mg1—Mg2—Mg1—Mg2—] and [—Mg3—Mg4—Mg3—Mg4—] along the c-axis. Further, each [—Mg1—Mg2—Mg1—Mg2—] chain connects to four adjacent [—Mg3—Mg4—Mg3—Mg4—] chains by the ligands L3 and L4 to form a 3D skeleton. The coordinated and free DMA, methanol, water molecules settle in the cages of the 3D framework(Fig. 2A). The solvent accessible volume is 43.6% if all the solvent molecules are removed according to the calculation performed by PLATON analysis. Topologically, when regarding the 1, 4-NDC2- ligands and each dinuclear secondary building unit as 3-connected and 5-connected nodes respectively, the structure of compound 1 could be simplified as a typical fsc-3, 5-C2/c topology, Fig. 2B.
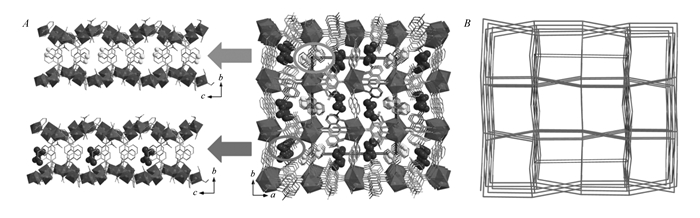 图2
View of the 3D framework of compound 1 along the c axis showing the cages in which the DMA and methanol molecules are filled(A) and the topology of compound 1(B)
Figure2.
View of the 3D framework of compound 1 along the c axis showing the cages in which the DMA and methanol molecules are filled(A) and the topology of compound 1(B)
图2
View of the 3D framework of compound 1 along the c axis showing the cages in which the DMA and methanol molecules are filled(A) and the topology of compound 1(B)
Figure2.
View of the 3D framework of compound 1 along the c axis showing the cages in which the DMA and methanol molecules are filled(A) and the topology of compound 1(B)
2.2 Thermal stability
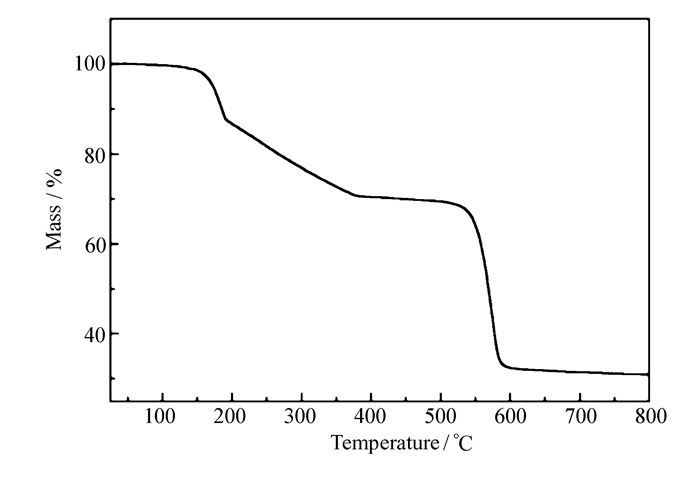
The phase purity of compound 1 was confirmed by PXRD(see Fig.S1 in Supporting Information) carried out with the polycrystalline sample of compound 1. Thermogravimetric analysis of compound 1 was performed under a N2 atmosphere from 25 to 800 ℃ with a heating rate of 10 ℃ /min on pure powdered sample. The thermogravimetric curve for compound 1 is shown in Fig. 3. The 9.01% mass loss from room temperature(RT) to ~180 ℃ should be attributed to the loss of the free guests(calcd. 8.84%); the 19.72% mass loss of compound 1 from 180 to 500 ℃ corresponds to the departure of the coordinated water, CH3OH and DMA molecules in the structure(calcd. 20.36%). The mass of the samples remained nearly constant from 600 to 800 ℃ and the characterization of PXRD suggested that the residual powder was MgO(see Fig.S2, in Supporting Information).
2.3 Fluorescence detection properties
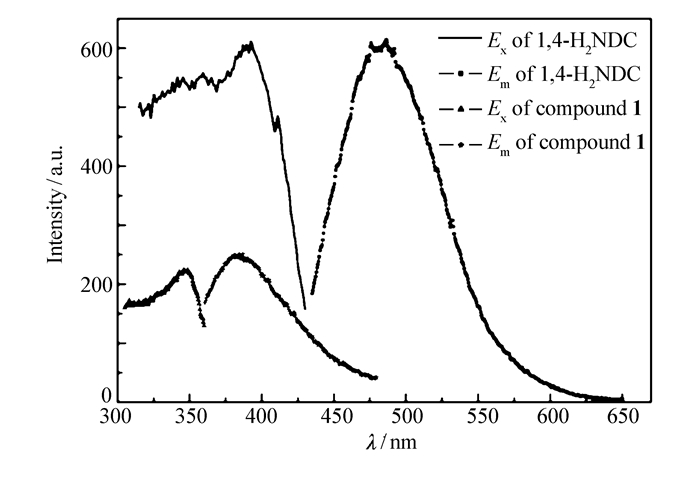
As shown in Fig. 4, the fluorescent spectra of compound 1 in the solid state exhibited a purple emission band with an intense peak maximum at 385 nm upon excitation at 345 nm at room temperature. Compared with the emission band of the free 1, 4-H2NDC ligand(λem=485 nm monitored at λex=390 nm), compound 1 showed blue-shift emission. The title compound should exhibit a ligand-centered emission due to the unique electron configuration of Mg2+. Further fluorescence measurement for compound 1 has been carried out to identify whether compound 1 has a luminescent response to volatile organic molecules. As shown in Fig. 5A, compound 1 was dispersed in five kinds of typically used solvents and it was interesting to see that the fluorescence intensities of compound 1 were heavily dependent on the identity of the organic solvent molecules.
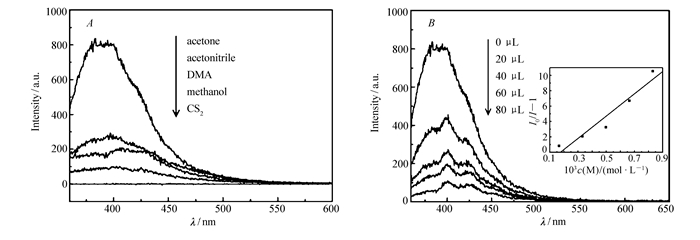 图5
Emission spectra of compound 1 dispersed in different solvents(A) and emission spectra of compound 1 dispersed in the acetone with various contents of CS2(inset is the SV plot for the quenching of compound 1 by CS2)(B)
Figure5.
Emission spectra of compound 1 dispersed in different solvents(A) and emission spectra of compound 1 dispersed in the acetone with various contents of CS2(inset is the SV plot for the quenching of compound 1 by CS2)(B)
图5
Emission spectra of compound 1 dispersed in different solvents(A) and emission spectra of compound 1 dispersed in the acetone with various contents of CS2(inset is the SV plot for the quenching of compound 1 by CS2)(B)
Figure5.
Emission spectra of compound 1 dispersed in different solvents(A) and emission spectra of compound 1 dispersed in the acetone with various contents of CS2(inset is the SV plot for the quenching of compound 1 by CS2)(B)
Notably, we found that compound 1 exhibited a significant quenching of fluorescence when dispersed in CS2. Since compound 1 demonstrated the strongest fluorescence emission in acetone, the acetone was chosen as the dispersed solvent to find the potential fluorescence detection of compound 1 for CS2. The sensing sensitivity towards CS2 was examined in detail through gradually increasing CS2 contents into the emulsions of compound 1 dispersed in acetone to monitor the emissive response. As depicted in Fig. 5B, the fluorescent intensity of compound 1 was almost completely quenched when only 80 μL CS2(0.4%(volume fraction)) was added, indicating that compound 1 was a benign candidate for selective sensing of CS2. Stern-Volmer equation(SV plot):I0/I=1+Ksv×[M] was applied to judge the quenching effect(I0 and I are the suspension luminescence intensity of compound 1 without and with addition of quencher, and [M] is the molarity of quencher and Ksv is the quenching constant)[24]. As shown in Fig. 5B, the SV plot displays a good linear behavior and the Ksv constant calculated from the experimental data is 1.45×103 L/mol. Compared to the former reports detecting CS2 based on the Mg-MOF luminescence intensity, the quenching concentration of CS2 for the title compound is slightly higher[17]. This could be attributed to the larger channels in the former reported Mg-MOFs, which facilitate the interactions between CS2 and MOFs[16-17]. Further investigation is still required for exploring the mechanism of fluorescence quenching in compound 1.
3 Conclusions
In summary, a novel 3D Mg-MOF, namely [Mg4(1, 4-NDC)4(DMA)2(CH3OH)2(H2O)2]·DMA·CH3OH has been synthesized under solvothermal conditions and characterized. Fluorescence study indicates that the title compound shows a highly sensitive fluorescent response for CS2 with a low concentration. Future work will continue to study the construction of fluorescent Mg-MOFs, explore their sensing properties towards the harmful volatile organic compounds and aim at a deep understanding of the relationship of structure and property.
Supporting Information [PXRD patterns] is available free of charge on the website of Chinese Journal of Applied Chemistry(http://yyhx.ciac.jl.cn/).
-
-
[1]
Chuang W L, Huang C C, Chen C J. Carbon Disulfide Encephalopathy:Cerebral Microangiopathy[J]. Neurotoxicology, 2007, 28(2): 387-393. doi: 10.1016/j.neuro.2006.10.008
-
[2]
Wang S, Irving G, Jiang L L. Oxidative Stress Mediated Hippocampal Neuron Apoptosis Participated in Carbon Disulfide-Induced Rats Cognitive Dysfunction[J]. Neurochem Res, 2017, 42(2): 583-594. doi: 10.1007/s11064-016-2113-8
-
[3]
Ciaffoni L, Peverall R, Ritchie G A D. Laser Spectroscopy on Volatile Sulfur Compounds:Possibilities for Breath Analysis[J]. J Breath Res, 2011, 5(2): 024002. doi: 10.1088/1752-7155/5/2/024002
-
[4]
Furton K G, Myers L J. The Scientific Foundation and Efficacy of the Use of Canines as Chemical Detectors for Explosives[J]. Talanta, 2001, 54(3): 487-500. doi: 10.1016/S0039-9140(00)00546-4
-
[5]
Lu W, Xiao P, Liu Z Z. Reaction-Driven Self-Assembled Micellar Nanoprobes for Ratiometric Fluorescence Detection of CS2with High Selectivity and Sensitivity[J]. ACS Appl Mater Interfaces, 2016, 8(31): 20100-20109. doi: 10.1021/acsami.6b06472
-
[6]
Zhang R K, Li G K, Hu Y F. Simple and Excellent Selective Chemiluminescence-Based CS2 On-Line Detection System for Rapid Analysis of Sulfur-Containing Compounds in Complex Samples[J]. Anal Chem, 2015, 87(11): 5649-5655. doi: 10.1021/acs.analchem.5b00722
-
[7]
Heine J, Muller-Buschbaum K. Engineering Metal-Based Luminescence in Coordination Polymers and Metal-Organic Frameworks[J]. Chem Soc Rev, 2013, 42(24): 9232-9242. doi: 10.1039/c3cs60232j
-
[8]
Hu Z C, Deibert B J, Li J. Luminescent Metal-Organic Frameworks for Chemical Sensing and Explosive Detection[J]. Chem Soc Rev, 2014, 43(16): 5815-5840. doi: 10.1039/C4CS00010B
-
[9]
Banerjee D, Hu Z C, Li J. Luminescent Metal-Organic Frameworks as Explosive Sensors[J]. Dalton Trans, 2014, 43(28): 10668-10685. doi: 10.1039/C4DT01196A
-
[10]
Kreno L E, Leong K, Farha O K. Metal-Organic Framework Materials as Chemical Sensors[J]. Chem Rev, 2012, 112(2): 1105-1125. doi: 10.1021/cr200324t
-
[11]
Cui Y J, Yue Y F, Qian G D. Luminescent Functional Metal-Organic Frameworks[J]. Chem Rev, 2012, 112(2): 1126-1162. doi: 10.1021/cr200101d
-
[12]
Meyer L V, Schoenfeld F, Zurawski A. A Blue Luminescent MOF as a Rapid Turn-Off/Turn-On Detector for H2O, O-2 and CH2Cl2, MeCN:∞3Ce(Im)3ImH ·ImH[J]. Dalton Trans, 2015, 44(9): 4070-4079. doi: 10.1039/C4DT03578J
-
[13]
Xu F, Wang H, Teat S J. Synthesis, Structure and Enhanced Photoluminescence Properties of Two Robust, Water Stable Calcium and Magnesium Coordination Networks[J]. Dalton Trans, 2015, 44(47): 20459-20463. doi: 10.1039/C5DT03705K
-
[14]
Jayaramulu K, Kanoo P, George S J. Tunable Emission From a Porous Metal-Organic Framework by Employing an Excited-State Intramolecular Proton Transfer Responsive Ligand[J]. Chem Commun, 2010, 46(42): 7906-7908. doi: 10.1039/c0cc02069a
-
[15]
Brown J W, Henderson B L, Kiesz M D. Photophysical Pore Control in an Azobenzene-Containing Metal-Organic Framework[J]. Chem Sci, 2013, 4(7): 2858-2864. doi: 10.1039/c3sc21659d
-
[16]
Wu Z F, Tan B, Feng M L. A Magnesium-Carboxylate Framework Showing Luminescent Sensing for CS2 and Nitroaromatic Compounds[J]. J Solid State Chem, 2015, 223: 59-64. doi: 10.1016/j.jssc.2014.06.018
-
[17]
Wu Z F, Tan B, Feng M L. A Magnesium MOF as a Sensitive Fluorescence Sensor for CS2 and Nitroaromatic Compounds[J]. J Mater Chem A, 2014, 2(18): 6426-6431. doi: 10.1039/C3TA15071B
-
[18]
Douvali A, Tsipis A C, Eliseeva S V. Turn-On Luminescence Sensing and Real-Time Detection of Traces of Water in Organic Solvents by a Flexible Metal-Organic Framework[J]. Angew Chem Int Ed, 2015, 54(5): 1651-1656. doi: 10.1002/anie.201410612
-
[19]
Sheldrick G M. Crystal Structure Refinement with SHELXL[J]. Acta Crystallogr, 2015, 71: 3-8.
-
[20]
Zhai Q G, Lin Q P, Wu T Z. Induction of Trimeric[Mg3(OH)(CO2)6] in a Porous Framework by a Desymmetrized Tritopic Ligand[J]. Dalton Trans, 2012, 41(10): 2866-2868. doi: 10.1039/c2dt12215d
-
[21]
Guo Z Y, Li G H, Zhou L. Magnesium-based 3D Metal-Organic Framework Exhibiting Hydrogen-Sorption Hysteresis[J]. Inorg Chem, 2009, 48(17): 8069-8071. doi: 10.1021/ic901056d
-
[22]
Zhai Q G, Bu X, Zhao X. Advancing Magnesium-Organic Porous Materials Through New Magnesium Cluster Chemistry[J]. Cryst Growth Des, 2016, 16(3): 1261-1267. doi: 10.1021/acs.cgd.5b01297
-
[23]
Rood J A, Noll B C, Henderson K W. Synthesis, Structural Characterization, Gas Sorption and Guest-Exchange Studies of the Lightweight, Porous Metal-Organic Framework Alpha-[Mg3(O2CH)6][J]. Inorg Chem, 2006, 45(14): 5521-5528. doi: 10.1021/ic060543v
-
[24]
Lakowicz J R. Principles of Fluorescence Spectroscopy[M]. New York:Plenum Press, 1983:260-266.
-
[1]
-
Table 1. Crystallographic data and structural refinement details for compound 1
Empirical formula C63H67Mg4N3O24 Formula mass 1 347.431 00(2) T/K 100(2) Crystal system Monoclinic Space group P21/c a/nm 2.060 90(12) b/nm 2.210 14(13) c/nm 1.503 85(10) α/(°) 90 β/(°) 111.399(3) γ/(°) 90 V/nm3 6.377 6(7) Z 4 Dcalc./(g·cm-3) 1.403 λ/nm 0.071 073 μ/mm-1 0.142 F(000) 2 824 Reflections measured 28 773 Independent reflections 12 199 No. of parameters 878 GOF on F2 1.033 R1[I > 2σ(I)]a 0.059 6 wR2[I > 2σ(I)]a 0.122 5 R1[all data] 0.089 9 wR2[all data] 0.146 1 CCDC 1552691 a.R1=Σ‖Fo|-|Fc‖/Σ|Fo|, wR2={Σw[(Fo)2-(Fc)2]2/Σw[(Fo)2]2}1/2. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 1
- 文章访问数: 1035
- HTML全文浏览量: 88


 下载:
下载:




 下载:
下载:

