
以6-甲基-2, 3, 5-吡啶三酸为配体的过渡金属配合物的合成、结构与性质
-
关键词:
- 6-甲基-2, 3, 5-吡啶三酸
- / 配位聚合物
- / 荧光性质
English
Syntheses, Structures, and Properties of Transition Metal Complexes Constructed from 6-Methyl-2, 3, 5-pyridinetricarboxylic Acid
-
The design and synthesis of novel coordination polymers have received significant attention due to their intriguing structures and potential applications in host-guest chemistry, catalysis, chemical sensors, magnetic and fluorescent materials[1-5]. Many metal-organic frameworks, which consist of metal ions and multidentate organic ligands containing O-or N-donors, have been reported during the past several years[6-8]. However, it is still a big challenge to predictably prepare the desired networks because many factors such as the coordination geometry of metal ions, the structural characteristics of ligands, the solvent system, and the counter anions, influence the final structures[9-11]. To date, the most effective approach for the synthesis of desirable complexes is still to adopt appropriate bridging ligands, because changes in substituent group, symmetry, and flexibility of organic ligands can result in materials bearing diverse architectures and functions.
Recently, we have systematically investigated a series of coordination networks constructed from pyridine-2, 3, 5, 6-tetracarboxylaic acid (H4pdtc), which display versatile architectures[12-14]. In this context, as the continuance of this research, we choose 6-methyl-2, 3, 5-pyridinetricarboxylic acid (H3mptc) as organic ligand, which has been prepared via slight change on the synthesis of H4pdtc[15]. Compared to H4pdtc, H3mptc possesses a lower symmetry because 6-methyl group has replaced the corresponding carboxyl group, which may result in the formation of unusual complexes. To our knowledge, only a few lanthanide coordination complexes based on H3mptc have been reported and its coordination chemistry remains largely unexplored[15].
Herein, we have successfully synthesized two new complexes {[Cu (Hmptc)(H2O)]·2H2O}n (1) and {Cd (Hmptc)(H2O)}n (2). It is noteworthy that complexes 1~2 are reported for the first time from transition metal ions and H3mptc and complex 1 presents a two-dimensional (2D) rectangle channels. The luminescent properties of complex 2 have also been investigated.
1 Experimental
1.1 General
H3mptc was synthesized according to the reported method[15]. The other reagents were commercially available and used without further purification. Elemental analysis (C, H and N) was carried out with a Perkin-Elmer 240 CHN elemental analyzer. IR spectra were obtained with a Bruker TENOR 27 instrument in the range of 4 000~400 cm-1 on a KBr pellet. Thermal gravimetric analysis (TGA) curve was obtained from a NETZSCH TG 209 thermal analyzer in a static air atmosphere with a heating rate of 10 ℃·min-1. Fluorescence spectra were measured on a F-4500 FL fluorescence spectrophotometer.
1.2 Synthesis of the complexes
Complex 1: A mixture of CuCl2·2H2O (25.7 mg, 0.15 mmol), H3mptc (33.8 mg, 0.15 mmol), H2O (10 mL) was stirred at room temperature for 2 h and then filtered. Blue crystals of 1 were obtained after the filtrate was allowed to stand at room temperature for one week. Yield: 0.011 g, 32% based on Cu. Anal. Calcd. for C9H11CuNO9(%): C, 31.72; H, 3.25; N, 4.11. Found (%): C, 31.75; H, 3.19; N, 4.07. IR (KBr pellet, cm-1): 3491s, 1 719s, 1 647s, 1 572vs, 1 398m, 1 278m, 1 140m, 814w, 675w.
Complex 2: A mixture of Cd (NO3)2·4H2O (46.3 mg, 0.15 mmol), H3mptc (22.5 mg, 0.1 mmol), H2O (10 mL) was stirred at room temperature for 2 h and then filtered. Colorless crystals of 2 were obtained after the filtrate was allowed to stand at room temperature for four weeks. Yield: 0.012 g, 34% based on Cd. Anal. Calcd. for C9H7CdNO7(%): C, 30.57; H, 1.99; N, 3.96. Found (%): C, 30.60; H, 1.92; N, 3.95. IR (KBr pellet, cm-1): 3 437s, 1 700s, 1 639vs, 1 566m, 1 440m, 1 370m, 1 286m, 1 147m, 675w, 811w.
1.3 X-ray crystallography
The single crystal X-ray diffraction data collections for complexes 1~2 were performed with a BRUKER SMART-1000 CCD diffractometer, equipped with graphite-monochromatized Mo Kα radiation with a radiation wavelength of 0.071 073 nm, using ω-φ scan. The structures were solved by direct methods and refined anisotropically with a full-matrix least-squares technique based on F2 using the SHELXS-97 and SHELXL-97 programs[16]. Anisotropic thermal para-meters were assigned to all non-hydrogen atoms. The organic hydrogen atoms were generated geometrically; the hydrogen atoms of the water molecules were located from different maps and refined with isotropic temperature factors. The disordered free solvent molecules are unavoidable, of which the hydrogen atoms cannot be confirmed for the improper electron density around the central atom. Analytical expressions of neutral-atom scattering factors were employed, and anomalous dispersion corrections were incorporated. Crystal data collection and refinement details for complexes 1~2 are summarized in Table 1. Selected bond lengths and angles for complexes 1 and 2 are listed in Table 2.
Complex 1 2 Empirical formula C9H11CuNO9 C9H7CdNO7 Formula weight 340.73 353.57 Crystal system Orthorhombic Monoclinic Space group Pccn P21/n a/nm 1.605 8(3) 1.162 9(10) b/nm 0.979 4(2) 0.694 5(6) c/nm 1.460 1(3) 1.320 1(10) β/(°) 101.615(13) V/nm3 2.297 7(8) 1.044(3) Z 8 4 Dc/(Mg·m-3) 1.97 2.249 μ/mm-1 1.951 2.12 F(000) 1 384 688 θrange/(°) 2.44~25.01 2.13~24.99 Reflections collected, unique 12 865, 1 991 8 366, 1 836 (Rint=0.113 0) (Rint=0.046 4) Goodness of fit on F2 1.244 1.141 Final R* indices [I > 2σ(I)] R1=0.084 9, wR2=0.161 9 R1=0.060 3, wR2=0.154 9 R*indices (all data) R1=0.091 4, wR2=0.165 0 R1=0.064 3, wR2=0.156 9 Parameters 187 171 Observed reflections [I > 2σ(I)] 1 991 1 836 Largest diff. peak and hole/(e·nm-3) 737 and -690 3 172 and -2 200 *R1=Σ‖Fo|-|Fc|/|Fo|;wR2=[Σw(Fo2-Fc2)2]/Σw(Fo2)2]1/2 Table 1. Crystal data and structure refinement information for complexes 1~2Complex 1 Cu (1)-O (1) 0.193 4(5) O (3)-Cu (1)ⅱ 0.232 0(5) Cu (1)-O (4)ⅰ 0.196 2(5) Cu (1)-O (7) 0.197 7(5) Cu (1)-N (1) 0.205 1(6) O (7)-Cu (1)-N (1) 168.9 (2) O (1)-Cu (1)-O (4)ⅰ 160.1(2) O (4)ⅰ-Cu (1)-O (3)ⅱ 92.44(9) O (1)-Cu (1)-O (7) 86.5(2) O (1)-Cu (1)-O (3)ⅱ 106.58(19) O (4)ⅰ-Cu (1)-O (7) 89.3(2) O (7)-Cu (1)-O (3)ⅱ 85.02(19) O (1)-Cu (1)-N (1) 82.8(2) N (1)-Cu (1)-O (3)ⅱ 95.22(19) O (4)ⅰ-Cu (1)-N (1) 101.8 (2) Complex 2 Cd (1)-O (7) 0.226 0(8) Cd (1)-O (5)ⅰ 0.229 8(7) Cd (1)-O (1)ⅱ 0.230 6(7) Cd (1)-N (1)ⅰ 0.231 1(8) Cd (1)-O (1) 0.234 6(7) Cd (1)-O (2)ⅲ 0.248 6(8) Cd (1)-O (2)ⅱ 0.263 4(8) O (7)-Cd (1)-O (5)ⅰ 93.1(3) O (7)-Cd (1)-O (1)ⅱ 84.5(3) O (5)ⅰ-Cd (1)-O (1)ⅱ 145.6(2) O (7)-Cd (1)-N (1)ⅰ 165.6(3) O (5)ⅰ-Cd (1)-N (1)ⅰ 73.1(3) O (1)ⅱ-Cd (1)-N (1)ⅰ 104.5(3) O (7)-Cd (1)-O (1) 85.7(3) O (5)ⅰ-Cd (1)-O (1) 95.4(2) O (1)ⅱ-Cd (1)-O (1) 118.55(17) N (1)ⅰ-Cd (1)-O (1) 99.3(3) O (7)-Cd (1)-O (2)ⅲ 94.2(3) O (5)ⅰ-Cd (1)-O (2)ⅲ 76.2(2) O (1)ⅱ-Cd (1)-O (2)ⅲ 69.7(2) N (1)ⅰ-Cd (1)-O (2)ⅲ 78.9(3) O (1)-Cd (1)-O (2)ⅲ 171.6(2) O (7)-Cd (1)-O (2)ⅱ 81.5(3) O (5)ⅰ-Cd (1)-O (2)ⅱ 161.4(2) O (1)ⅱ-Cd (1)-O (2)ⅱ 52.0(2) N (1)ⅰ-Cd (1)-O (2)ⅱ 112.9(3) O (1)-Cd (1)-O (2)ⅱ 66.6(2) O (2)ⅲ-Cd (1)-O (2)ⅱ 121.7(2) Symmetry code: ⅰx, 1.5-y, z+0.5;ⅱ1-x, 2-y, -z for 1; ⅰ2-x, 2-y, -z; ⅱ2.5-x, 0.5+y, 0.5-z; ⅲx, y+1, z for 2 Table 2. Selected bond lengths (nm) and angles (°) for complexes 1~2CCDC: 906071, 1; 906072, 2.
2 Results and discussion
2.1 Structure description
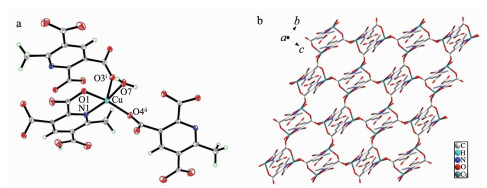
All the complexes, once isolated, are air-stable and can retain their structural integrity at room temperature for a considerable length of time. The single crystal X-ray diffraction analysis reveals the asymmetrical unit of 1 consists of one crystallographically independent copper ion, one Hmptc2- ligand and three water molecules, and O8 is from disordered water molecules (Fig. 1a). Each Cu (Ⅱ) center is surrounded with a tetragonal pyramidal geometry and is coordinated by four oxygen atoms and one nitrogen atom, in which two oxygen atoms (O3A, O4B) are from two different Hmptc2- ligands; one oxygen atom (O7) is from coordinated water molecule; the other two are bidentate chelate O and N atoms. The bond distances and angles involving the metal ions are collected in Table 2. The Cu-O distances are in the range of 0.1 932(1)~0.231 1(1) nm and Cu-N distance is 0.204 8(1) nm, in good agreement with previous studies[17].
For the Hmptc2- ligand, there is one carboxyl group that is not deprotonated. The Hmptc2- ligand bounds to three Cu (Ⅱ) through chelating and bridging modes: one carboxylate group adopts a bidentate bridging coordination mode connecting two Cu (Ⅱ) ions, whereas another carboxylate group and one pyridine nitrogen chelate with one Cu (Ⅱ) ion bidentately. Normally, the carboxyl group coordinated with metal is dehydrogenated. Meanwhile, the C-O distances of the carboxyl group possessing hydrogen atom are significantly different, and hydrogen atom is generally located in the oxygen atom of longer C-O bond length. In complex 1, the C9-O5 and C9-O6 distances are 0.129 7 and 0.123 5 nm, respectively, so H5 is connected to O5.
On the basis of the connection modes, two Cu (Ⅱ) centers are bridged by two different Hmptc2- ligands to form a dinuclear unit consists of a 12-membered ring. And then O4 from carboxylate groups (O4-C8-O3) acts as nodes to connect the dinuclear units to form the infinite 2D layer structure in the bc plane (Fig. 1b). What is interesting is that complex 1 shows an infinite channel along a-axis, the hole is 1.008 24 nm×0.842 65 nm.
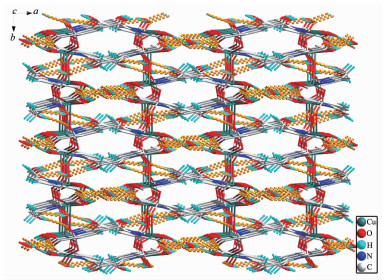
In addition, the 2D layer structures are further assembled into a 3D supramolecular framework through the rich hydrogen-bonding interactions involving the carboxylate oxygen, the coordinated and uncoordinated water molecules (Fig. 2).
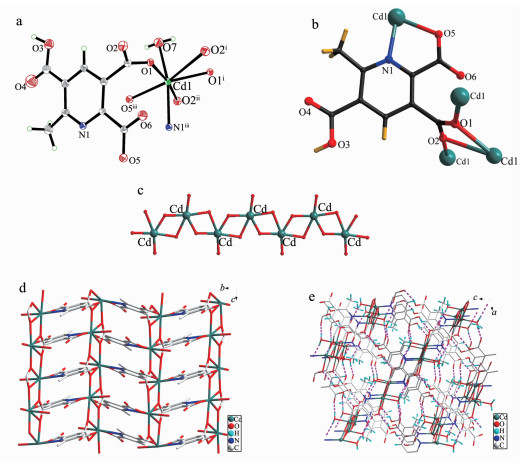
Complex 2 exhibits a 2D coordination framework crystallizing in monoclinic space group P21/n and was connected into 3D networks by supramolecular interactions. The asymmetrical unit of 2 contains one Cd (Ⅱ) atom, one Hmptc2- ligand and one coordinated water molecule (Fig. 3a). The Cd (Ⅱ) atom is seven-coordinated and in a distorted pentagonal bipyramidal coordination geometry. Five carboxylate oxygen atoms (O1, O1A, O2A, O2B, O5C) from different Hmptc2- ligands comprise the equatorial plane, while another two atoms (O7 and N1) occupy the axial positions with a trans angle of 165.6(3)°. The Cd-O distances are in the range of 0.226 0(1)~0.263 4(1) nm and Cd-N distance is 0.231 1(1) nm. The distance of 0.263 4(1) nm is a little long, but it is a nonnegligible interaction, which is in good agreement with previous studies[18-19].
The Hmptc2- ligand in complex 2 exhibits a tetradentate chelating and bridging coordination modes: a carboxylate group and a pyridine nitrogen chelate with one Cd (Ⅱ) ion bidentately; another carboxylate group adopts a chelate/bridge tetradentate coordination mode connecting three Cd (Ⅱ) metal ion (Fig. 3b). In the crystal structure, neighboring Cd (Ⅱ) centers are connected to each other by the carboxylate groups to form an infinite one-dimensional Cd-O grid chain that runs along the a-axis with a Cd…Cd separation of 0.402 78(2) nm (Fig. 3c). These one-dimensional (1D) chains are parallel to each other and further linked together by Hmptc2- ligands to generate a 0.792 56 nm×0.993 04 nm 2D grid network in the bc plane (Fig. 3d). Moreover, O…O hydrogen bonds between carboxylate oxygen atoms and lattice water molecules further extend 2D layered network into a 3D supramolecular framework along the ac plane (Fig. 3e).
2.2 Thermal gravimetric analysis
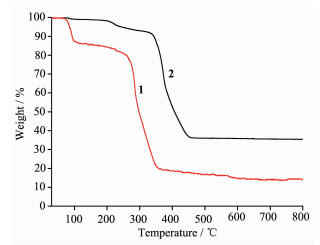
To study the thermal stabilities of the two complexes, thermal gravimetric analysis of complexes 1~2 was performed under the atmosphere of air in the temperature range of 30~800 ℃. As shown in Fig. 4, complex 1 first lost all water molecules below 160 ℃; the weight loss found of 15.45% was consistent with that calculated (15.86%). After the loss of water molecules, a continuously weight loss occurs in the temperature range of 160~350 ℃, which is due to the degradation of Hmptc2- ligand. The residue, 22.38%, is expected to be the mixture of CuO and Cu2O. The TG curve of 2 indicates the first weight loss is about 4.99% from 30 to ca. 225 ℃, corresponding to the loss of coordinated water molecules, which is in agreement with the calculated value (5.09%). The second weight loss, 59.19%, occurs in the temperature range of 225~460 ℃, which is due to the degradation of Hmptc2- ligand (Calcd. 58.59%). The residue, 35.82%, is expected to be CdO, which is in agreement with the calculated value, 36.82%.
2.3 Fluorescence
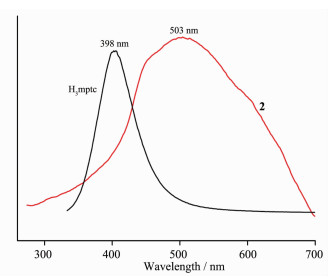
Luminescent properties of compounds containing d10 metal centers have attracted more interest due to their potential applications in chemical sensors, photochemistry, electroluminescent display, and so on[20-21]. Thus, solid-state emission spectra of the complex 2 have been investigated at room temperature (Fig. 7). It can be observed that intense blue/green fluorescent emissions occur at 503 nm (λex=340 nm) for 2. To further analyze the nature of the emission band, the photoluminescent properties of H3mptc have also been explored in this research. Free H3mptc ligand fluoresces with the emission peaks at 398 nm upon excitation at 320 nm. Obviously, complex 2 exhibits one small red shift compared to the band shown by the free ligand. This may be due to the chelating and/or bridging effect of the ligand to the metal centers, which effectively increases the rigidity of the ligand and reduces the loss of energy via a radiationless decay[22-23].
According to the literature, the Cd (Ⅱ) ions are difficult to oxidize or reduce because of the d10 configuration. As a result, the emission of the complex 2 is neither metal-to-ligand charge transfer (MLCT) nor ligand -to-metal charge transfer (LMCT) in nature[24]. On the basis of the empirical data, such emission of complex 2 may be owing to the intraligand transition.
3 Conclusions
In summary, we have successfully prepared two new coordination polymers via using H3mptc as the organic linker. Different structures of complexes 1 and 2 imply that the H3mptc ligand is of the ability to adjust its coordination modes and configuration in different reaction systems. The structural characteristics of 1 and 2 are unusual compared to other Cu or Cd complexes constructed from the tetracarboxylate or tricarboxylate analogues[25-27]. The introduction of 6-methyl group can decrease the symmetry of the organic ligand, which may result in the formation of unusual MOF. Furthermore, complexes 1 and 2 still have a protonated carboxylic acid moiety for the acidic condition, which may make low dimensional structure of complexes. Further experiments are in progress aiming at designing new coordination polymers based on H3mptc and other transition metals in order to better understand the nature of luminescence properties in these systems.
-
-
[1]
Wang F Q, Weng D F, Zheng X J, et al. Inorg. Chim. Acta, 2007, 360(6):2029-2038 doi: 10.1016/j.ica.2006.10.023
-
[2]
Yaghi O M, O'Keeffe M, Ockwig N W, et al. Nature, 2003, 423(6941):705-714 doi: 10.1038/nature01650
-
[3]
Halder G J, Kepert C J, Moubaraki B. Science, 2002, 298: 1762-1765 doi: 10.1126/science.1075948
-
[4]
Pan L, Olson D H, Ciemnolonski L R, et al. Angew. Chem., 2006, 118:632-635 doi: 10.1002/(ISSN)1521-3757
-
[5]
Liu Y Y, Ma J F, Yang J, et al. Inorg. Chem., 2007, 46:3027-3037 doi: 10.1021/ic061575l
-
[6]
Li J R, Timmons D J, Zhou H C. J. Am. Chem. Soc., 2009, 131:6368-6369 doi: 10.1021/ja901731z
-
[7]
Ke C H, Lin G R, Kuo B C, et al. Cryst. Growth Des., 2012, 12:3758-3765 doi: 10.1021/cg300559p
-
[8]
Kreno L E, Leong K, Farha O K, et al. Chem. Rev., 2012, 112:1105-1125 doi: 10.1021/cr200324t
-
[9]
Forster P M, Stock N, Cheetham A K. Angew. Chem. Int. Ed., 2005, 44:7608-7611 doi: 10.1002/(ISSN)1521-3773
-
[10]
Barnett S A, Champness N R. Coord. Chem. Rev., 2003, 246:145-168 doi: 10.1016/S0010-8545(03)00121-8
-
[11]
Chu Q, Liu G X, Huang Y Q, et al. Dalton Trans., 2007, 38: 4302-4311
-
[12]
Yang A H, Zhang H, Yin P, et al. Inorg. Chem. Commun., 2010, 13:1304-1308 doi: 10.1016/j.inoche.2010.07.022
-
[13]
Yang A H, Zhang H, Gao H L, et al. Cryst. Growth Des., 2008, 8:3354-3359 doi: 10.1021/cg800291p
-
[14]
Yan S T, Shi L X, Sun F F, et al. CrystEngComm, 2010, 12: 3437-3440 doi: 10.1039/c002573a
-
[15]
Yang A H, Zhao L H, Quan Y P, et al. Cryst. Growth Des., 2010, 10:218-223 doi: 10.1021/cg9008575
-
[16]
(a) Sheldrick G M. SHELXL-97, Program for the Solution of Crystal Structures, University of Göttingen, Göttingen, Germany, 1997.
(b) Sheldrick G M. SHELXL-97, Program for the Refinement of Crystal Structures, University of Göttingen, Göttingen, Germany, 1997. -
[17]
Yordanova S, Temiz H T, Boyaci I H, et al. J. Mol. Struct., 2015, 1101:50-56 doi: 10.1016/j.molstruc.2015.08.012
-
[18]
Chen X L, Zhang B, Hu H M, et al. Cryst. Growth Des., 2008, 8:3706-3712 doi: 10.1021/cg8003257
-
[19]
Dai J C, Wu X T, Fu Z Y, et al. Inorg. Chem., 2002, 41: 1391-1396 doi: 10.1021/ic010794y
-
[20]
Zheng S L, Yang J H, Yu X L, et al. Inorg. Chem., 2004, 43: 830-838 doi: 10.1021/ic034847i
-
[21]
Zheng S L, Zhang J P, Chen X M, et al. Chem.-Eur. J., 2003, 9:3888-3896 doi: 10.1002/(ISSN)1521-3765
-
[22]
Yersin H, Vogler A. Photochemistry and Photophysics of Coordination Compounds. Berlin:Springer-Verlag, 1987.
-
[23]
Valeur B. Molecular Fluorescence: Principles and Applications. Weinheim: WILEY-VCH Verlag GmbH, 2002.
-
[24]
Allendorf M D, Bauer C A, Bhakta R K, et al. Chem. Soc. Rev., 2009, 38:1330-1352 doi: 10.1039/b802352m
-
[25]
Yang A H, Quan Y P, Zhao L H, et al. J. Coord. Chem., 2009, 62:3306-3313 doi: 10.1080/00958970903046593
-
[26]
Wang L Y, Wang J G, Xie C Z. J. Coord. Chem., 2008, 61: 3401-3409 doi: 10.1080/00958970802051058
-
[27]
Wang H S, Ma J G, Zhai B, et al. J. Mol. Struct., 2007, 829: 1-7 doi: 10.1016/j.molstruc.2006.05.049
-
[1]
-
Figure 3 (a) The coordination environment of the Cd (Ⅱ) ion in 2 with 50% thermal ellipsoid probability; (b) The coordination mode of Hmptc2- in 2; (c) View of 1D Cd-O grid chain; (d) 2D grid structure of 2; (e) 3D packing diagram of 2 connected by hydrogen bonds
In (a): Symmetry codes: ⅰ2-x, 2-y, -z; ⅱ2.5-x, 0.5+y, 0.5-z; ⅲx, y+1, z; In (d): the hydrogen atoms are omitted for clarity
Table 1. Crystal data and structure refinement information for complexes 1~2
Complex 1 2 Empirical formula C9H11CuNO9 C9H7CdNO7 Formula weight 340.73 353.57 Crystal system Orthorhombic Monoclinic Space group Pccn P21/n a/nm 1.605 8(3) 1.162 9(10) b/nm 0.979 4(2) 0.694 5(6) c/nm 1.460 1(3) 1.320 1(10) β/(°) 101.615(13) V/nm3 2.297 7(8) 1.044(3) Z 8 4 Dc/(Mg·m-3) 1.97 2.249 μ/mm-1 1.951 2.12 F(000) 1 384 688 θrange/(°) 2.44~25.01 2.13~24.99 Reflections collected, unique 12 865, 1 991 8 366, 1 836 (Rint=0.113 0) (Rint=0.046 4) Goodness of fit on F2 1.244 1.141 Final R* indices [I > 2σ(I)] R1=0.084 9, wR2=0.161 9 R1=0.060 3, wR2=0.154 9 R*indices (all data) R1=0.091 4, wR2=0.165 0 R1=0.064 3, wR2=0.156 9 Parameters 187 171 Observed reflections [I > 2σ(I)] 1 991 1 836 Largest diff. peak and hole/(e·nm-3) 737 and -690 3 172 and -2 200 *R1=Σ‖Fo|-|Fc|/|Fo|;wR2=[Σw(Fo2-Fc2)2]/Σw(Fo2)2]1/2 Table 2. Selected bond lengths (nm) and angles (°) for complexes 1~2
Complex 1 Cu (1)-O (1) 0.193 4(5) O (3)-Cu (1)ⅱ 0.232 0(5) Cu (1)-O (4)ⅰ 0.196 2(5) Cu (1)-O (7) 0.197 7(5) Cu (1)-N (1) 0.205 1(6) O (7)-Cu (1)-N (1) 168.9 (2) O (1)-Cu (1)-O (4)ⅰ 160.1(2) O (4)ⅰ-Cu (1)-O (3)ⅱ 92.44(9) O (1)-Cu (1)-O (7) 86.5(2) O (1)-Cu (1)-O (3)ⅱ 106.58(19) O (4)ⅰ-Cu (1)-O (7) 89.3(2) O (7)-Cu (1)-O (3)ⅱ 85.02(19) O (1)-Cu (1)-N (1) 82.8(2) N (1)-Cu (1)-O (3)ⅱ 95.22(19) O (4)ⅰ-Cu (1)-N (1) 101.8 (2) Complex 2 Cd (1)-O (7) 0.226 0(8) Cd (1)-O (5)ⅰ 0.229 8(7) Cd (1)-O (1)ⅱ 0.230 6(7) Cd (1)-N (1)ⅰ 0.231 1(8) Cd (1)-O (1) 0.234 6(7) Cd (1)-O (2)ⅲ 0.248 6(8) Cd (1)-O (2)ⅱ 0.263 4(8) O (7)-Cd (1)-O (5)ⅰ 93.1(3) O (7)-Cd (1)-O (1)ⅱ 84.5(3) O (5)ⅰ-Cd (1)-O (1)ⅱ 145.6(2) O (7)-Cd (1)-N (1)ⅰ 165.6(3) O (5)ⅰ-Cd (1)-N (1)ⅰ 73.1(3) O (1)ⅱ-Cd (1)-N (1)ⅰ 104.5(3) O (7)-Cd (1)-O (1) 85.7(3) O (5)ⅰ-Cd (1)-O (1) 95.4(2) O (1)ⅱ-Cd (1)-O (1) 118.55(17) N (1)ⅰ-Cd (1)-O (1) 99.3(3) O (7)-Cd (1)-O (2)ⅲ 94.2(3) O (5)ⅰ-Cd (1)-O (2)ⅲ 76.2(2) O (1)ⅱ-Cd (1)-O (2)ⅲ 69.7(2) N (1)ⅰ-Cd (1)-O (2)ⅲ 78.9(3) O (1)-Cd (1)-O (2)ⅲ 171.6(2) O (7)-Cd (1)-O (2)ⅱ 81.5(3) O (5)ⅰ-Cd (1)-O (2)ⅱ 161.4(2) O (1)ⅱ-Cd (1)-O (2)ⅱ 52.0(2) N (1)ⅰ-Cd (1)-O (2)ⅱ 112.9(3) O (1)-Cd (1)-O (2)ⅱ 66.6(2) O (2)ⅲ-Cd (1)-O (2)ⅱ 121.7(2) Symmetry code: ⅰx, 1.5-y, z+0.5;ⅱ1-x, 2-y, -z for 1; ⅰ2-x, 2-y, -z; ⅱ2.5-x, 0.5+y, 0.5-z; ⅲx, y+1, z for 2 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 1
- 文章访问数: 398
- HTML全文浏览量: 51


 下载:
下载:




 下载:
下载:

