
一对手性铕(Ⅲ)配合物的合成和铁电性质
English
Syntheses and Ferroelectric Properties of a Couple of Chiral Europium(Ⅲ) Complexes
-
Recently, multifunctional materials based on lanthanide complexes have received much attention due to their potential applications in optoelectronics[1-3]. The introduction of chiral environments in lanthanide complexes would lead to a diversification of configuration and crystallize in a polar space group. Thus, interesting properties, such as ferroelectricity, piezoelectricity, triboluminescence, circular polarized luminescence (CPL) and second harmonic generation (SHG) would be expected[4-7].
Ferroelectric materials possessing a spontaneous electric polarization that can be reversed by the application of an external electric field, are of great interest in the field of optics and electronics[8]. Compared to the typical inorganic ABO3-type ferroelectrics, molecular-based ferroelectric materials show some advantages: larger spontaneous polarization, facile chemical vapor deposition (CVD) and convenient structural modification. Furthermore, owing to the high coordination numbers and the interaction between a central metal ion and its ligand field (LF), the structures of lanthanide complexes could be easily distorted and spontaneous dipole moments can be induced, possibly resulting in interesting multi-ferroic properties.
In previous studies, we have studied multifunc-tional properties (luminescence, ferroelectricity, mag-netism and so on) of chiral lanthanide complexes incorporated pinene functionalized with aromatic amine derivatives as well as solvent-induced or temperature-induced switching of configurations and properties of these complexes[9-12]. A novel Eu(Ⅲ) complex Eu(TTA)3L1a (1a) (TTA=thenoyltrifluoroacetonate, L1a=(+)-4, 5-bis(pinene)-2, 2′-bipyridine) with a bispi-nene-containing bipyridyl ligand was used as high efficient luminescent down-shifting material and lead to a significant improvement of the spectral response of c-Si PV module in the UV region[13].
As an extension of these work, the crystal structure and ferroelectric property of 1a has been studied in this paper. The ccompound 1a crystallizes in a noncentrosymmetric space group (P1), and a distorted square-antiprism configuration exists around the central Eu(Ⅲ) ion, satisfying the prerequisites of ferroelectrics. As expected, complex 1a shows a distinct electric hysteresis loop, making it as potential multifunctional molecular materials. The enantiomer Eu(TTA)3L1b (1b) (L1b=(-)-4, 5-bis(pinene)-2, 2′-bipyri-dine) was also prepared, and chiral environments are confirmed by a mirror symmetry of circular dichroism (CD) spectra of 1a and 1b.
1 Experimental
1.1 Chemicals and synthesis
All reagents were purchased from commercial suppliers and used as received. Compounds 1a and 1b were prepared according to our published methods as follows[13]: L1a or L1b (344 mg, 1 mmol) was added to a stirred solution of Eu(TTA)3·2H2O (851 mg, 1 mmol) in ethanol (40 mL). The resulting mixture was stirred at room temperature for 24 h. The precipitate was collected by filtration to yield the desired product. Crystals of compound 1a suitable for X-ray crystallo-graphic analysis were obtained by slow evaporation over a period of 3 weeks of their CH2Cl2/CH3CH2OH solution.
1.2 Chemical and physical characterizations
Single-crystal X-ray diffraction measurements were carried out on a Bruker SMART APEX CCD based on diffractometer operating at room tempera-ture. Intensities were collected with graphite mono-chromatized Mo Kα radiation (λ=0.071 073 nm) oper-ating at 50 kV and 30 mA, using ω-2θ scan mode. The data reduction was made with the Bruker SAINT package[14]. Absorption corrections were performed using the SADABS program[15]. The structures were solved by direct methods and refined on F2 by full-matrix least-squares using SHELXL-97 with aniso-tropic displacement parameters for all non-hydrogen atoms in all two structures[16]. Hydrogen atoms bonded to the carbon atoms were placed in calculated positions and refined as riding mode, with dC-H=0.093 nm (methane) or 0.096 nm (methyl) and Uiso(H)=1.2Ueq (Cmethane) or Uiso(H)=1.5Ueq (Cmethyl). All computations were carried out using the SHELXTL-97 program package[16-17]. The absorption spectra of complexes 1a and 1b in CH2Cl2 solution were recorded on a Shimazu UV-3100 spectrometer. CD spectra were recorded by a Jasco J-810 spectropolarimeter. The Electric hysteresis loops were recorded by a Ferroelectric Tester Precision Premier Ⅱ by using powder samples in pellets at room temperature.
CCDC: 818185, 1a.
2 Results and discussion
2.1 Crystal structure
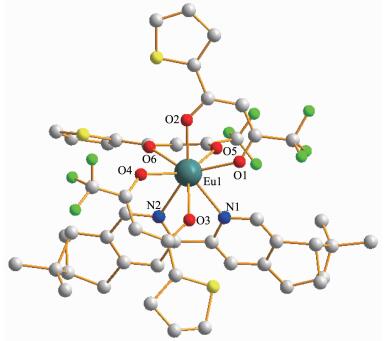
The crystal data and structure refinements for ccompound 1a are provided in Table 1. Similar to those europium(Ⅲ) complexes reported previously[4, 12], ccompound 1a is a mononuclear neutral europium(Ⅲ)compound and crystalizes in triclinic system, space group P1. As shown in Fig. 1, each molecule contains three β-diketonate anions, one (+)-4, 5-bis(pinene)-2, 2′-bipyridine, and one eight-coordinated Eu(Ⅲ) ion. Each of the three diketonate anions provides two donor O atoms to coordinate to the Eu(Ⅲ) ion. The other two coordination sites of the Eu(Ⅲ) ion are occupied by two N atoms of the chiral bipyridine derivative to complete the eight-coordinate configuration.
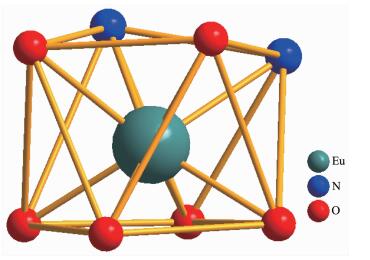
Empirical formula C48H40EuF9N2O6S3 Formula weight 1 159.96 Crystal system Triclinic Space group P1 T/K 293(2) a/ nm 1.066 9(5) b/ nm 1.076 9(5) c/ nm 2.354 9(10) α/(°) 88.832(6) β/(°) 89.872(5) γ/(°) 66.146(5) V/nm3 2.474 0(19) Z 2 Dc / (Mg·m-3) 1.557 F(000) 1 164 Collected reflection 10 292 Unique reflection 7 702 Parameter 1 142 R1, wR2a [I > 2σ(I)] 0.078, 0.188 μ / mm-1 1.477 GOF 1.133 a R1=∑||Fo|-|Fc||/∑|Fo|, wR2=[∑w(|Fo2|-|Fc2|)2/∑w(|Fo2|)2]1/2 Selected bond lengths and angles are summarized in Table 2. The Eu-O bond lengths range from 0.231 8 to 0.238 5 nm, while the two Eu-N bond lengths are 0.258 7(16) and 0.255 6(15) nm, respectively. Eight bonds with different lengths give rise to a strongly distorted square-antiprism environment around the Eu(Ⅲ) ion (Fig. 2). The O1-O2-O3-O4 (bottom plane) and O5-O6-N1-N2 atoms (top plane) comprise the two square-basic planes of the antiprism with mean deviations of 0.002 78 and 0.013 97 nm from each plane, and their dihedral angle is 3.442°. For a regular square antiprism, two square planes are parallel to each other and one square rotates 45° from the other. However, in 1a, the top plane rotates only 41.4° from the bottom plane.
Eu(1)-O(1) 0.233 2(12) Eu(1)-O(2) 0.235 4(16) Eu(1)-O(3) 0.236 4(13) Eu(1)-O(4) 0.231 8(12) Eu(1)-O(5) 0.232 6(12) Eu(1)-O(6) 0.238 5(13) Eu(2)-O(7) 0.234 3(13) Eu(2)-O(8) 0.235 3(14) Eu(2)-O(9) 0.234 6(13) Eu(2)-O(10) 0.241 1(13) Eu(2)-O(11) 0.236 7(13) Eu(2)-O(12) 0.234 8(12) Eu(1)-N(1) 0.258 7(16) Eu(1)-N(2) 0.255 6(15) Eu(2)-N(3) 0.263 9(16) Eu(2)-N(4) 0.255 9(16) O(1)-Eu(1)-O(2) 7.07(6) O(1)-Eu(1)-O(3) 8.37(5) O(1)-Eu(1)-O(6) 13.93(5) O(1)-Eu(1)-N(2) 14.07(5) O(1)-Eu(1)-N(1) 7.93(5) O(2)-Eu(1)-O(3) 11.80(6) O(2)-Eu(1)-O(6) 8.20(6) O(2)-Eu(1)-N(2) 14.84(6) O(2)-Eu(1)-N(1) 14.65(5) N(2)-Eu(1)-N(1) 6.23(5) O(3)-Eu(1)-O(6) 13.67(4) O(3)-Eu(1)-N(2) 7.71(5) O(3)-Eu(1)-N(1) 7.19(5) O(4)-Eu(1)-N(1) 13.40(5) O(4)-Eu(1)-O(5) 14.79(5) O(4)-Eu(1)-O(1) 12.02(5) O(4)-Eu(1)-O(2) 7.62(5) O(4)-Eu(1)-O(3) 7.00(5) O(4)-Eu(1)-O(6) 7.96(5) O(4)-Eu(1)-N(2) 8.47(5) O(5)-Eu(1)-O(1) 7.84(5) O(5)-Eu(1)-O(2) 8.77(5) O(5)-Eu(1)-O(3) 14.16(5) O(5)-Eu(1)-O(6) 7.06(5) O(5)-Eu(1)-N(2) 9.59(5) O(5)-Eu(1)-N(1) 7.16(5) O(6)-Eu(1)-N(2) 6.99(5) O(6)-Eu(1)-N(1) 11.36(5) O(7)-Eu(2)-O(9) 7.76(5) O(7)-Eu(2)-O(12) 12.03(5) O(7)-Eu(2)-O(8) 7.14(5) O(7)-Eu(2)-O(11) 8.13(5) O(7)-Eu(2)-O(10) 14.22(5) O(7)-Eu(2)-N(4) 13.69(5) O(7)-Eu(2)-N(3) 7.82(5) O(8)-Eu(2)-O(10) 8.64(5) O(8)-Eu(2)-N(4) 15.12(5) O(8)-Eu(2)-O(11) 11.80(5) O(8)-Eu(2)-N(3) 14.48(5) O(9)-Eu(2)-O(8) 8.48(5) O(9)-Eu(2)-O(11) 14.17(4) O(9)-Eu(2)-O(12) 14.72(5) O(9)-Eu(2)-O(10) 7.01(5) O(9)-Eu(2)-N(4) 10.45(5) O(9)-Eu(2)-N(3) 7.14(5) O(10)-Eu(2)-N(4) 7.19(5) O(10)-Eu(2)-N(3) 10.84(5) O(11)-Eu(2)-O(10) 13.65(4) O(11)-Eu(2)-N(4) 7.09(5) O(11)-Eu(2)-N(3) 7.31(5) O(12)-Eu(2)-O(8) 7.68(4) O(12)-Eu(2)-O(11) 7.10(4) O(12)-Eu(2)-O(10) 8.16(5) O(12)-Eu(2)-N(4) 8.14(5) O(12)-Eu(2)-N(3) 13.57(5) N(4)-Eu(2)-N(3) 6.27(5) 2.2 Circular dichroism (CD) spectra
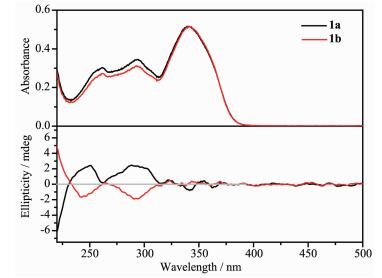
The chirality of complexes 1a and 1b is confirmed by measuring circular dichroism (CD) spectra (Fig. 3). Two distinct Cotton effect at 245 and 292 nm can be observed, and the spectra of 1a and 1b are almost mirror-symmetric. These features indicate that 1a and 1b are chiral and enantiomeric to each other.
2.3 Ferroelectric properties
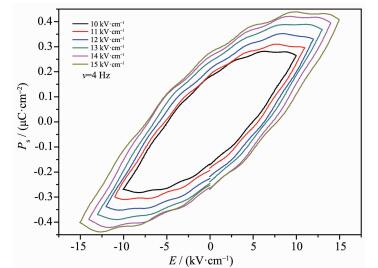
Since complexes 1a crystalizes in a polar space group (P1), the ferroelectric properties of 1a were investigated at 298 K with compressed powder samples as shown in Fig. 4. The ferroelectric measurements reveal that complex 1a indeed displays an obvious ferroelectric behavior and almost reaches the saturated polarization status with a remnant polarization (Pr) of ca. 0.27 μC·cm-2 and Ec of ca. 7.2 kV·cm-1 by applying an electric field of 15 kV·cm-1 at 4 Hz. The saturation spontaneous polarization (Ps) is about 0.43 μC·cm-2, which exceeds the value of typical ferroelectrics NaKC4H4O6·4H2O (Rosal salt, Ps=0.25 μC·cm-2).
3 Conclusions
In summary, a couple of pinene-containing chiral europium(Ⅲ) complexes 1a and 1b were prepared. The chirality of complexes 1a and 1b is confirmed by measuring circular dichroism (CD) spectra. Complexes 1a crystalizes in P1 space group of triclinic system. The coordination polyhedron of the Eu(Ⅲ) ion in 1a can be described as a siginificantly distorted square-antiprism. The complex 1a exhibited distinct ferroele-ctric behaviors with a remnant polarization (Pr) of ca. 0.27 μC·cm-2 and Ec of ca. 7.2 kV·cm-1 by applying an electric field of 15 kV·cm-1 at 4 Hz.
-
-
[1]
Walton J W, Bourdolle A, Butler S J, et al. Chem. Commun., 2013, 49(16):1600-1602 doi: 10.1039/c2cc35247h
-
[2]
Kotova O, Kitchen J A, Lincheneau C, et al. Chem. Eur. J., 2013, 19(48):16181-16186 doi: 10.1002/chem.v19.48
-
[3]
Carr R, Di Bari L, Lo Piano S, et al. Dalton Trans., 2012, 41 (42):13154-13158 doi: 10.1039/c2dt30143a
-
[4]
Li D P, Li C H, Wang J, et al. Eur. J. Inorg. Chem., 2009 (32):4844-4849
-
[5]
Bozoklu G, Gateau C, Imbert D, et al. J. Am. Chem. Soc., 2012, 134(20):8372-8375 doi: 10.1021/ja3020814
-
[6]
Harada T, Tsumatori H, Nishiyama K, et al. Inorg. Chem., 2012, 51(12):6476-6485 doi: 10.1021/ic202467f
-
[7]
Li X L, Chen C L, Xiao H P, et al. Dalton Trans., 2013, 42 (43):15317-15325 doi: 10.1039/c3dt51743h
-
[8]
Hang T, Zhang W, Ye H Y, et al. Chem. Soc. Rev., 2011, 40 (7):3577-3598 doi: 10.1039/c0cs00226g
-
[9]
Li D P, Wang T W, Li C H, et al. Chem. Commun., 2010, 46 (17):2929-2931 doi: 10.1039/b924547b
-
[10]
Li D P, Zhang X P, Wang T W, et al. Chem. Commun., 2011, 47(24):6867-6869 doi: 10.1039/c1cc11659b
-
[11]
Liu J, Zhang X P, W T, et al. Inorg. Chem., 2012, 51(16): 8649-8651 doi: 10.1021/ic3012475
-
[12]
Li X L, Chen K, Liu Y, et al. Angew. Chem. Int. Ed., 2007, 46(36):6820-6823 doi: 10.1002/(ISSN)1521-3773
-
[13]
Liu J, Wang K, Zheng W, et al. Prog. Photovoltaics Res. Appl., 2013, 21(4):668-675
-
[14]
SAINT-Plus, Ver. 6. 02, Bruker Analytical X-ray System, Madison, WI, 1999.
-
[15]
Sheldrick G M. SADABS, an Empirical Absorption Correction Program, Bruker Analytical X-ray Systems, Madison, WI, 1996.
-
[16]
Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997.
-
[17]
Sheldrick G M. Acta Crystallogr. Sect. A: Found. Crystallogr., 2008, 64:112-122 doi: 10.1107/S0108767307043930
-
[1]
-
Table 1. Crystal data and structure refinements for complex 1a
Empirical formula C48H40EuF9N2O6S3 Formula weight 1 159.96 Crystal system Triclinic Space group P1 T/K 293(2) a/ nm 1.066 9(5) b/ nm 1.076 9(5) c/ nm 2.354 9(10) α/(°) 88.832(6) β/(°) 89.872(5) γ/(°) 66.146(5) V/nm3 2.474 0(19) Z 2 Dc / (Mg·m-3) 1.557 F(000) 1 164 Collected reflection 10 292 Unique reflection 7 702 Parameter 1 142 R1, wR2a [I > 2σ(I)] 0.078, 0.188 μ / mm-1 1.477 GOF 1.133 a R1=∑||Fo|-|Fc||/∑|Fo|, wR2=[∑w(|Fo2|-|Fc2|)2/∑w(|Fo2|)2]1/2 Table 2. Selected bond lengths (nm) and angles (°) for 1a
Eu(1)-O(1) 0.233 2(12) Eu(1)-O(2) 0.235 4(16) Eu(1)-O(3) 0.236 4(13) Eu(1)-O(4) 0.231 8(12) Eu(1)-O(5) 0.232 6(12) Eu(1)-O(6) 0.238 5(13) Eu(2)-O(7) 0.234 3(13) Eu(2)-O(8) 0.235 3(14) Eu(2)-O(9) 0.234 6(13) Eu(2)-O(10) 0.241 1(13) Eu(2)-O(11) 0.236 7(13) Eu(2)-O(12) 0.234 8(12) Eu(1)-N(1) 0.258 7(16) Eu(1)-N(2) 0.255 6(15) Eu(2)-N(3) 0.263 9(16) Eu(2)-N(4) 0.255 9(16) O(1)-Eu(1)-O(2) 7.07(6) O(1)-Eu(1)-O(3) 8.37(5) O(1)-Eu(1)-O(6) 13.93(5) O(1)-Eu(1)-N(2) 14.07(5) O(1)-Eu(1)-N(1) 7.93(5) O(2)-Eu(1)-O(3) 11.80(6) O(2)-Eu(1)-O(6) 8.20(6) O(2)-Eu(1)-N(2) 14.84(6) O(2)-Eu(1)-N(1) 14.65(5) N(2)-Eu(1)-N(1) 6.23(5) O(3)-Eu(1)-O(6) 13.67(4) O(3)-Eu(1)-N(2) 7.71(5) O(3)-Eu(1)-N(1) 7.19(5) O(4)-Eu(1)-N(1) 13.40(5) O(4)-Eu(1)-O(5) 14.79(5) O(4)-Eu(1)-O(1) 12.02(5) O(4)-Eu(1)-O(2) 7.62(5) O(4)-Eu(1)-O(3) 7.00(5) O(4)-Eu(1)-O(6) 7.96(5) O(4)-Eu(1)-N(2) 8.47(5) O(5)-Eu(1)-O(1) 7.84(5) O(5)-Eu(1)-O(2) 8.77(5) O(5)-Eu(1)-O(3) 14.16(5) O(5)-Eu(1)-O(6) 7.06(5) O(5)-Eu(1)-N(2) 9.59(5) O(5)-Eu(1)-N(1) 7.16(5) O(6)-Eu(1)-N(2) 6.99(5) O(6)-Eu(1)-N(1) 11.36(5) O(7)-Eu(2)-O(9) 7.76(5) O(7)-Eu(2)-O(12) 12.03(5) O(7)-Eu(2)-O(8) 7.14(5) O(7)-Eu(2)-O(11) 8.13(5) O(7)-Eu(2)-O(10) 14.22(5) O(7)-Eu(2)-N(4) 13.69(5) O(7)-Eu(2)-N(3) 7.82(5) O(8)-Eu(2)-O(10) 8.64(5) O(8)-Eu(2)-N(4) 15.12(5) O(8)-Eu(2)-O(11) 11.80(5) O(8)-Eu(2)-N(3) 14.48(5) O(9)-Eu(2)-O(8) 8.48(5) O(9)-Eu(2)-O(11) 14.17(4) O(9)-Eu(2)-O(12) 14.72(5) O(9)-Eu(2)-O(10) 7.01(5) O(9)-Eu(2)-N(4) 10.45(5) O(9)-Eu(2)-N(3) 7.14(5) O(10)-Eu(2)-N(4) 7.19(5) O(10)-Eu(2)-N(3) 10.84(5) O(11)-Eu(2)-O(10) 13.65(4) O(11)-Eu(2)-N(4) 7.09(5) O(11)-Eu(2)-N(3) 7.31(5) O(12)-Eu(2)-O(8) 7.68(4) O(12)-Eu(2)-O(11) 7.10(4) O(12)-Eu(2)-O(10) 8.16(5) O(12)-Eu(2)-N(4) 8.14(5) O(12)-Eu(2)-N(3) 13.57(5) N(4)-Eu(2)-N(3) 6.27(5) -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 4
- 文章访问数: 788
- HTML全文浏览量: 78


 下载:
下载:



 下载:
下载:

