 Figure Scheme 1.
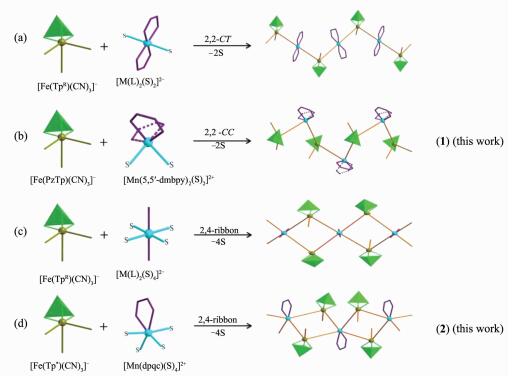
Different topologies based on different synthetic strategies of cyanide building blocks fac-[Fe(TpR)CN)3]- in the Fe(Ⅲ)-M(Ⅱ) bimetallic chains
Figure Scheme 1.
Different topologies based on different synthetic strategies of cyanide building blocks fac-[Fe(TpR)CN)3]- in the Fe(Ⅲ)-M(Ⅱ) bimetallic chains

氰基桥联的Fe(Ⅲ)-Mn(Ⅱ)双金属链的可控组装和磁性作用的调控
English
Tuning Assembly and Magnetic Interactions of Cyano-bridged Fe(Ⅲ)-Mn(Ⅱ) Bimetallic Chains
-
Key words:
- cyano-bridged
- / chain
- / ferrimagnetic
- / antiferromagnetic
-
0 Introduction
Cyano-bridged molecule-based magnetic materials have been a research field of rapid expansion in recent decades, because the cyano-bridge not only directs the formation of predictable structure but also can efficiently transfer magnetic interactions[1-5]. Meanwhile, since the first experimental one-dimensional (1D) system displaying slow magnetic dynamics was reported by Gatteschi and co-workers[6], single-chain magnets (SCMs) have attracted increasing interest because they exhibit the same slow magnetic relaxation as well as with possibly higher magnetic transition temperature than the single-molecule magnets (SMMs)[7-10]. Therefore, the rational design and synthesis of the low dimensional cyanometallate compounds with the property of the SCMs has become a particularly important subject. A rational synthetic strategy is to use the capped building blocks [Fe(L)(CN)x]y-(x=2~5), where L is a variety of bidentate, tridentate or tetradentate ligands and so on[4, 5, 11-15]. Based on these building blocks, a great number of low dimensional cyanide-bridged compounds have been prepared; some of them are SCMs[16-19]. Among them, the use of fac-[Fe(TpR)CN)3]- as the cyanometallate building block has attracted our attention. The above building block exhibits a stable topology, and directs the formation of predictable structure through rational design. As a consequence, its use often leads to form the tetranuclear {Fe2M2} square systems[20-22], 2, 2-CT(C=cis; T=trans) single chain[23-25] and double 4, 2-ribbon like bimetallic chain[16, 26-27] (Scheme 1). Most of the reported studies focused on Fe(Ⅲ)-M(Ⅱ) (M=Fe, Co, Ni and Cu) bimetallic assemblies. However, a few Fe(Ⅲ)-Mn(Ⅱ) bimetallic systems were reported based on the fac-[Fe(TpR)CN)3]- [28-33], and they all exhibited the antiferromagnetic behavior. Numerous papers dealing with Fe(Ⅲ)-M analogous systems showed that the magnetic interactions strength is highly sensitive to metal-ligand distances, M-C≡N and M′-N≡C angles and torsion angles[24-25, 34-36]. In the reported Fe(Ⅲ)-Mn(Ⅱ) systems, the bending of the Mn-N≡C bond angles is larger in chain systems than that of in polynuclear systems (Table 1). Therefore, if the bending of the Mn-N≡C bond angles becomes large, the decrease of the antiferromagnetic interactions could be realized, leading to the ferromagnetic or ferrimagnetic behaviors. The introduction of steric hindrance ligands may induce the distortion of the structure, realizing the control of the magnetic interactions. Herein, we selected different bulky [Fe(PzTp)CN)3]- and [Fe(Tp*)CN)3]- as the building blocks, 5,5′-dmbpy (5,5′-dmbpy=5,5′-dimethyl-2, 2′-bipyridine) and dpqc (dpqc=dipyrido[3, 2-a: 2′, 3′-c]-(6, 7, 8, 9-tetrahydro)phenazine) as the second ligands. Two cyano-bridged chains {[Fe(Ⅲ)(PzTp)(CN)3][Mn(Ⅱ)(5,5′-dmbpy)2]ClO4}n (1) and {[Fe(Ⅲ)(Tp*)(CN)3]2Mn(Ⅱ)(dpqc)·CH3OH·H2O}n (2) were synthesized. Compound 1 exhibits a 1D 2, 2-CC helix chain structure and shows the ferrimagnetic behavior. Compound 2 has a novel 4, 2-ribbon double chain structure and displays the antiferromagnetic interactions. As far as we know, such a novel architecture has never been reported previously.
 Figure Scheme 1.
Different topologies based on different synthetic strategies of cyanide building blocks fac-[Fe(TpR)CN)3]- in the Fe(Ⅲ)-M(Ⅱ) bimetallic chains
Figure Scheme 1.
Different topologies based on different synthetic strategies of cyanide building blocks fac-[Fe(TpR)CN)3]- in the Fe(Ⅲ)-M(Ⅱ) bimetallic chains
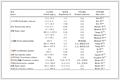 Table 1.
Mn-N≡C bond angles and related magnetic behaviors for Fe(Ⅲ)-Mn(Ⅱ) systems constructed from the tricyanide precursors fac-[Fe(TpR)CN)3]-
Table 1.
Mn-N≡C bond angles and related magnetic behaviors for Fe(Ⅲ)-Mn(Ⅱ) systems constructed from the tricyanide precursors fac-[Fe(TpR)CN)3]-
Compound Structure ∠Mn-N≡C/(°) Magnetic behavior Ref. a trinuclear 167.3(2) antiferromagnetic 28 b tetranuclear 161.1(2), 172.0(2) antiferromagnetic 28 c tetranuclear 177.4(4), 175.3(4) antiferromagnetic 29 d hexanuclear 155.5(4)~170.9(4) antiferromagnetic 30 e double chain 156.1(2), 159.0(2) antiferromagnetic 30 f double chain 154.9(3), 168.3(3) antiferromagnetic 31 g hexanuclear 155.3(3)~162.6(3) antiferromagnetic 31 h tetranuclear 160.1(2), 73.3(2) antiferromagnetic 32 i double chain 144.0-156.0 antiferromagnetic 33 j double chain 147.6-153.1 antiferromagnetic 33 1 single chain 140.2(4), 164.6(4) ferrimagnetic this work 2 double chain 156.6(5)~164.1(5) antiferromagnetic this work a: {[Fe(HB(pz)3)(CN)3]2[Mn(MeOH)4]·2MeOH}n; b: {[Mn2Fe2(HB(pz)3)2(CN)6(4, 4′-bipyridine)2](ClO4)2·4MeCN}n; c: {[(Tp*)Fe(Ⅲ)(CN)3][Mn(Ⅱ)(DMF)4]2(OTf)·2DMF}n; d: {[(Tp)Fe(Ⅲ)(CN)3]2[Mn(Ⅱ)(DMF)2(H2O)]}n; e: {[(Tp)Fe(Ⅲ)(CN)3]2[Mn(Ⅱ)(DMF)2]}n; f: {[Mn(bpym)(H2O)]2[Fe(HB(pz)3)(CN)3]4}n; g: {[Mn(bpym)(H2O)]2[Fe(B(pz)4)(CN)3]4·4H2O}n; h: {[Fe(Ⅲ)(B(pz)4)(CN)2(μ-CN)][Mn(Ⅱ)(bpy)2]2(ClO4)2·CH3CN}n; i: {[Fe(Tp)(CN)3]2[Mn(bib)]·CH3OH·2H2O}n; j: {[Fe(pzTp)(CN)3]2[Mn(bib)]·3H2O}n 1 Experimental
1.1 Materials and physical measurements
All chemical reagents were acquired from commercial sources and were used as received without further purification. Bu4N[Fe(PzTp)(CN)3], Bu4N[Fe(Tp*)(CN)3] and the ligand dpqc were synthesized according to the literature method[37-38]. Elemental analyses were performed on an Elementar Vario EL Ⅲ analyzer. IR spectra were recorded on a Bruker AXS TENSOR-27 FTIR spectrometer with KBr pellets in the range of 400~4 000 cm-1. Magnetic measurements of the samples were performed on a Quantum Design SQUID (MPMSXL-7) magnetometer. Data were corrected for the diamagnetic contribution calculated from Pascal constants.
1.2 Synthesis of {[Fe(Ⅲ)(PzTp)(CN)3][Mn(Ⅱ)(5,5′-dmbpy)2]ClO4}n (1)
A 6.0 mL aqueous solution of Mn(ClO4)2·6H2O (0.03 mmol) was placed at the bottom of a test tube, a mixture of methanol and water (1:2, V/V, 6 mL) was gently layered on the top of the solution, and then a 6.0 mL methanol solution of Bu4N[Fe(PzTp)(CN)3] (0.03 mmol) and 5,5′-dmbpy (0.06 mmol) was carefully added as the third layer. After few weeks, red block crystals of 1 were collected, washed with water and dried in air. Yield: 58% based on Mn(ClO4)2·6H2O. Anal. Calcd. for C39H36BClFeMnN15O4(%): C 50.05, H 3.88, N 22.45; Found(%): C 50.10, H 3.98, N 22.37. IR (KBr, cm-1): 3 123(w), 2 927(w), 2 141(s), 2 136(s), 1 610(m), 1 572(m), 1 504(m), 1 482(s), 1 436(m), 1 406(s), 1 391(s), 1316(s), 1 241(m), 1 211(s), 1 106(s), 917(m), 857(s), 766(s), 623(s), 488(m), 413(s).
1.3 Synthesis of {[Fe(Ⅲ)(Tp*)(CN)3]2Mn(Ⅱ)(dpqc)·CH3OH·H2O}n (2)
The compound was obtained with a similar procedure to that of 1, except using Bu4N[Fe(Tp*)(CN)3] (0.06 mmol) and dpqc (0.03 mmol) to replace Bu4N[Fe(PzTp)(CN)3] and 5,5′-dmbpy, respectively. The black red plate crystals of 2 were collected after several weeks, washed with water and dried in air. Yield: 45% based on Mn(ClO4)2·6H2O. Anal. Calcd. for C55H64B2Fe2MnN22O2(%): C 52.70, H 5.15, N 24.58; Found(%): C 52.65, H 5.19, N 24.67. IR (KBr, cm-1): 3 416(br), 2 927(m), 2 859(w), 2 528(m), 2 142(s), 2128(w), 1 632(m), 1 542(s), 1 452(m), 1 414(m), 1 376(s), 1 308(m), 1 203(s), 1 060(s), 857(m), 819(m), 789(m), 736(m), 691(m), 638(m), 570(w), 435(m).
1.4 X-ray Crystallography
The data were collected on a Bruker Smart APEX (Ⅱ) X-diffractometer equipped with graphite monochromated Mo Kα radiation (λ=0.071 073 nm) using the SMART and SAINT[39] programs at 298 K for compounds 1 and 2. Final unit cell parameters were based on all observed reflections from integration of all frame data. The structures were solved in the space group by direct method and refined by the full-matrix least-squares using SHELXTL-97 fitting on F2 [40]. For compounds 1 and 2, all non-hydrogen atoms were refined anisotropically. The hydrogen atoms of organic ligands were located geometrically and fixed isotropic thermal parameters. Attempts to add the hydrogen atoms for the solvent water molecules in the crystal structure of compound 2 through Fourier electron density were failed. The ClO4- group in compound 1 was disordered; therefore, large thermal displacement parameters were found for these atoms and refined with partial occupancy. In compound 2, the solvent water molecule (O1W) was disordered, which was split over two sites and refined with partial occupancy. The crystal data and details of the structure refinement of compounds 1 and 2 are summarized in Table 2. Selected bond distances and angles of compounds 1 and 2 are listed in Table 3.
Compound 1 2 Formula C39H36BClFeMnN15O4 C55H64B2Fe2MnN22O2 Formula weight 935.88 1 253.53 Crystal system Monoclinic Triclinic Space group P21/c P1 a/nm 1.266 91(10) 1.3724 6(5) b/nm 1.176 12(9) 1.5940 9(6) c/nm 2.871 31(19) 1.6489 6(6) α/(°) 90 83.450 7(17) β/(°) 94.744(5) 79.194 9(15) γ/(°) 90 69.771 5(15) V/nm3 4.263 7(5) 3.320 4(2) Z 4 2 Dc/(g·cm-3) 1.458 1.252 F(000) 1 920 1 298 Reflections collected 26 548 77 019 Unique reflections (Rint) 7 498(0.072 6) 11 679(0.122 2) Goodness-of-fit on F2 1.037 1.045 Final R indicesa,b[I > 2σ(I) R1=0.059 7, wR2=0.135 4 R1=0.071 0, wR2=0.208 5 R indicesa,b(all data) R1=0.096 0, wR2=0.148 6 R1=0.141 6, wR2=0.233 0 \begin{document}$^{\rm{a}}{\mathit{R}_{\rm{1}}}{\rm{ = }}\sum {\rm{(|}}{\mathit{F}_{\rm{o}}}{\rm{| - |}}{\mathit{F}_{\rm{c}}}{\rm{|)/}}\sum {\rm{|}}{\mathit{F}_{\rm{o}}}{\rm{|;}}{\;^{\rm{b}}}\mathit{w}{\mathit{R}_{\rm{2}}}{\rm{ = [}}\sum \mathit{w}{{\rm{(|}}{\mathit{F}_{\rm{o}}}{\rm{|-|}}{\mathit{F}_{\rm{c}}}{\rm{|)}}^{\rm{2}}}{\rm{/}}\sum \mathit{wF}_{\rm{o}}^{\rm{2}}{{\rm{]}}^{{\rm{1/2}}}} $\end{document} 1 Fe(1)-C(39) 0.192 8(5) Fe(1)-N(3) 0.196 8(4) Mn(1)-N(12) 0.225 4(3) Fe(1)-C(38) 0.193 1(6) Fe(1)-N(5) 0.197 4(4) Mn(1)-N(10) 0.227 8(4) Fe(1)-C(37) 0.193 4(5) Mn(1)-N(14) 0.220 2(4) Mn(1)-N(9) 0.228 4(4) Fe(1)-N(1) 0.196 1(4) Mn(1)-N(15)ⅰ 0.223 1(4) Mn(1)-N(11) 0.231 8(4) N(15)-C(37)-Fe(1) 171.4(4) W(13)-C(38)-Fe(1) 176.6(5) C(39)-N(14)-Mn(1) 164.6(4) N(14)-C(39)-Fe(l) 173.0(4) C(37)-N(15)-Mn(1)ⅱ 140.2(4) 2 Fe(1)-C(18) 0.190 5(6) Fe(2)-C(34) 0.191 8(7) Mn(1)-N(9) 0.215 3(5) Fe(l)-C(17) 0.191 5(6) Fe(2)-C(35) 0.193 1(6) Mn(1)-N(18) 0.218 1(5) Fe(1)-C(16) 0.1931(7) Fe(2)-C(36) 0.193 5(6) Mn(1)-N(8)ⅰ 0.221 8(5) Fe(1)-N(3) 0.198 8(5) Fe(2)-N(14) 0.199 9(4) Mn(1)-N(17)ⅱ 0.222 8(5) Fe(1)-N(5) 0.200 0(5) Fe(2)-N(10) 0.200 5(5) Mn(1)-N(19) 0.228 6(5) Fe(1)-N(1) 0.200 6(5) Fe(2)-N(12) 0.201 7(5) Mn(1)-N(20) 0.230 6(5) N(7)-C(16)-Fe(1) 178.6(6) N(17)-C(35)-Fe(2) 176.5(5) C(18)-N(9)-Mn(1) 160.5(4) N(8)-C(17)-Fe(1) 175.0(5) N(18)-C(36)-Fe(2) 178.2(5) C(35)-N(17)-Mn(1)ⅱ 164.1(5) N(9)-C(18)-Fe(1) 174.3(5) C(17)-N(8)-Mn(1)ⅰ 156.6(5) C(36)-N(18)-Mn(1) 156.2(5) N(16)-C(34)-Fe(2) 177.7(6) Symmetry codes: ⅰ-x, y-1/2, -z+1/2; ⅱ-x, y+1/2, -z+1/2 for 1; ⅰ-x+1, -y+1, -z+2; ⅱ-x, -y+1, -z+2 for 2 CCDC: 1449127, 1; 1449128, 2.
2 Results and discussion
2.1 Crystal structural description
2.2 Magnetic properties
2.1.1 Crystal structure of 1
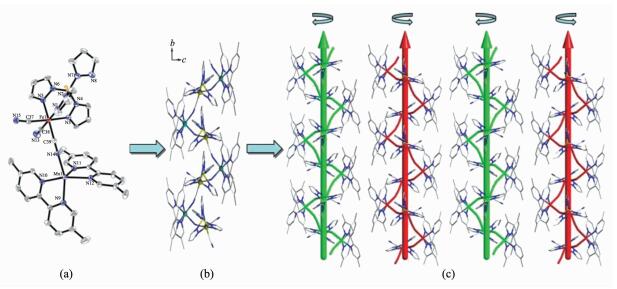
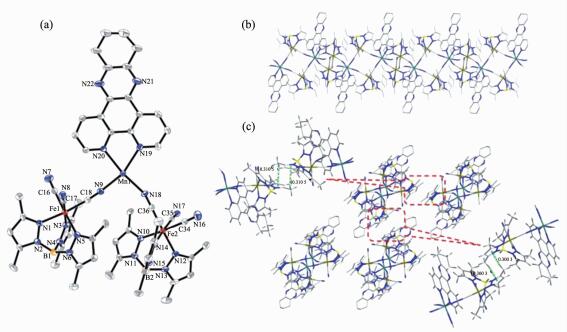
Single-crystal X-ray diffraction analysis revealed that 1 crystallized in the monoclinic space group P21/c. The crystal structure consists of a cyano-bridged 2, 2-CC zigzag chain (Fig. 1b). The 2, 2-CC chain is made up of a cyano-bridged alternating [Fe(Ⅲ)(PzTp)(CN)3]--[Mn(5,5′-dmbpy)2]2+ fragment. Within the chain, each [Fe(Ⅲ)(PzTp)(CN)3]- entity connects two [Mn(5,5′-dmbpy)2]2+ motifs with two of its three cyanide groups in cis positions, and each [Mn(5,5′-dmbpy)2]2+ unit links two [Fe(Ⅲ)(PzTp)(CN)3]- ions in cis modes. Interestingly, such connection mode results in forming a left-and right-handed helices along the b-axis (Fig. 1c). Because both left-and right-handed helices are alternatively arranged, the whole structure is meso-meric. Each central Fe(Ⅲ) environment can be described as a distorted octahedron, comprising three C atoms from terminal CN ligands and three N atoms from the tridentate ligand PzTp. The Fe-Ccyanide (0.192 8(5)~0.193 4(5) nm) and Fe-NPzTp (0.196 1(4)~0.197 4(4) nm) bond lengths are in good agreement with those observed previously in the related LS Fe(Ⅲ) compounds[28]. The Fe-C≡N bond angles in the range of 171.4(4)°~176.6(5)° depart slightly from linearity. In the [Mn(5,5′-dmbpy)2]2+ unit, each Mn(Ⅱ) ion is also octahedral coordination. Four N atoms are from two bidentate 5,5′-dmbpy ligands, and the remaining coordination sites of each six-coordinated Mn(Ⅱ) ion are occupied by the bridging cyanide building blocks. The Mn-Ncyanide distances (0.220 2(4) and 0.223 1(4) nm) are shorter than those of the Mn-Nbpy (0.225 4(3)~0.231 8(4) nm), which are comparable with related HS Mn(Ⅱ) compounds previously reported[32]. The Mn-N≡C bond angles deviate significantly from linearity with the angles of C(39)-N(14)-Mn(1) 164.6(4)° and C(37)-N(15)-Mn(1)ⅱ 140.2(4)° (Symmetry codes: ⅱ-x, y+1/2, -z+1/2). The neighboring chains are linked together through C-H…π interactions between methyl hydrogen and pyridine rings of 5,5′-dmbpy ligands (d=0.310 4 nm), resulting in a 2D supramolecular structure (Fig. 2). The shortest intrachain Fe…Mn, Fe…Fe and Mn…Mn distances are 0.491, 0.741 and 1.176 nm, respectively, while the nearest interchain Fe…Mn, Fe…Fe and Mn…Mn distances are 1.078, 1.021 and 0.969 nm, respectively, indicating that the interchain magnetic interactions are very weak.
2.1.2 Crystal structure of 2
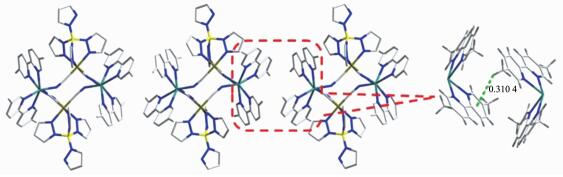
Single-crystal X-ray diffraction analysis revealed that 2 crystallized in the triclinic space group P1. The crystal structure comprises a neutral cyano-bridged 2, 4-ribbon like double-zigzag chain (Fig. 3b). Within the chain, the basic structural unit is a Mn(Ⅱ)2(CN)4Fe(Ⅲ)2 square with each Mn(Ⅱ) shared by two adjacent squares. Within each square, the [Fe(Ⅲ)(Tp*)(CN)3]- unit binds two Mn(Ⅱ) through two of its three cyanide groups, while each [Mn(Ⅱ)(dpqc)]2+ unit links four [Fe(Ⅲ)(Tp*)(CN)3]- units. The square units exhibit two orientations of their mean planes (Fe(Ⅲ)2Mn(Ⅱ)2), showing an approximately perpendicular with the dihedral angle of 83.44° owing to the steric effect of the bulky [Fe(Ⅲ)(Tp*)(CN)3]- building block, which is rare for the double-zigzag chain reported in the literature[16]. Each Fe(Ⅲ) center adopts a slightly distorted octahedral configuration consisting of three cyanide carbon atoms and three nitrogen atoms of Tp*- anion. The Fe-Ccyanide bond lengths range from 0.190 5(6) to 0.193 5(6) nm, and the Fe-NTp* distances are in the range of 0.198 8(5)~0.201 7(5) nm, respectively. The Fe-C≡N linkages are closer to linearity with bond angles of 174.3(5)°~178.6(6)°. Such characteristics of the bond lengths and bond angles indicate that the iron center is low-spin Fe(Ⅲ)[28]. Each Mn(Ⅱ) center is located in a distorted N6 octahedral coordination environment with four nitrogen atoms from four cyanide groups and two nitrogen atoms from a dpqc ligand. Similar to 1, the Mn-Ncyanide bond distances (0.215 3(5)~0.222 8(5) nm) are also shorter than the Mn-Ndpqc bond distances (0.228 6(5) and 0.230 6(5) nm). These values are in good agreement with the cyano-bridged Fe(Ⅲ)-Mn(Ⅱ) compounds[32]. The Mn-N≡C bond angles range from 156.2(5)° to 164.1(5)°. The maximum deviation of the Mn-N≡C angles from linearity is smaller than that of 1 (23.8° for 2 vs 39.2° for 1). The adjacent chains are linked to form a 3D supramolecular structure with assistance of C-H…π interactions between methyl hydrogen and pyrazole rings of Tp* ligands (d=0.300 3 nm and 0.310 5 nm) (Fig. 3c). The shortest intrachain Fe…Mn, Fe…Fe and Mn…Mn distances are 0.512, 0.734 and 0.697 nm, respectively. Whereas, the shortest interchain Fe…Mn, Fe…Fe and Mn…Mn distances are 1.198, 0.872 and 1.323 nm.
It is worth noting that the structures of compounds 1 and 2 are quite different from the reported chains structures incorporating the anionic building block, fac-[Fe(TpR)CN)3]-. Self-assembly of the anionic building block, fac-[Fe(TpR)CN)3]- and fully solvated metal ions [M(S)6]2+ or partially blocked metal cationic units [M(L)x(S)y]2+ (L=monodentate, bidentate or tetradentate ligand; S=solvent molecule; x+y=6) frequently results in the formation of chains with two different topologies (Scheme 1): 2, 2-CT chain and 2, 4-ribbon chain. In the reported 2, 2-CT chain (Scheme 1a), the four coordination sites of the metal(Ⅱ) ion are occupied by two trans-positioned bidentate ligands or a tetradentate Schiff base ligand, and the remaining two sites are filled by two fac-[Fe(TpR)CN)3]- units in trans positions[23-25]. In the 2, 4-ribbon chain (Scheme 1c), each metal(Ⅱ) center is coordinated by four cyanide nitrogen atoms from four fac-[Fe(TpR)CN)3]- units and two monodentate ligands or two solvent molecules[26-27, 30]. In the present work (Scheme 1b), in the [Mn(5,5′-dmbpy)2]2+ unit, each Mn(Ⅱ) ion is coordinated by two bidentate ligands in cis positions, the remaining sites are occupied by the bridging cyanide building blocks in cis positions. Such similar structure was only reported by Oshio′s group incor-porating a tetradentate N-donoring ligand and the [Fe(Tp)CN)3]- building block[19]. Different from the classical 2, 4-ribbon chain, each Mn(Ⅱ) ion in complex 2 is coordinated by a bidentate ligand in cis position instead of two monodentate ligands in trans-axial positions (Scheme 1d). As far as we know, such a novel architecture representing a new net topology has never been reported previously.
2.2.2 Magnetic properties of 2
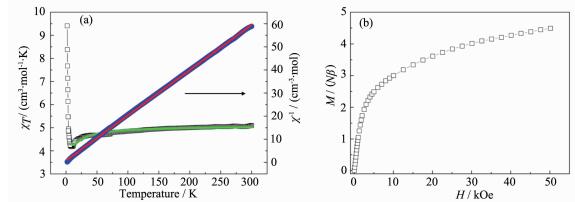
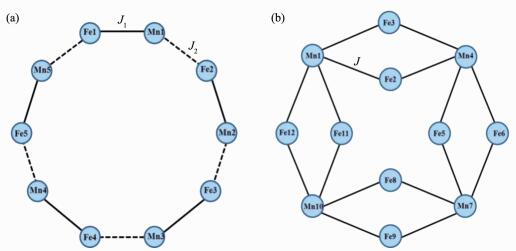
The temperature dependence of the magnetic susceptibility of 2 was measured in the range of 2~300 K at 1 000 Oe (Fig. 6a). The χT value per Fe2Mn unit at 300 K is 6.01 cm3·mol-1·K, which is close to but somewhat larger than the spin-only value of 5.13 cm3·mol-1·K for the uncorrelated two LS Fe(Ⅲ) (S=1/2) and one HS Mn(Ⅱ)(S=5/2) with g=2.00. As the temperature is lowered, the χT value decreases very smoothly down to 5.23 cm3·mol-1·K at 60 K. Upon further lowing, the χT value abruptly decreases to reach a minimum value of 0.43 cm3·mol-1·K at 2.0 K. The magnetic susceptibility data above 15 K obey the Curie-Weiss law, which give a Curie constant of 6.19 cm3·mol-1·K and a Weiss temperature of -12.00 K. To simulate the magnetic susceptibility of compound 2, the double chain also can be fitted with a 12-atom Fe8Mn4 ring mode (Fig. 5b). The MAGPACK program was used to fit based on the Hamiltonian
$H{\rm{ = - 2}}{J_{\rm{1}}}({S_{{\rm{Mn1}}}}{S_{{\rm{Fe2}}}}{\rm{ + }}{S_{{\rm{Mn1}}}}{S_{{\rm{Fe3}}}}{\rm{ + }}{S_{{\rm{Fe2}}}}{S_{{\rm{Mn4}}}}{\rm{ + }}{S_{{\rm{Fe3}}}}{S_{{\rm{Mn4}}}}{\rm{ + }}{S_{{\rm{Mn4}}}}{S_{{\rm{Fe5}}}}{\rm{ + }}{S_{{\rm{Mn4}}}}{S_{{\rm{Fe6}}}}{\rm{ + }}{S_{{\rm{Fe5}}}}{S_{{\rm{Mn7}}}}{\rm{ + }}{S_{{\rm{Fe6}}}}{S_{{\rm{Mn7}}}} $ $ {\rm{ + }}{S_{{\rm{Mn7}}}}{S_{{\rm{Fe8}}}}{\rm{ + }}{S_{{\rm{Mn7}}}}{S_{{\rm{Fe9}}}}{\rm{ + }}{S_{{\rm{Fe8}}}}{S_{{\rm{Mn10}}}}{\rm{ + }}{S_{{\rm{Fe9}}}}{S_{{\rm{Mn10}}}}{\rm{ + }}{S_{{\rm{Mn10}}}}{S_{{\rm{Fe11}}}}{\rm{ + }}{S_{{\rm{Mn10}}}}{S_{{\rm{Fe12}}}}{\rm{ + }}{S_{{\rm{Fe11}}}}{S_{{\rm{Mn1}}}}{\rm{ + }}{S_{{\rm{Fe12}}}}{S_{{\rm{Mn1}}}})$ . The best-fit parameters with J=-8.61 and g=2.21 with the agreement factor R=3.00×10-5 show a good curve match. The negative Weiss temperature and coupling parameters indicate the presence of intrachain antiferromagnetic coupling between neighbouring Fe(Ⅲ) and Mn(Ⅱ) ions. As shown in the Fig. 6b, the field-dependent magnetization of 2 was measured at 1.8 K in the field (0~50 kOe). The magnetization increases linearly with the applied magnetic field, reaching a value of 2.48Nβ at 50 kOe, which is lower than the saturation magnetization value of 3.0Nβ expected for MS=g(SMn-SFe) with g=2.00. The result further indicates that the dominant intrachain antiferromagnetic interactions were transmitted via the cyanide bridge.Investigation of the magnetic properties of compounds 1 and 2 indicates that they have different magnetic behaviors. Compound 1 shows ferrimagnetic behavior in low temperature region, whereas compound 2 indicates the presence of the typically antiferromagnetic coupling. The different magnetic properties of compounds 1 and 2 may result from the different bent Mn-N≡C bond angles and interchain C-H…π stacking interactions. Generally, the bending of the Mn-N≡C bond angles diminishes the overlap of the spin-orbit coupling and then reduces the magnetic interactions. The maximum deviation of the Mn-N≡C angles from linearity in 1 (39.2°) is the largest in comparison with the reported cyano-bridged Fe(Ⅲ)-Mn(Ⅱ) compounds (Table 1), therefore, the intrachain antiferromagnetic coupling of compound 1 is weak and then it exhibits the ferrimagnetic behavior. For compound 2, the Mn-N≡C bond angles are in the reported range of 144°~170°, thus it exhibits the typically antiferromagnetic coupling.
2.2.1 Magnetic properties of 1
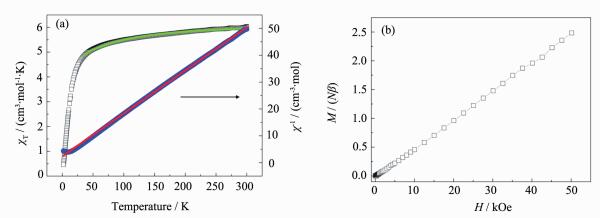
The magnetic susceptibility data of 1 were measured at 1 000 Oe in the temperature range of 2~300 K (Fig. 4a). The χT value is 5.10 cm3·mol-1·K at 300 K, which is slightly larger than the spin-only value of 4.75 cm3·mol-1·K expected for an uncoupled LS Fe(Ⅲ) (S=1/2) and one HS Mn(Ⅱ) (S=5/2) assuming that g=2.00. As the temperature is lowered, the χT value undergoes a gradual reduction, reaching a minimum value of 4.18 cm3·mol-1·K at 10 K. Below the temperature, it increases rapidly up to 9.41 cm3·mol-1·K at 2.0 K. The overall magnetic behavior indicates the typical of a ferrimagnetic situation within a chain. The magnetic susceptibility data are fitted by the Curie-Weiss law in the temperature range of 2~300 K, which give a Curie constant of 5.10 cm3·mol-1·K and a Weiss temperature of -3.64 K. The negative Weiss temperature demonstrates the antiferromagnetic coupling interactions between the paramagnetic centers. When the magnetic behavior of the 1D chain was simulated through the MAGPACK program[41], the 1D chain can be treated as a ring mode[42]. Because of the presence of two different Mn-N≡C bridging angles in compound 1, 2J coupling parameters were used to simulate the experimental magnetic susceptibility. Therefore, we consider the 1D zigzag chain as a 10-atom Fe5Mn5 ring with the Hamiltonian
$ \mathit{H}{\rm{ = - 2}}{\mathit{J}_{\rm{1}}}{\rm{(}}{\mathit{S}_{{\rm{Fe1}}}}{\mathit{S}_{{\rm{Mn1}}}}{\rm{ + }}{\mathit{S}_{{\rm{Fe2}}}}{\mathit{S}_{{\rm{Mn2}}}}{\rm{ + }}{\mathit{S}_{{\rm{Fe3}}}}{\mathit{S}_{{\rm{Mn3}}}}{\rm{ + }}{\mathit{S}_{{\rm{Fe4}}}}{\mathit{S}_{{\rm{Mn4}}}}{\rm{ + }}{\mathit{S}_{{\rm{Fe5}}}}{\mathit{S}_{{\rm{Mn5}}}}{\rm{) - 2}}{\mathit{J}_{\rm{2}}}{\rm{(}}{\mathit{S}_{{\rm{Mn1}}}}{\mathit{S}_{{\rm{Fe2}}}}{\rm{ + }}{\mathit{S}_{{\rm{Mn2}}}}{\mathit{S}_{{\rm{Fe3}}}}{\rm{ + }}{\mathit{S}_{{\rm{Mn3}}}}{\mathit{S}_{{\rm{Fe4}}}}{\rm{ + }}{\mathit{S}_{{\rm{Mn4}}}}{\mathit{S}_{{\rm{Fe5}}}}{\rm{ + }}{\mathit{S}_{{\rm{Mn5}}}}{\mathit{S}_{{\rm{Fe1}}}}{\rm{)}}$ , where SFe=1/2 and SMn=5/2. Using this model (Fig. 5a), the magnetic susceptibility data were fitted by the MAGPACK program in the temperature range of 10~300 K, which gave J1=-1.68, J2=-2.42 and g=2.07 with the agreement factor R=1.05×10-4. The coupling parameters also indicate the antiferromagnetic interactions. The field dependence of the magnetization at 1.8 K was measured in the field range from 0 to 50 kOe (Fig. 4b). The magnetization value at 50 kOe is 4.48Nβ, which is a little larger than the ferrimagnetic results of 4.0Nβ calculated from MS=g(SMn-SFe) with g=2.00. The large saturation magnetization may originate from the existence of the significant orbital contributions of the LS Fe(Ⅲ) ions.3 Conclusions
In summary, different steric hindrance ligands were introduced into Fe(Ⅲ)-Mn(Ⅱ) system to induce the distortion of the structures and finally realized the adjustment of the magnetic interactions. A cyano-bridged 1D FeMn 2, 2-CC helix single chain 1 and a 1D Fe2Mn 2, 4-ribbon double chain 2 were successfully synthesized via tunable assembly, and the structure of compound 2 has never been reported in the previous literature. Investigation of the magnetic properties of compounds 1 and 2 indicates the bending of Mn-N≡C bond angles and interchain C-H…π stacking interactions play important roles in adjusting the magnetic interactions. Therefore, compound 1 shows ferrimagnetic behavior because of its largest bending of Mn-N≡C bond angles, whereas compound 2 exhibits the presence of the antiferromagnetic coupling.
-
-
[1]
Sato O, Iyoda T, Fujishima A, et al. Science, 1996, 272:704 -705 doi: 10.1126/science.272.5262.704
-
[2]
Shatruk M, Avendano C, Dunbar K R. Prog. Inorg. Chem., 2009, 56:155-274
-
[3]
Ohba M, kawa H. Coord. Chem. Rev., 2000, 198:313-328 doi: 10.1016/S0010-8545(00)00233-2
-
[4]
Wang S, Ding X H, Li Y H, et al. Coord. Chem. Rev., 2012, 256:439-464 doi: 10.1016/j.ccr.2011.10.029
-
[5]
Wang S, Ding X H, Zuo J L, et al. Coord. Chem. Rev., 2011, 255:1713-1732 doi: 10.1016/j.ccr.2011.01.057
-
[6]
Caneschi A, Gatteschi D, Lalioti N, et al. Angew. Chem. Int. Ed., 2001, 40:1760-1763 doi: 10.1002/(ISSN)1521-3773
-
[7]
Coulon C, Miyasaka H, Clerac R. Struct. Bond., 2006, 122: 163-206 doi: 10.1007/b104234
-
[8]
Miyasaka H, Julve M, Yamashita M, et al. Inorg. Chem., 2009, 48:3420-3437 doi: 10.1021/ic802050j
-
[9]
Sun H L, Wang Z M, Gao S. Coord. Chem. Rev., 2010, 254: 1081-1100 doi: 10.1016/j.ccr.2010.02.010
-
[10]
Lescouëzec R, Toma L M, Vaissermann J, et al. Coord. Chem. Rev., 2005, 249:2691-2729 doi: 10.1016/j.ccr.2005.09.017
-
[11]
Nihei M, Ui M, Yokota M, et al. Angew. Chem. Int. Ed., 2005, 44:6484-6487 doi: 10.1002/(ISSN)1521-3773
-
[12]
Shen X P, Zhou H B, Yan J H, et al. Inorg. Chem., 2014, 53:116-127 doi: 10.1021/ic401753y
-
[13]
Liu T, Zhang Y J, Kanegawa S, et al. J. Am. Chem. Soc., 2010, 132:8250-8251 doi: 10.1021/ja1027953
-
[14]
Wen H R, Wang C F, Zuo J L, et al. Inorg. Chem., 2006, 45: 582-590 doi: 10.1021/ic0511468
-
[15]
Kim J I, Kwak H Y, Yoon J H, et al. Inorg. Chem., 2009, 48:2956-2966 doi: 10.1021/ic802033q
-
[16]
Dong D P, Liu T, Kanegawa S, et al. Angew. Chem. Int. Ed., 2012, 51:5119-5123 doi: 10.1002/anie.201105987
-
[17]
Liu T, Zheng H, Kang S, et al. Nat. Commun., 2013, 4:2826 -2833 http://www.ncbi.nlm.nih.gov/pubmed/24253737
-
[18]
Shao D, Zhang S L, Zhao X H, et al. Chem. Commun., 2015,51:4360-4363 doi: 10.1039/C4CC10003D
-
[19]
Hoshino N, Iijima F, Newton G N, et al. Nat. Chem., 2012, 4:921-926 doi: 10.1038/nchem.1455
-
[20]
Liu W, Wang C F, Li Y Z, et al. Inorg. Chem., 2006, 45: 10058-10065 doi: 10.1021/ic061347r
-
[21]
Zhang Y Z, Mallik U P, Clérac R, et al. Polyhedron, 2013, 52:115-121 doi: 10.1016/j.poly.2012.10.039
-
[22]
Zhang Y Z, Ferko P, Siretanu D, et al. J. Am. Chem. Soc., 2014, 136:16854-16864 doi: 10.1021/ja508280n
-
[23]
Wen H R, Tang Y Z, Liu C M, et al. Inorg. Chem., 2009, 48: 10177-10185 doi: 10.1021/ic901224f
-
[24]
Kwak H Y, Ryu D W, Lee J W, et al. Inorg. Chem., 2010, 49:4632-4642 doi: 10.1021/ic100301q
-
[25]
Dong D P, Zhang Y J, Zheng H, et al. Dalton Trans., 2013, 42:7693-7698 doi: 10.1039/c3dt50273b
-
[26]
Wen H R, Wang C F, Song Y, et al. Inorg. Chem., 2006, 45: 8942-8949 doi: 10.1021/ic060928d
-
[27]
Mitsumoto K, Ui M, Nihei M, et al. CrystEngComm, 2010, 12:2697-2699 doi: 10.1039/c004351f
-
[28]
Kim J, Han S J, Cho I-K, et al. Polyhedron, 2004, 23:1333-1339 doi: 10.1016/j.poly.2004.02.026
-
[29]
Li D F, Parkin S, Wang G B, et al. Inorg. Chem., 2005, 44: 4903-4905 doi: 10.1021/ic048367i
-
[30]
Jiang L, Feng X L, Lu T B, et al. Inorg. Chem., 2006, 45: 5018-5026 doi: 10.1021/ic052168x
-
[31]
Gheorghe R, Kalisz M, Clérac R, et al. Inorg. Chem., 2010, 49:11045-11056 doi: 10.1021/ic1015725
-
[32]
Pardo E, Verdaguer M, Herson P, et al. Inorg. Chem., 2011, 50:6250-6262 doi: 10.1021/ic200616p
-
[33]
郑慧, 徐杨, 段春迎.无机化学学报, 2015, 31 (7):1460-1466 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20150728&journal_id=wjhxxbcnZHENG Hui, XU Yang, DUAN Chun-Ying. Chinese J. Inorg. Chem., 2015, 31 (7):1460-1466 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20150728&journal_id=wjhxxbcn
-
[34]
Wang S, Zuo J L, Zhou H C, et al. Eur. J. Inorg. Chem., 2004, 3681-3687 doi: 10.1002/ejic.200400121/full
-
[35]
Ni Z H, Kou H Z, Zhang L F, et al. Angew. Chem. Int. Ed., 2005, 44:7742-7745 doi: 10.1002/(ISSN)1521-3773
-
[36]
Costa V, Lescouëzec R, Vaissermann J, et al. Inorg. Chim. Acta, 2008, 361:3912-3918 doi: 10.1016/j.ica.2008.03.044
-
[37]
Gu Z G, Liu W, Yang Q F, et al. Inorg. Chem., 2007, 46: 3236-3244 doi: 10.1021/ic062267q
-
[38]
Ma L L, Ge K, Zhang R, et al. Eur. J. Med. Chem., 2014, 87:624-630 doi: 10.1016/j.ejmech.2014.10.002
-
[39]
SMART, SAINT and XPREP, Area Detectr and Data Integration and Reduction Software, Bruker Analytical Instruments Inc. , Madison, WI, 1995.
-
[40]
Sheldrick G M. SHELXS-97, Program for X-ray Crystal Structure Solution and Refinement, University of Göttingen, Germany, 1997.
-
[41]
Borrás-Almenar J J, Clemente-Juan J M, Coronado E, et al. MAGPACK, J. Comput. Chem., 2001, 22:985-991 doi: 10.1002/jcc.1059
-
[42]
Kou H Z, Ni Z H, Liu C M, et al. New J. Chem., 2009, 33: 2296-2299 doi: 10.1039/b9nj00316a
-
[1]
-
Figure 1 (a) ORTEP representation of a selected unit of compound 1 with thermal ellipsoids drawn at the 30% probability level; (b) Side view of a 1D single-zigzag chain of compound 1 along the b-axis; (c) Packing structure of the left-or right-handed helical chains
All H atoms and ClO4- anions are omitted for clarity
Figure 3 (a) ORTEP representation of a selected unit of compound 2 with thermal ellipsoids drawn at the 30% probability level; (b) Side view of a 1D double-zigzag chain along the b-axis; (c) 3D supramolecular structure of compound 2 via the C-H…π stacking interactions
All H atoms and solvent molecules are omitted for clarity
Table 1. Mn-N≡C bond angles and related magnetic behaviors for Fe(Ⅲ)-Mn(Ⅱ) systems constructed from the tricyanide precursors fac-[Fe(TpR)CN)3]-
Compound Structure ∠Mn-N≡C/(°) Magnetic behavior Ref. a trinuclear 167.3(2) antiferromagnetic 28 b tetranuclear 161.1(2), 172.0(2) antiferromagnetic 28 c tetranuclear 177.4(4), 175.3(4) antiferromagnetic 29 d hexanuclear 155.5(4)~170.9(4) antiferromagnetic 30 e double chain 156.1(2), 159.0(2) antiferromagnetic 30 f double chain 154.9(3), 168.3(3) antiferromagnetic 31 g hexanuclear 155.3(3)~162.6(3) antiferromagnetic 31 h tetranuclear 160.1(2), 73.3(2) antiferromagnetic 32 i double chain 144.0-156.0 antiferromagnetic 33 j double chain 147.6-153.1 antiferromagnetic 33 1 single chain 140.2(4), 164.6(4) ferrimagnetic this work 2 double chain 156.6(5)~164.1(5) antiferromagnetic this work a: {[Fe(HB(pz)3)(CN)3]2[Mn(MeOH)4]·2MeOH}n; b: {[Mn2Fe2(HB(pz)3)2(CN)6(4, 4′-bipyridine)2](ClO4)2·4MeCN}n; c: {[(Tp*)Fe(Ⅲ)(CN)3][Mn(Ⅱ)(DMF)4]2(OTf)·2DMF}n; d: {[(Tp)Fe(Ⅲ)(CN)3]2[Mn(Ⅱ)(DMF)2(H2O)]}n; e: {[(Tp)Fe(Ⅲ)(CN)3]2[Mn(Ⅱ)(DMF)2]}n; f: {[Mn(bpym)(H2O)]2[Fe(HB(pz)3)(CN)3]4}n; g: {[Mn(bpym)(H2O)]2[Fe(B(pz)4)(CN)3]4·4H2O}n; h: {[Fe(Ⅲ)(B(pz)4)(CN)2(μ-CN)][Mn(Ⅱ)(bpy)2]2(ClO4)2·CH3CN}n; i: {[Fe(Tp)(CN)3]2[Mn(bib)]·CH3OH·2H2O}n; j: {[Fe(pzTp)(CN)3]2[Mn(bib)]·3H2O}n Table 2. Crystal data and structure refinements for compounds 1 and 2
Compound 1 2 Formula C39H36BClFeMnN15O4 C55H64B2Fe2MnN22O2 Formula weight 935.88 1 253.53 Crystal system Monoclinic Triclinic Space group P21/c P1 a/nm 1.266 91(10) 1.3724 6(5) b/nm 1.176 12(9) 1.5940 9(6) c/nm 2.871 31(19) 1.6489 6(6) α/(°) 90 83.450 7(17) β/(°) 94.744(5) 79.194 9(15) γ/(°) 90 69.771 5(15) V/nm3 4.263 7(5) 3.320 4(2) Z 4 2 Dc/(g·cm-3) 1.458 1.252 F(000) 1 920 1 298 Reflections collected 26 548 77 019 Unique reflections (Rint) 7 498(0.072 6) 11 679(0.122 2) Goodness-of-fit on F2 1.037 1.045 Final R indicesa,b[I > 2σ(I) R1=0.059 7, wR2=0.135 4 R1=0.071 0, wR2=0.208 5 R indicesa,b(all data) R1=0.096 0, wR2=0.148 6 R1=0.141 6, wR2=0.233 0 \begin{document}$^{\rm{a}}{\mathit{R}_{\rm{1}}}{\rm{ = }}\sum {\rm{(|}}{\mathit{F}_{\rm{o}}}{\rm{| - |}}{\mathit{F}_{\rm{c}}}{\rm{|)/}}\sum {\rm{|}}{\mathit{F}_{\rm{o}}}{\rm{|;}}{\;^{\rm{b}}}\mathit{w}{\mathit{R}_{\rm{2}}}{\rm{ = [}}\sum \mathit{w}{{\rm{(|}}{\mathit{F}_{\rm{o}}}{\rm{|-|}}{\mathit{F}_{\rm{c}}}{\rm{|)}}^{\rm{2}}}{\rm{/}}\sum \mathit{wF}_{\rm{o}}^{\rm{2}}{{\rm{]}}^{{\rm{1/2}}}} $\end{document} Table 3. Selected bond lengths (nm) and angles (°) for compounds 1 and 2
1 Fe(1)-C(39) 0.192 8(5) Fe(1)-N(3) 0.196 8(4) Mn(1)-N(12) 0.225 4(3) Fe(1)-C(38) 0.193 1(6) Fe(1)-N(5) 0.197 4(4) Mn(1)-N(10) 0.227 8(4) Fe(1)-C(37) 0.193 4(5) Mn(1)-N(14) 0.220 2(4) Mn(1)-N(9) 0.228 4(4) Fe(1)-N(1) 0.196 1(4) Mn(1)-N(15)ⅰ 0.223 1(4) Mn(1)-N(11) 0.231 8(4) N(15)-C(37)-Fe(1) 171.4(4) W(13)-C(38)-Fe(1) 176.6(5) C(39)-N(14)-Mn(1) 164.6(4) N(14)-C(39)-Fe(l) 173.0(4) C(37)-N(15)-Mn(1)ⅱ 140.2(4) 2 Fe(1)-C(18) 0.190 5(6) Fe(2)-C(34) 0.191 8(7) Mn(1)-N(9) 0.215 3(5) Fe(l)-C(17) 0.191 5(6) Fe(2)-C(35) 0.193 1(6) Mn(1)-N(18) 0.218 1(5) Fe(1)-C(16) 0.1931(7) Fe(2)-C(36) 0.193 5(6) Mn(1)-N(8)ⅰ 0.221 8(5) Fe(1)-N(3) 0.198 8(5) Fe(2)-N(14) 0.199 9(4) Mn(1)-N(17)ⅱ 0.222 8(5) Fe(1)-N(5) 0.200 0(5) Fe(2)-N(10) 0.200 5(5) Mn(1)-N(19) 0.228 6(5) Fe(1)-N(1) 0.200 6(5) Fe(2)-N(12) 0.201 7(5) Mn(1)-N(20) 0.230 6(5) N(7)-C(16)-Fe(1) 178.6(6) N(17)-C(35)-Fe(2) 176.5(5) C(18)-N(9)-Mn(1) 160.5(4) N(8)-C(17)-Fe(1) 175.0(5) N(18)-C(36)-Fe(2) 178.2(5) C(35)-N(17)-Mn(1)ⅱ 164.1(5) N(9)-C(18)-Fe(1) 174.3(5) C(17)-N(8)-Mn(1)ⅰ 156.6(5) C(36)-N(18)-Mn(1) 156.2(5) N(16)-C(34)-Fe(2) 177.7(6) Symmetry codes: ⅰ-x, y-1/2, -z+1/2; ⅱ-x, y+1/2, -z+1/2 for 1; ⅰ-x+1, -y+1, -z+2; ⅱ-x, -y+1, -z+2 for 2 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 1
- 文章访问数: 694
- HTML全文浏览量: 70


 下载:
下载:






 下载:
下载:

