
一个具有eea拓扑和高甲烷存储量的金属有机骨架材料
-
关键词:
- Cu(Ⅱ)配合物
- / eea拓扑的网络结构
- / CH4吸附
English
An eea Topological Metal-Organic Framework with High Methane Uptake
-
Key words:
- Cu(Ⅱ) complex
- / eea topology
- / CH4 storage
-
Storage of natural gas (NG), comprising mainly methane, has attracted wide attention due to the concerns over national and regional energy security, ground-level air quality, and climate change[1-2]. Considering its naturally abundant and relatively environmentally friendly compared to conventional liquid hydrocarbon fuels, methane has been regarded as one of the most attractive and promising alternative source of clean energy[3-5]. However, owing to its low volumetric energy density at standard conditions, the efficient storage of methane in a limited volume has become a major challenge for its on-board applications[4, 6]. Compared with the compressed natural gas (CNG) realized at high pressure and liquefied natural gas (LNG) stored at low temperature, adsorptive natural gas (ANG) is a possible and effective approach, in which the high storage of methane can be achieved near room temperature and at moderate pressures through efficient packing of the fluid molecules in nanospaces of the porous materials[5, 7-9].
Different from the low surface area of zeolites and the difficulties in tuning of structures for activated carbons[10-11], metal-organic frameworks (MOFs), which have emerged as an intriguing class of porous material, combine with the advantages of ultra-high surface, versatile and designable structures, high pore volume and tunable pores, et al.[12-18]. Thus, this kind of material which displays the great potential applications in the gas storage would be developed to discover more fascinating methane storage properties. Moreover, the MOF materials available to the real-word CH4 on-board applications are still rare. Hence, further exploring MOFs with good CH4 storage properties is still a great task and challenge for synthetic chemists.
We are of great interest in the design and construction of 3D porous functional MOFs from both symmetric and unsymmetric multidentate organic ligands with promising gas adsorptions, i.e., CH4 storage[19-30]. Recently, bifunctional organic ligands with two carboxyl groups and one nitrogen donor have been largely used to construct MOFs for the investigations of their CO2 capture properties[23, 25-26, 29]. However, for the study of methane storage, MOFs based on this type of ligands are still rarely developed[28]. Thus, in this paper, we utilized a bifunctional organic ligand of 5-(quinolin-6-yl)isophthalic acid (H2L) and dicopper-paddlewheel units to assemble a new 3D porous MOF, {[CuL]·x(Solvent)}n (1). In this work, we achieved an eea topological MOF with the point (Schläfli) symbol of {42.6}2{44.62.86.103}. Remarkably, desolvated 1 exhibits a high CH4 adsorption enthalpy and high methane uptake amount at high pressure and 298 K.
1 Experimental
1.1 Materials and methods
All reagents were obtained from commercial vendors and, unless otherwise noted, were used without further purification (CuCl2·2H2O, Sinopharm Chemical Reagent, AR, 99%; 6-bromoquinoline, TCI, GC, 95%; 3, 5-bis(methoxy-carbonyl)phenylboronic acid, Ark, ≥95%). The FT-IR spectra were obtained in the 4 000~400 cm-1 on a VECTOR TM 22 spectrometer using KBr pellets. 1H NMR spectra were recorded on a Bruker DRX-300 spectrometer with tetramethylsilane as an internal reference. Thermal gravimetric analyses (TGA) were performed under N2 atmosphere (100 mL·min-1) with a heating rate of 5 ℃·min-1 using a 2960 SDT thermogravimetric analyzer. Powder X-ray diffraction (PXRD) data were collected over the 2θ range 5°~50° on a Bruker D8 ADVANCE X-ray diffractometer using Cu Kα radiation (λ=0.154 18 nm) at 40 kV and 40 mA.
1.2 Synthesis of 5-(quinolin-6-yl)isophthalic acid (H2L)
To a solution of 6-bromoquinoline (2.1 g, 10 mmol) in 125 mL of toluene was added the mixture of 3, 5-bis(methoxy-carbonyl)phenylboronic acid (3.2 g, 12 mmol) in 30 mL of ethanol, followed by the addition of a solution of Na2CO3 (3.5 g, 33 mmol) in 10 mL of water. This solution was degassed using N2 for 10 min, and then Pd(PPh3)4 (0.5 g, 0.43 mmol) was added. The resulting reaction mixture was stirred at 90 ℃ under N2 overnight. The solvent was then removed using rotary evaporation, and the residue was dissolved in CH2Cl2 and washed with water. The organic layer was subsequently dried over MgSO4, filtered, concentrated, and purified by silica gel flash column chromatography with an eluent of acetone/petroleum ether (1:10, V/V). The product was hydrolyzed by refluxing in 2 mol·L-1 aqueous KOH followed by acidification with 37%(w/w) HCl to afford a white solid of H2L. Yield: 1.7 g (58%). FT-IR (KBr, cm-1): 1 720, 1 595, 1 406, 1 226, 1 068, 923, 896, 827, 802, 769, 705, 678, 474. 1H NMR (DMSO-d6): δ 13.51 (broad peak, COOH), 9.07 (d, 1H, ArH), 8.76 (d, 1H, ArH), 8.58 (s, 3H, ArH), 8.53 (s, 1H, ArH), 8.32 (d, 1H, ArH), 8.24 (d, 1H, ArH), 7.79 (m, 1H, ArH).
1.3 Synthesis of {[CuL]·x(Solvent)}n (1)
A solution of CuCl2·2H2O (25.0 mg, 0.147 mmol) in 0.5 mL of methanol was mixed with the H2L (10 mg, 0.034 mmol) in 1.5 mL of N, N-dimethylformamide. To this was added 0.08 mL of concentrated HNO3 with stirring. The mixture was sealed in a Pyrex tube and heated to 100 ℃ for 48 h. The green block crystals obtained were filtered and washed with DMF. Yield: 85%. Selected IR (cm-1): 1 646, 1 585, 1 507, 1 448, 1 380, 1 089, 839, 779, 730, 487.
1.4 Crystal structure determination
Single-crystal X-ray diffraction data were measured on a Bruker Apex Ⅱ CCD diffractometer at 150(2) K using graphite monochromated Mo Kα radiation (λ=0.071 073 nm). Data reduction was made with the Bruker SAINT program. The structures were solved by direct methods and refined with full-matrix least squares technique using the SHELXTL package[31]. Non-hydrogen atoms were refined with anisotropic displacement parameters during the final cycles. Organic hydrogen atoms were placed in calculated positions with isotropic displacement parameters set to 1.2Ueq of the attached atom. The unit cell includes a large region of disordered solvent molecules, which could not be modelled as discrete atomic sites. We employed PLATON/SQUEEZE[32] to calculate the diffraction contribution of the solvent molecules and, thereby, to produce a set of solvent-free diffraction intensities; structures were then refined again using the data generated. Crystal data and refinement conditions are shown in Table 1.
Empirical formula C17H9CuNO4 Formula weight 354.79 Crystal system Trigonal Space group R3c a/nm 1.867 89(8) b/nm 1.867 89(8) c/nm 6.925 9(11) Volume/nm3 20.927(4) Z 36 Dc/(Mg·m-3) 1.013 Absorption coefficient/mm-1 0.951 F(000) 6 444 Crystal size/mm 0.210×0.130×0.080 θ range for data collection/(°) 1.764~28.306 Index ranges -24 ≤ h ≤ 20, -24 ≤ k ≤ 24, -92 ≤ l ≤ 92 Reflection collected 48 147 Independent reflection 5 770 (Rint=0.153 5) Completeness to θ=20.879°/% 99.70 Refinement method Full-matrix least-squares on F2 Data, restraint, parameter 5 770, 0, 208 Goodness-of-fit on F2 0.983 Final R indices [I>2σ(I)] R1=0.045 0, wR2=0.104 5 R indices (all data) R1=0.109 0, wR2=0.134 1 Largest diff, peak and hole/(e·nm-3) 521 and -448 CCDC: 1559215, desolvated 1.
1.5 Gas sorption measurements
Low-pressure sorption isotherms of CH4 (99.999%) and N2 (99.999%) gas were performed on Quantachrome Autosorb IQ-2 surface area and pore size analyzer. High pressure excess adsorptions of CH4 gas (99.999%) were measured using a Rubotherm ISOSORP-HyGpra+V adsorption instrument over a pressure range of 0~10 MPa at 298 K. Before analysis, the as-synthesized samples of 1 were soaked in acetone for 3 days with acetone refreshing every 8 hours. Then, the acetone-exchanged sample was activated at 80 ℃ and under vacuum for 10 hours.
2 Results and discussion
2.1 Crystal structure of compound 1
Solvothermal reaction of CuCl2·2H2O with H2L in the solution of DMF/MeOH containing HNO3 afforded a high yield of green block crystals of compound 1. The single X-ray crystal diffraction reveals that 1 crystallizes in trigonal space group R3c. In 1, the dicopper-paddlewheel units are interconnected by the L2- organic ligand, leading to the formation of a 3D porous framework (Fig. 1). The structure consists of two types of cages: trigonal antiprism (cage A) and cuboctahedron (cage B), in which, the cage A is comprised by six Cu-paddlewheel units and six L2- with each side of the trigonal antiprism occupied by one organic ligand, and the cage B is consisted of twelve dicopper-paddlewheel units and six L2- with each triangular side of the cuboctahedron occupied by one organic ligand and leaving its rectangular sides serving as the windows of cage B, respectively (Fig. 1b). Then, each cage A is surrounded by eight cages B through face-sharing, and simultaneously, each cage B is extended through eight cages A by sharing their triangular faces, too, which leads to the formation of the three-dimensional porous framework of compound 1 (Fig. 1c, d). Determined by the van der Waals diameter of the inserted pseudo atom, the pore size of cage A and cage B is 0.74 and 0.9 nm, respectively. The total potential solvent accessible volumes in desolvated compound 1 calculated by the PLATON/SOLV program is ca. 49.6% and its framework density is 1.013 g·cm-3.
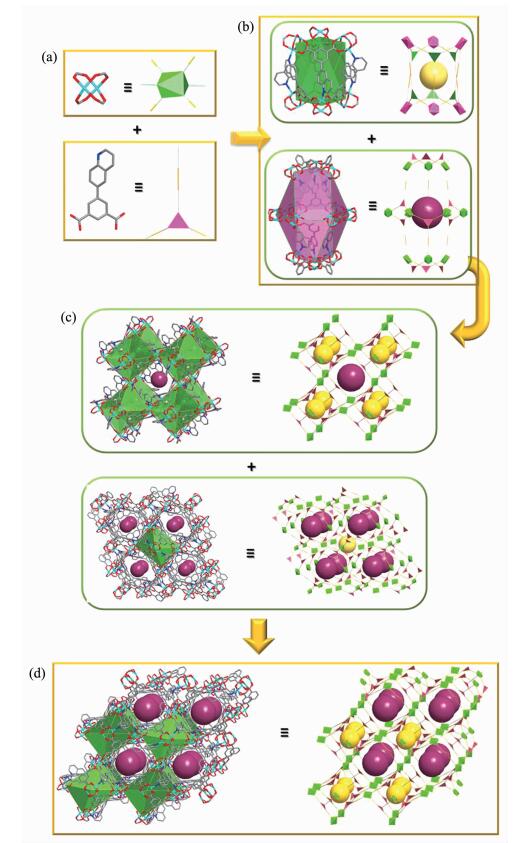 图1
(a) Inorganic (left) and organic (right) secondary building units (SBUs) in compound 1; (b) Trigonal antiprism (cage A, up) and cuboctahedron (cage B, bottom) in compound 1; (c) One cage A surrounded by eight cages B through face-sharing (up) and one cage B surrounded by eight cages A through face-sharing (bottom); (d) 3D porous framework (left) of compound 1 and its topological network (right)
Figure1.
(a) Inorganic (left) and organic (right) secondary building units (SBUs) in compound 1; (b) Trigonal antiprism (cage A, up) and cuboctahedron (cage B, bottom) in compound 1; (c) One cage A surrounded by eight cages B through face-sharing (up) and one cage B surrounded by eight cages A through face-sharing (bottom); (d) 3D porous framework (left) of compound 1 and its topological network (right)
图1
(a) Inorganic (left) and organic (right) secondary building units (SBUs) in compound 1; (b) Trigonal antiprism (cage A, up) and cuboctahedron (cage B, bottom) in compound 1; (c) One cage A surrounded by eight cages B through face-sharing (up) and one cage B surrounded by eight cages A through face-sharing (bottom); (d) 3D porous framework (left) of compound 1 and its topological network (right)
Figure1.
(a) Inorganic (left) and organic (right) secondary building units (SBUs) in compound 1; (b) Trigonal antiprism (cage A, up) and cuboctahedron (cage B, bottom) in compound 1; (c) One cage A surrounded by eight cages B through face-sharing (up) and one cage B surrounded by eight cages A through face-sharing (bottom); (d) 3D porous framework (left) of compound 1 and its topological network (right)
To better understand the structure, the dicopper-paddlewheel unit is simplified as 6-connected node, and the organic ligand serve as a 3-connected node, then 1 may be described as a (3, 6)-connected eea-topological network with its point (Schläfli) symbol of {42.6}2{44.62.86.103} (Fig. 1).
2.2 Thermal stabilities and powder X-ray diffraction
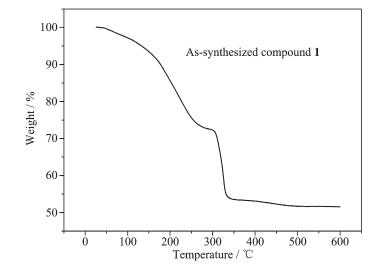
The thermogravimetric analysis (TGA) of as-synthesized compound 1 was performed to confirm its thermal stability. As shown in Fig. 2, 1 exhibits an obvious weight-loss process. The first stage is observed before 220 ℃, which corresponds to the removal of solvent molecules in the pores. The second one is around 320 ℃, which should be ascribed to the decomposition of the organic ligand and the collapse of the dicopper-paddlewheel unit.
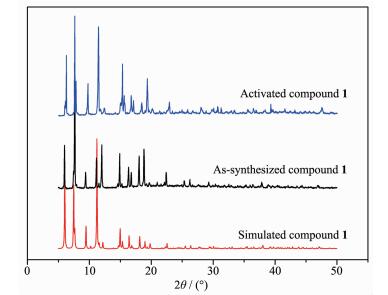
The powder X-ray diffraction (PXRD) has been measured to confirm the phase purity of the bulk sample (Fig. 3). The patterns of the as-synthesized sample are well coincident with the simulated one derived from the X-ray single crystal data, implying the phase purity of the bulk sample. In addition, the PXRD patterns of activated compound 1 that is coincident with the as-synthesized one also indicate the integrality of its framework after the removal of solvent molecules.
2.3 Surface area and porosity
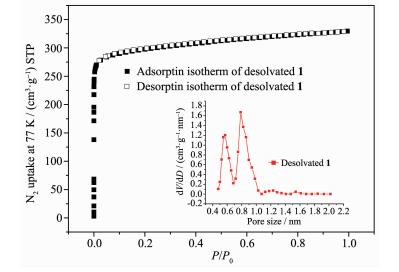
To evaluate the permanent porosity of desolvated compound 1, its N2 sorption isotherm at 77 K was measured. As a result, the N2 uptake is 330 cm3·g-1 (STP) at 100 kPa (Fig. 4). The N2 gas sorption shows a reversible type Ⅰ isotherm without hysteresis on desorption, which is characteristic of microporous material. The Brunaer-Emmett-Teller (BET) and Langmuir surface area of desolvated compound 1 were estimated to be 1 177 and 1 337 m2·g-1, respectively. Furthermore, its NLDFT (nonlocal density functional theory) pore diameters are 0.6 and 0.8 nm, which is in great agreement with the pore size as determined from the crystal structure. Based on N2 adsorption uptake at P/P0=0.974, the total pore volume of desolvated 1 was estimated to be 0.5 cm3·g-1.
2.4 Low pressure methane sorption
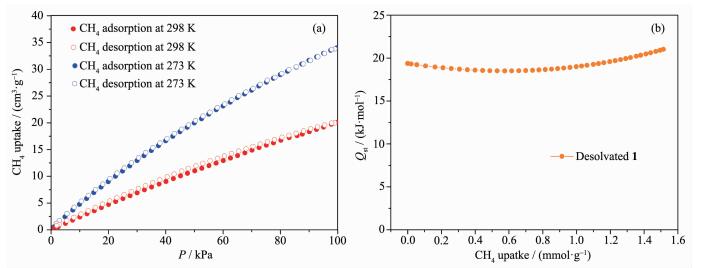
The high surface area of desolvated 1 prompted us to further investigate its methane adsorption property. The CH4 low-pressure (0~100 kPa) sorption isotherms of activated 1 were measured at 273 and 298 K (Fig. 5a), respectively. At 100 kPa, desolvated compound 1 takes up CH4 with the amount of 34 and 20 cm3·g-1 at 273 and 298 K, respectively. Based upon the experimental isotherm data at 273 and 298 K (Fig. 5b), the isosteric heat (Qst) of CH4 adsorption was calculated to be 19.4 kJ·mol-1 at zero loading by the virial method, indicating a relatively strong interaction between its frameworks and the adsorbed CH4 molecules. This value is close to the corresponding values found for most promising MOFs for methane storage[10].
2.5 High pressure methane adsorption
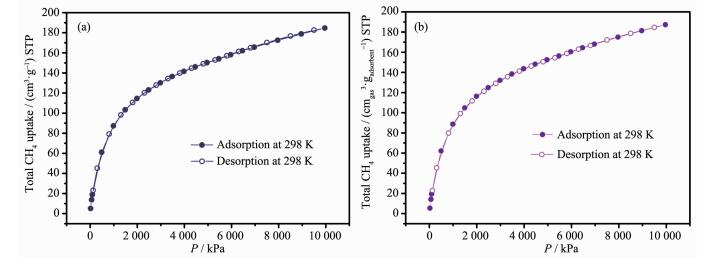
To further evaluate the methane storage capability of desolvated 1, its high-pressure CH4 sorption isotherms were also measured in the pressure range of 0~10 MPa at 298 K. As shown in Fig. 6, at 3 500 kPa, the moderately practical condition for CH4 storage, desolvated compound 1 shows a total gravimetric uptake of 137 cm3·g-1 (STP). Moreover, at 6 500 and 10 000 kPa, the total methane uptake amounts of desolvated 1 are further increased to 162 and 185 cm3·g-1 (STP), respectively. More interestingly, when the crystal density is considered, the total volumetric methane total uptakes of activated compound 1 are 138, 164 and 187 cmgas3 (STP)·cmads-3 at 3 500, 6 500 and 10 000 kPa, respectively. These values are among the relatively high range of the reported porous MOFs, which indicates this MOF might serve as a good potential candidate for methane storage application[8].
3 Conclusions
In conclusion, based upon an organic ligand of 5-(quinolin-6-yl)isophthalic acid, a new 3D porous MOF 1, has been successfully constructed with a rare (3, 6)-connected eea topological network, of which the point (Schläfli) symbol is {42.6}2{44.62.86.103}. More interestingly, this activated MOF performs a large CH4 adsorption enthalpy and high methane uptake amount at high pressure and 298 K.
-
-
[1]
Peng Y, Krungleviciute V, Eryazici I, et al. J. Am. Chem. Soc., 2013, 135:11887-11894 doi: 10.1021/ja4045289
-
[2]
Wu H, Simmons J M, Liu Y, et al. Chem. Eur. J., 2010, 16: 5205-5214 doi: 10.1002/chem.v16:17
-
[3]
He Y B, Zhou W, Qian G D, et al. Chem. Soc. Rev., 2014, 43:5657-5678 doi: 10.1039/C4CS00032C
-
[4]
He Y B, Zhou W, Yildirim T, et al. Energy Environ. Sci., 2013, 6:2735-2744 doi: 10.1039/c3ee41166d
-
[5]
Kowalczyk P, Solarz L, Do D D, et al. Langmuir, 2006, 22: 9035-9040 doi: 10.1021/la061925g
-
[6]
Mendoza-Cortés J L, Han S S, Furukawa H, et al. J. Phys. Chem. A, 2010, 114:10824-10833 doi: 10.1021/jp1044139
-
[7]
Wilmer C E, Farha O K, Yildirim T, et al. Energy Environ. Sci., 2013, 6:1158-1163 doi: 10.1039/c3ee24506c
-
[8]
Mason J A, Veenstra M, Long J R. Chem. Sci., 2014, 5:32-51 doi: 10.1039/C3SC52633J
-
[9]
Li B, Wen H M, Wang H L, et al. J. Am. Chem. Soc., 2014, 136:6207-6210 doi: 10.1021/ja501810r
-
[10]
Makal T A, Li J R, Lu W G, et al. Chem. Soc. Rev., 2012, 41:7761-7779 doi: 10.1039/c2cs35251f
-
[11]
Li J R, Kuppler R J, Zhou H C. Chem. Soc. Rev., 2009, 38: 1477-1504 doi: 10.1039/b802426j
-
[12]
Klein N, Senkovska I, Gedrich K, et al. Angew. Chem. Int. Ed., 2009, 48:9954-9957 doi: 10.1002/anie.v48:52
-
[13]
Gedrich K, Senkovska I, Klein N, et al. Angew. Chem. Int. Ed., 2010, 49:8489-8492 doi: 10.1002/anie.201001735
-
[14]
Ma S Q, Zhou H C. Chem. Commun., 2010, 46:44-53 doi: 10.1039/B916295J
-
[15]
Tan Y X, He Y P, Zhang J. Chem. Commun., 2011, 47: 10647-10649 doi: 10.1039/c1cc14118j
-
[16]
Perry J J, Perman J A, Zaworotko M J. Chem. Soc. Rev., 2009, 38:1400-1417 doi: 10.1039/b807086p
-
[17]
Yang E C, Ding B, Liu Z Y, et al. Cryst. Growth Des., 2012, 12:11851192 doi: 10.1021/cg2011666
-
[18]
Zhuang W, Yuan D, Liu D, et al. Chem. Mater., 2012, 24: 18-25 doi: 10.1021/cm2008889
-
[19]
Yun R R, Lu Z Y, Pan Y, et al. Angew. Chem., 2013, 125: 11492-11495 doi: 10.1002/ange.v125.43
-
[20]
Duan J G, Yang Z, Bai J F, et al. Chem. Commun., 2012, 48:3058-3060 doi: 10.1039/c2cc16231h
-
[21]
Zheng B S, Yang Z, Bai J F, et al. Chem. Commun., 2012, 48:7025-7027 doi: 10.1039/c2cc17593b
-
[22]
Zhang M X, Li B, Li Y Z, et al. Chem. Commun., 2016, 52: 7241-7244 doi: 10.1039/C6CC03198F
-
[23]
Yun R R, Duan J G, Bai J F, et al. Cryst. Growth Des., 2013, 13:24-26 doi: 10.1021/cg301577a
-
[24]
Zheng B S, Bai J F, Duan J G, et al. J. Am. Chem. Soc., 2011, 133:748-751 doi: 10.1021/ja110042b
-
[25]
Du L T, Lu Z Y, Zheng K Y, et al. J. Am. Chem. Soc., 2013, 135:562-565 doi: 10.1021/ja309992a
-
[26]
Jiang J J, Wang Q, Zhang M X, et al. Cryst. Growth Des., 2017, 17:2223-2227 doi: 10.1021/acs.cgd.7b00206
-
[27]
Lu Z Y, Bai J F, Hang C, et al. Chem. Eur. J., 2016, 22: 6277-6285 doi: 10.1002/chem.v22.18
-
[28]
Wang Q, Song X H, Zhang M X, et al. Cryst. Growth Des., 2016, 16:6156-6159 doi: 10.1021/acs.cgd.6b01265
-
[29]
Wang Q, Jiang J J, Zhang M X, et al. Cryst. Growth Des., 2017, 17:16-18 doi: 10.1021/acs.cgd.6b01484
-
[30]
Lu Z Y, Du L T, Tang K Z, et al. Cryst. Growth Des., 2013, 13:2252-2255 doi: 10.1021/cg400449c
-
[31]
Sheldrick G M. Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, A64:112-122 https://www.scienceopen.com/document?vid=9001a01f-495e-41c1-a9ff-560bde2378a2
-
[32]
Spek A L. J. Appl. Crystallogr., 2003, 36:7-13 doi: 10.1107/S0021889802022112
-
[1]
-
Figure 1 (a) Inorganic (left) and organic (right) secondary building units (SBUs) in compound 1; (b) Trigonal antiprism (cage A, up) and cuboctahedron (cage B, bottom) in compound 1; (c) One cage A surrounded by eight cages B through face-sharing (up) and one cage B surrounded by eight cages A through face-sharing (bottom); (d) 3D porous framework (left) of compound 1 and its topological network (right)
Hydrogen atoms are omitted for clarity; Cu: turquiose, C: gray, N: blue, O: red
Table 1. Crystal data and structure refinement for desolvated compound 1
Empirical formula C17H9CuNO4 Formula weight 354.79 Crystal system Trigonal Space group R3c a/nm 1.867 89(8) b/nm 1.867 89(8) c/nm 6.925 9(11) Volume/nm3 20.927(4) Z 36 Dc/(Mg·m-3) 1.013 Absorption coefficient/mm-1 0.951 F(000) 6 444 Crystal size/mm 0.210×0.130×0.080 θ range for data collection/(°) 1.764~28.306 Index ranges -24 ≤ h ≤ 20, -24 ≤ k ≤ 24, -92 ≤ l ≤ 92 Reflection collected 48 147 Independent reflection 5 770 (Rint=0.153 5) Completeness to θ=20.879°/% 99.70 Refinement method Full-matrix least-squares on F2 Data, restraint, parameter 5 770, 0, 208 Goodness-of-fit on F2 0.983 Final R indices [I>2σ(I)] R1=0.045 0, wR2=0.104 5 R indices (all data) R1=0.109 0, wR2=0.134 1 Largest diff, peak and hole/(e·nm-3) 521 and -448 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 1
- 文章访问数: 643
- HTML全文浏览量: 65


 下载:
下载:





 下载:
下载:

