
酰胺型配体铜、锌、银配合物的合成、结构及荧光性质
English
Syntheses, Crystal Structures and Fluorescence Properties of Cu(Ⅱ)/Zn(Ⅱ)/Ag(Ⅰ) Complexes with an Amide Type Ligand
-
Key words:
- Cu(Ⅱ) complex
- / Zn(Ⅱ) complex
- / Ag(Ⅰ) complex
- / amide type ligand
- / fluorescence
-
As one of the promising systems of amide open chain ligands, quinolinyloxy acetamides have been proved to be a well suited type of antenna for lanthanide(Ⅲ) ions. In particular, their Sm(Ⅲ) and Eu(Ⅲ) complexes could exhibit the characteristic emission of the Eu(Ⅲ) and Sm(Ⅲ) ions, respectively[1]. On the other hand, such ligands have been used for the recognition of important transition metal ions, such as Zn(Ⅱ), Cd(Ⅱ) or Hg(Ⅱ), due to the metal-induced fluorescence emi-ssion enhancement[2-3]. Our previous work also shows that this kind of ligands could coordinate to Cu(Ⅱ)/ Zn(Ⅱ) ions to form stable complexes[4-5]. However, their Ag(Ⅰ) complexes have been paid much less attention[4-5]. Thus, in this paper, five Cu(Ⅱ)/Zn(Ⅱ)/Ag(Ⅰ) complexes containing an amide type ligand L, namely, 2-(5-chlo-roquinolin-8-yloxy)-1-(pyrrolidin-1-yl)ethanone, were synthesized and characterized by X-ray diffraction. In addition, the fluorescence properties of all complexes are investigated in detail.
1 Experimental
1.1 Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received. The ligand L was prepared by the reported method[5]. Elemental analysis was carried out on an Elemental Vario EL analyzer. The IR spectra (ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer. The UV spectra were recorded on a Purkinje General TU-1800 spec-trophotometer. Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer, in the measurements of emission and excitation spectra the pass width is 5 nm.
1.2 Preparations of the complexes
The ligand L (0.1 mmol) and CuCl2 (0.1 mmol) were dissolved in an acetone solution (5 mL). After stirring for about 1 h, the mixture was filtered and set aside to crystallize at room temperature. The crystals suitable for single crystal X-ray analyses are obtained after few days. The syntheses of 2~5 are similar to that of 1, while using ZnCl2, Zn(NO3)2, AgClO4 and AgBF4 instead of CuCl2, respectively.
1: yellow blocks. Yield: 59% (based on L). Anal. Calcd. for C18H21N2O3Cl3Cu(%): C, 44.72; H, 4.38; N, 5.80. Found(%): C, 44.62; H, 4.51; N, 5.78. IR (KBr, cm-1): ν(C=O)acetone 1 710, ν(C=O) 1 623, ν(C=N) 1585, ν(Ar-O-C) 1 250.
2: colorless blocks. Yield: 68% (based on L). Anal. Calcd. for C18H21N2O3Cl3Zn(%): C, 44.56; H, 4.36; N, 5.77. Found(%): C, 44.67; H, 4.49; N, 5.62. IR (KBr, cm-1): ν(C=O)acetone 1 713, ν(C=O) 1 626, ν(C=N) 1586, ν(Ar-O-C) 1 241.
3: colorless rods. Yield 72% (based on L). Anal. Calcd. for C16.5H18N4O8.5ClZn(%): C, 38.92; H, 3.56; N, 11.00. Found(%): C, 39.11; H, 3.80; N, 10.76. IR (KBr, cm-1): ν(C=O)acetone 1 711, ν(C=O) 1 633, ν(C=N) 1 588, ν(Ar-O-C) 1 245, ν1(NO3) 1 484, ν(NO3) 1 384 and 1 291.
4: colorless blocks. Yield 79% (based on L). Anal. Calcd. for C30H30N4O8Cl3Ag(%): C, 45.68; H, 3.83; N, 7.10. Found(%): C, 45.78; H, 3.92; N, 7.02. IR (KBr, cm-1): ν(C=O) 1 642, ν(C=N) 1 586, ν(Ar-O-C) 1 239.
5: colorless plates. Yield 76% (based on L). Anal. Calcd. for C30H30N4O4Cl2BF4Ag(%): C, 46.42; H, 3.90; N, 7.22. Found(%): C, 46.57; H, 3.79; N, 7.42. IR (KBr, cm-1): ν(C=O) 1 636, ν(C=N) 1 586, ν(Ar-O-C) 1 238.
1.3 X-ray crystallography
The X-ray diffraction measurements for complexes 1~5 were performed on a Bruker SMART APEX Ⅱ CCD diffractometer equipped with a graphite monochromatized Mo Kα radiation (λ=0.071 073 nm) by using φ-ω scan mode. Semi-empirical absorption correction was applied to the intensity data using the SADABS program[6]. The structures were solved by direct methods and refined by fullmatrixleast-square on F2 using the SHELXTL-97 program[7]. All non-hydrogen atoms were refined anisotropically. All the H atoms were positioned geometrically and refined using a riding model. SQUEEZE procedure was applied to deal with the lattice acetone molecules of complexes 2 and 3. Details of the crystal parameters, data collection and refinements for complexes 1~5 are summarized in Table 1.
1 2 3 4 5 Empirical formula C18H21N2O3Cl3Cu C18H21N2O3Cl3Zn C16.5H18N4O8.5ClZn C30H30N4O8Cl3Ag C30H30N4O4Cl2BF4Ag Formula weight 483.26 485.09 509.17 788.80 776.16 T/K 296(2) 296(2) 296(2) 296(2) 296(2) Crystal system Monoclinic Orthorhombic Monoclinic Monoclinic Monoclinic Space group P21/c Pbcn P21/c P2/c C2/c a/nm 2.277 1(3) 1.476 9(2) 0.861 74(19) 1.397 6(2) 2.283 9(9) b/nm 1.515 5(2) 1.205 84(17) 2.231 8(5) 0.849 08(12) 0.848 9(3) c/nm 1.217 71(18) 2.228 5(3) 1.088 1(2) 1.571 80(16) 1.571 1(6) β/(°) 95.176(3) 90.360(4) 122.486(9) 91.076(8) V/nm3 4.184 9(11) 3.968 8(10) 2.092 6(8) 1.573 3(4) 3.046(2) Z 8 8 4 2 4 Dc/(g·cm-3) 1.534 1.624 1.616 1.665 1.693 Unique 7 350 3 504 3 683 2 764 2 687 Rint 0.033 6 0.032 2 0.050 8 0.022 7 0.072 5 GOF 1.061 1.024 1.061 1.081 1.063 R indices [I > 2σ(I)] R1=0.066 6,
wR2=0.202 0R1=0.034 7,
wR2=0.107 2R1=0.050 5,
wR2=0.105 4R1=0.038 9,
wR2=0.103 2R1=0.048 9,
wR2=0.086 8R indices (all data) R1=0.086 7,
wR2=0.214 2R1=0.042 1,
wR2=0.111 1R1=0.079 6,
wR2=0.113 8R1=0.046 3,
wR2=0.109 4R1=0.114 2,
wR2=0.102 9CCDC: 1484068, 1; 1484069, 2; 1484070, 3; 1484071, 4; 1484072, 5.
2 Results and discussion
2.1 Crystal structures of the complexes
Complexes 1 and 2 are isostructural, while crystallize in monoclinic and orthorhombic, space group P21/c and Pbcn, respectively. As shown in Fig. 1a and 1b, in each complex, the metal ion is five-coordinated by one amide ligand with NO2 donor set and two chloride anions. It is noted that there are two independent complex molecules in the asymmetric unit of 1. According to the Addison rule[8], the geometric index τ is 0.178 or 0.235 and 0.303 in complexes 1 and 2, respectively, indicating that the coordination geometry of metal ion is a distorted tetragonal pyramid. By contrast, the Zn(Ⅱ) ion in complex 3 (Fig. 1c) is surrounded by one tridentate ligand L, one monoden-tate and one bidentate nitrate anions, giving a distorted octahedral coordination geometry. In addition, the second O atom, O7, of the monodentate NO3- group, forms a weak bond with the Zn(Ⅱ) ion (Zn1-O7 0.286 5 nm), thus the coordination octahedral is largely deviated from the ideal one. Except with different lattice solvent molecules, the structures of complexes 1~3 are similar as those derived from the same ligand L and metal salts in acetonitrile solution[5].
Complexes 4 and 5 are isostructural and the ratio of metal ion and ligand is 1:2, while with perchlorate and tetrafluoroborate as counter ions, respectively. As shown in Fig. 1d and 1e, the asymmetric unit of each complex contains a half of the molecule with the Ag(Ⅰ) ion situated on the two-fold rotational axis. The Ag(Ⅰ) ion is coordinated by two amide ligands with NO2 donor set, and possesses a distorted octahedral coor-dination geometry. The Ag-N/O bond lengths in complexes 4 are similar as those found in complex 5, and comparable to the Ag(Ⅰ) complexes with same donor sets[9]. As expected, there are no classical hydrogen bonds in the crystals of 1~5.
1 Cu1-N1 0.203 2(6) Cu1-01 0.223 7(5) Cu1-02 0.203 6(5) Cu1-C12 0.221 0(2) Cu1-C13 0.223 0(2) Cu2-N3 0.202 9(6) Cu2-O3 0.223 9(5) Cu2-O4 0.204 2(5) Cu2-C15 0.223 1(2) Cu2-C16 0.223 1(2) N1-Cu1-C13 95.04(17) O2-Cu1-Ol 73.58(18) N1-Cu1-O2 148.8(2) O2-Cu1-C13 94.13(16) C12-Cu1-Ol 116.33(15) N1-Cu1-C12 96.12(18) C12-Cu1-C13 138.09(9) C13-Cu1-Ol 105.57(16) O2-Cu1-C12 96.73(15) N1-Cu1-Ol 75.3(2) N3-Cu2-O3 75.5(2) N3-Cu2-O4 148.7(2) N3-Cu2-C15 97.35(18) O4-Cu2-O3 73.38(18) N3-Cu2-C16 95.18(18) O4-Cu2-C15 94.49(18) C16-Cu2-O3 107.5(2) O4-Cu2-C16 96.86(18) C16-Cu2-C15 134.63(10) C15-Cu2-O3 117.8(2) 2 ZN1-N1 0.214 1(2) ZN1-Ol 0.230 3(2) ZN1-O2 0.207 34(19) ZN1-C12 0.222 44(9) ZN1-C13 0.222 50(8) C13-ZN1-Ol 121.81(6) O2-ZN1-N1 140.02(9) O2-ZN1-C13 99.43(6) O2-ZN1-Ol 69.63(7) O2-ZN1-C12 98.79(7) N1-ZN1-C13 99.17(7) N1-ZN1-Ol 70.47(8) N1-ZN1-C12 100.76(7) C12-ZN1-C13 122.01(4) C12-ZN1-Ol 116.17(7) 3 Zn1-N1 0.208 5(3) Zn1-O1 0.220 8(3) Zn1-O2 0.203 4(3) ZN1-O3 0.234 9(4) ZN1-O4 0.209 8(3) ZN1-O6 0.201 1(3) O6-ZN1-O2 97.57(12) N1-ZN1-O4 105.93(14) O6-ZN1-O3 141.06(13) O6-ZN1-N1 102.70(12) O6-ZN1-O1 120.88(11) O2-ZN1-O3 92.01(12) O2-ZN1-N1 146.27(12) O2-ZN1-O1 72.96(10) N1-ZN1-O3 88.93(12) O6-ZN1-O4 84.31(13) N1-ZN1-O1 73.52(12) O4-ZN1-O3 56.75(13) O2-ZN1-O4 102.64(13) O4-ZN1-O1 154.61(12) O1-ZN1-O3 98.03(12) 4 Ag1-N1 0.232 6(3) Ag1-O1 0.257 3(2) Ag1-O2 0.249 1(3) N1ⅰ-Ag1-N1 129.47(14) O2ⅰ-mg1-O2 107.25(16) O2ⅰ-Ag1-O1 109.98(9) N1ⅰ-Agl-O2 84.95(10) N1ⅰ-mg1-O1 121.10(9) O2-Ag1-O1 61.80(8) N1-Ag1-O2 126.45(9) N1-mg1-O1 65.01(9) O1ⅰ-Ag1-O1 167.33(12) 5 Ag1-N1 0.232 6(4) Ag1-O1 0.261 3(3) Ag1-O2 0.247 9(4) N1ⅰ-Agl-N1 124.7(2) O2-Ag1-O2ⅰ 114.31(19) O2-Ag1-O1 61.18(12) N1ⅰ-Ag1-O2 85.67(13) N1ⅰ-Ag1-O1 119.46(13) O2ⅰ-Ag1-O1 114.50(12) N1-Ag1-O2 125.38(13) N1-Ag1-Ol 64.26(13) O1ⅰ-Ag1-O1 172.87 Symmetry codes: ⅰ-x, y, -z+1/2 2.2 IR spectra
The IR spectra of free ligand L show three band at 1 682, 1 598 and 1 254 cm-1, attributable to ν(C=O), ν(C=N) and ν(Ar-O-C), respectively[5]. They shifts to lower wavenumber in the complexes 1~5, indicating that carbonyl oxygen, ethereal oxygen and quinoline nitrogen atoms take part in coordination[9]. In addition, the intense absorption bands in the spectra of complex 3 associated with the asymmetric stretching appear at 1 384 or 1 291 cm-1 (ν4) and 1 484 cm-1 (ν1), clearly establishing the existence of monodentate and bidentate NO3- ligands, respectively[8]. The ν(C=O) bands in complexes 1~3 at around 1 710 cm-1 are corresponding to the crystal acetone molecules. It is in accordance with the result of the crystal structure study.
2.3 UV spectra
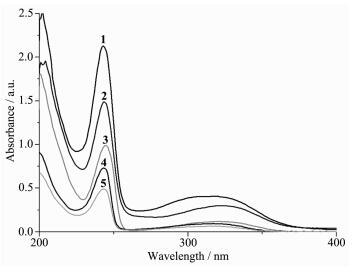
The UV spectra complexes 1~5 in acetonitrile solution (concentration: 1×10-5 mol·L-1) were measured at room temperature (Fig. 2). The UV spectra of complexes 1~5 are quite similar as that of the ligand L with two bands at 244 nm (ε=156 000 L·mol-1·cm-1) and 316 nm (ε=19 500 L·mol-1·cm-1)][5], each of them features two main bands located at 244 nm(ε=212 886, 149 054, 99 927, 73 871 and 50 073 L·mol-1·cm-1 for complexes 1~5, respectively) and 318 nm(ε=40 755, 29 625, 11 770, 9 390 and 6 450 L·mol-1·cm-1 for complexes 1~5, respectively). Such two bands should be assigned to characteristic π-π* transitions centered on quinoline ring and the acetamide unit of the amide ligand L, respectively[5, 8].
2.4 Fluorescence spectra
The fluorescence spectra of complexes 1~5 have also been studied in acetonitrile solution (concentration: 1×10-5 mol·L-1) at room temperature. The results show that the emission spectra of complexes 1, 2, 4 and 5 exhibit only one main peak at 410 nm when excited at 320 nm, which is similar as that of the ligand L[5]. However, the emission band of complex 3 red-shifts to 430 nm under same tested condition, indicating the energy transferring from the ligand L to the Zn(Ⅱ) ion[8]. It should be noted that complexes 2 and 3 exhibit quite different fluorescence emission even they have same centre Zn(Ⅱ) ion, primarily related with the anion effect (chloride for 2, while nitrate for 3)[10].
-
-
[1]
毛盼东, 徐君, 吴伟娜, 等.无机化学学报, 2016, 32: 677-682 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20160417&journal_id=wjhxxbcnMAO Pan-Dong, XU Jun, WU Wei-Na, et al. Chinese J. Inorg. Chem., 2016, 32: 677-682 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20160417&journal_id=wjhxxbcn
-
[2]
Song K C, Kim J S, Park S M, et al. Org. Lett., 2006, 8:3413 -3416 doi: 10.1021/ol060788b
-
[3]
Zhou X, Li P, Shi Z, et al. Inorg. Chem., 2012, 51:9226-9231 doi: 10.1021/ic300661c
-
[4]
蔡红新, 吴伟娜, 王元.无机化学学报, 2013, 29: 845-849 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20130429&journal_id=wjhxxbcnCAI Hong-Xin, WU Wei-Na, WANG Yuan. Chinese J. Inorg. Chem., 2013, 29: 845-849 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20130429&journal_id=wjhxxbcn
-
[5]
毛盼东, 闫玲玲, 吴伟娜, 等.无机化学学报, 2016, 32:1476-1480 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20160823&journal_id=wjhxxbcnMAO Pan-Dong, YAN Ling-Ling, WU Wei-Na, et al. Chinese J. Inorg. Chem., 2016, 32:1476-1480 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20160823&journal_id=wjhxxbcn
-
[6]
Sheldrick G M. SADABS, University of Göttingen, Germany, 1996.
-
[7]
Sheldrick G M. SHELX-97, Program for the Solution and the Refinement of Crystal Structures, University of Göttingen, Germany, 1997.
-
[8]
李晓静, 吴伟娜, 徐周庆, 等.无机化学学报, 2015, 31:2256-2271 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20151123&journal_id=wjhxxbcnLI Xiao-Jing, WU Wei-Na, XU Zhou-Qing, et al. Chinese J. Inorg. Chem., 2015, 31:2256-2271 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20151123&journal_id=wjhxxbcn
-
[9]
Wang J, Qi Q, Cheng L, et al. Inorg. Chem. Commun., 2015, 58:5-8 doi: 10.1016/j.inoche.2015.05.013
-
[10]
Tavman A, Çinarli A. Inorg. Chim. Acta, 2014, 421:481-488 doi: 10.1016/j.ica.2014.07.036
-
[1]
-
Table 1. Selected crystallographic data for complexes 1~5
1 2 3 4 5 Empirical formula C18H21N2O3Cl3Cu C18H21N2O3Cl3Zn C16.5H18N4O8.5ClZn C30H30N4O8Cl3Ag C30H30N4O4Cl2BF4Ag Formula weight 483.26 485.09 509.17 788.80 776.16 T/K 296(2) 296(2) 296(2) 296(2) 296(2) Crystal system Monoclinic Orthorhombic Monoclinic Monoclinic Monoclinic Space group P21/c Pbcn P21/c P2/c C2/c a/nm 2.277 1(3) 1.476 9(2) 0.861 74(19) 1.397 6(2) 2.283 9(9) b/nm 1.515 5(2) 1.205 84(17) 2.231 8(5) 0.849 08(12) 0.848 9(3) c/nm 1.217 71(18) 2.228 5(3) 1.088 1(2) 1.571 80(16) 1.571 1(6) β/(°) 95.176(3) 90.360(4) 122.486(9) 91.076(8) V/nm3 4.184 9(11) 3.968 8(10) 2.092 6(8) 1.573 3(4) 3.046(2) Z 8 8 4 2 4 Dc/(g·cm-3) 1.534 1.624 1.616 1.665 1.693 Unique 7 350 3 504 3 683 2 764 2 687 Rint 0.033 6 0.032 2 0.050 8 0.022 7 0.072 5 GOF 1.061 1.024 1.061 1.081 1.063 R indices [I > 2σ(I)] R1=0.066 6,
wR2=0.202 0R1=0.034 7,
wR2=0.107 2R1=0.050 5,
wR2=0.105 4R1=0.038 9,
wR2=0.103 2R1=0.048 9,
wR2=0.086 8R indices (all data) R1=0.086 7,
wR2=0.214 2R1=0.042 1,
wR2=0.111 1R1=0.079 6,
wR2=0.113 8R1=0.046 3,
wR2=0.109 4R1=0.114 2,
wR2=0.102 9Table 2. Selected bond lengths (nm) and angles (°) in complexes 1~5
1 Cu1-N1 0.203 2(6) Cu1-01 0.223 7(5) Cu1-02 0.203 6(5) Cu1-C12 0.221 0(2) Cu1-C13 0.223 0(2) Cu2-N3 0.202 9(6) Cu2-O3 0.223 9(5) Cu2-O4 0.204 2(5) Cu2-C15 0.223 1(2) Cu2-C16 0.223 1(2) N1-Cu1-C13 95.04(17) O2-Cu1-Ol 73.58(18) N1-Cu1-O2 148.8(2) O2-Cu1-C13 94.13(16) C12-Cu1-Ol 116.33(15) N1-Cu1-C12 96.12(18) C12-Cu1-C13 138.09(9) C13-Cu1-Ol 105.57(16) O2-Cu1-C12 96.73(15) N1-Cu1-Ol 75.3(2) N3-Cu2-O3 75.5(2) N3-Cu2-O4 148.7(2) N3-Cu2-C15 97.35(18) O4-Cu2-O3 73.38(18) N3-Cu2-C16 95.18(18) O4-Cu2-C15 94.49(18) C16-Cu2-O3 107.5(2) O4-Cu2-C16 96.86(18) C16-Cu2-C15 134.63(10) C15-Cu2-O3 117.8(2) 2 ZN1-N1 0.214 1(2) ZN1-Ol 0.230 3(2) ZN1-O2 0.207 34(19) ZN1-C12 0.222 44(9) ZN1-C13 0.222 50(8) C13-ZN1-Ol 121.81(6) O2-ZN1-N1 140.02(9) O2-ZN1-C13 99.43(6) O2-ZN1-Ol 69.63(7) O2-ZN1-C12 98.79(7) N1-ZN1-C13 99.17(7) N1-ZN1-Ol 70.47(8) N1-ZN1-C12 100.76(7) C12-ZN1-C13 122.01(4) C12-ZN1-Ol 116.17(7) 3 Zn1-N1 0.208 5(3) Zn1-O1 0.220 8(3) Zn1-O2 0.203 4(3) ZN1-O3 0.234 9(4) ZN1-O4 0.209 8(3) ZN1-O6 0.201 1(3) O6-ZN1-O2 97.57(12) N1-ZN1-O4 105.93(14) O6-ZN1-O3 141.06(13) O6-ZN1-N1 102.70(12) O6-ZN1-O1 120.88(11) O2-ZN1-O3 92.01(12) O2-ZN1-N1 146.27(12) O2-ZN1-O1 72.96(10) N1-ZN1-O3 88.93(12) O6-ZN1-O4 84.31(13) N1-ZN1-O1 73.52(12) O4-ZN1-O3 56.75(13) O2-ZN1-O4 102.64(13) O4-ZN1-O1 154.61(12) O1-ZN1-O3 98.03(12) 4 Ag1-N1 0.232 6(3) Ag1-O1 0.257 3(2) Ag1-O2 0.249 1(3) N1ⅰ-Ag1-N1 129.47(14) O2ⅰ-mg1-O2 107.25(16) O2ⅰ-Ag1-O1 109.98(9) N1ⅰ-Agl-O2 84.95(10) N1ⅰ-mg1-O1 121.10(9) O2-Ag1-O1 61.80(8) N1-Ag1-O2 126.45(9) N1-mg1-O1 65.01(9) O1ⅰ-Ag1-O1 167.33(12) 5 Ag1-N1 0.232 6(4) Ag1-O1 0.261 3(3) Ag1-O2 0.247 9(4) N1ⅰ-Agl-N1 124.7(2) O2-Ag1-O2ⅰ 114.31(19) O2-Ag1-O1 61.18(12) N1ⅰ-Ag1-O2 85.67(13) N1ⅰ-Ag1-O1 119.46(13) O2ⅰ-Ag1-O1 114.50(12) N1-Ag1-O2 125.38(13) N1-Ag1-Ol 64.26(13) O1ⅰ-Ag1-O1 172.87 Symmetry codes: ⅰ-x, y, -z+1/2 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 2
- 文章访问数: 613
- HTML全文浏览量: 92




 下载:
下载:


 下载:
下载:

