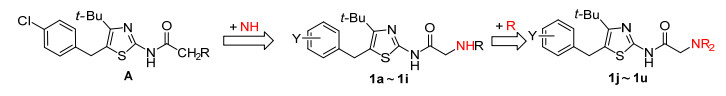
 图 图式 1
目标化合物1的设计思路
Figure 图式 1.
Design route of title compounds 1
图 图式 1
目标化合物1的设计思路
Figure 图式 1.
Design route of title compounds 1

Citation: Tang Yuting, Ding Na, Wu Zhilin, Ye Jiao, Shen Kun, Hu Aixi. Synthesis and Antitumor Activity of N-[4-(t-Butyl)-5-benzylthiazol-2-yl]amininoacetamides[J]. Chinese Journal of Organic Chemistry, 2017, 37(3): 675-682. doi: 10.6023/cjoc201610018

N-(4-叔丁基-5-苄基噻唑-2-基) 氨基乙酰胺的合成与抗肿瘤活性
English
Synthesis and Antitumor Activity of N-[4-(t-Butyl)-5-benzylthiazol-2-yl]amininoacetamides
-
Key words:
- thiazoleacetamide
- / morpholine
- / synthesis
- / antitumor activity
-
噻唑类化合物因具有抗癌[1~8]、抗菌[9]、抗病毒[10]、抗炎[11]、抗痉挛[12]、降血糖[13]和降血压[14]等广泛生物活性而备受国内外学者关注.近年来, 以2-氨基噻唑为母体, 通过酰化引入其他潜在活性的基团, 以期获得具有良好抗肿瘤活性的新化合物的思路也愈趋成为研究热点. Rostom等[15]研究了以2-氨基-4-甲基噻唑-5-甲酸乙酯为关键中间体, 分别经芳香酰化、磺酰化以及氯乙酰化引入诸如吗啉和N-甲基哌嗪等活性基团, 合成了一系列噻唑酰胺衍生物, 部分化合物经体外抗癌活性测试显示良好活性. Gurdal等[16]研究了以2-氨基-6-甲基苯并噻唑为原料, 经氯乙酰化引入哌嗪类基团得到的化合物对MCF-7有良好抑制活性.崔建国等[17]研究了以去氢表雄酮为原料, 通过微波一步合成法及常规2步合成方法合成一系列去氢表雄酮噻唑衍生物, 部分化合物经体外抗癌活性测试显示良好活性.胡艾希等[18]研究了N-(4-叔丁基-5-苄基噻唑-2-基) 酰胺和4-叔丁基-5-(2-硝基乙基)-2-酰氨基噻唑的合成与抗癌药活性.
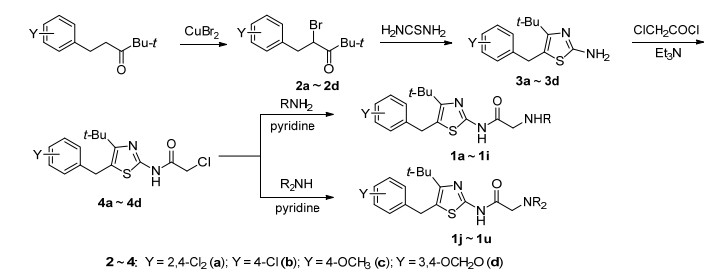
前文[19]报道了N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]脂肪酰胺 (A) 具有抗癌活性, 但活性不强.为了寻找到高活性的抗癌化合物, 本文采用衍生法, 在化合物A酰基的α-位插入氨基, 设计得到N-(4-叔丁基-5-苄基噻唑-2-基) 氨基乙酰胺 (1a~1u), 设计思路见Scheme 1.以4, 4-二甲基-1-芳基-3-戊酮为原料, 经4-叔丁基-5-苄基-2-氨基噻唑 (3), 再经氯乙酰化和取代反应合成21个N-(4-叔丁基-5-苄基噻唑-2-基) 氨基乙酰胺 (1), 并进行体外抗肿瘤活性筛选, 目标化合物的合成路线见Scheme 2.
1 结果与讨论
1.1 目标化合物的合成
化合物3[19]在二氯甲烷作溶剂, 三乙胺作缚酸剂, 冰浴下与氯乙酰氯反应制得N-(4-叔丁基-5-苄基噻唑-2-基)-2-氯乙酰胺 (4).实验发现, 不加催化剂, 反应时间较长; 氯乙酰氯滴加越快, 二酰化副产物越多.因此, 该反应加催化量4-二甲氨基吡啶 (DMAP), 氯乙酰氯缓慢滴加, 冰浴反应4 h, 得中间体4a, 4b, 4c和4d, 收率分别为85.6%, 92.7%, 44.6%和82%.
化合物4分别与一级胺、二级胺、吗啉和哌啶, 在四氢呋喃中, 吡啶或三乙胺作缚酸剂, 室温下反应, 得到21个N-(4-叔丁基-5-苄基噻唑-2-基) 氨基乙酰胺衍生物1a~1u.
1.2 抗肿瘤活性初步评价
以5-氟尿嘧啶为阳性对照, 化合物1a~1u对A549(非小细胞肺癌细胞株)、Hela (宫颈癌细胞株) 和MCF-7(乳腺癌细胞株) 分别进行体外抗肿瘤活性测试; 抗肿瘤活性结果列入表 1.
Compd. Y R/R2 IC50/(μmol•L-1) A549 Hela MCF-7 1a 2, 4-Cl2 CH3 55.6±3.4 38.2±5.3 36.2±2.2 1b 2, 4-Cl2 Et 45.6±2.0 46.5±8.3 28.9±0.5 1c 2, 4-Cl2 n-Bu 89.3±1.9 49.7±4.2 25.2±1.2 1d 4-Cl CH3 41.2±7.2 51.0±7.4 35.0±2.6 1e 4-Cl Et 19.2±8.4 28.0±0.9 30.0±1.1 1f 4-Cl n-Bu 18.1±5.8 47.6±18.1 11.8±2.0 1g 4-OCH3 CH3 45.4±10.0 35.7±4.7 33.6±10.0 1h 4-OCH3 Et 55.9±6.5 42.1±6.2 52.3±8.9 1i 4-OCH3 n-Bu 26.4±3.4 12.9±1.3 32.2±1.2 1j 2, 4-Cl2 Et2 192.6±8.2 397.2±61.1 205.4±0.8 1k 2, 4-Cl2 n-Bu2 NA NA 456.2±14.8 1l 4-Cl Et2 261.5±114.4 274.9±10.2 139.4±26.8 1m 4-OCH3 Et2 NA 196.5±0.8 NA 1n 2, 4-Cl2 (CH2)5 333.8±68.1 NA NA 1o 4-Cl (CH2)5 379.5±170.1 136.7±42.9 NA 1p 4-OCH3 (CH2)5 NA 243.6±26.4 484.8±47.1 1q 2, 4-Cl2 O (CH2CH2)2 188.5±6.1 NA NA 1r 4-Cl O (CH2CH2)2 343.2±93.4 51.5±9.8 176.6±13.2 1s 4-OCH3 O (CH2CH2)2 441.7±7.6 265.1±45.3 379.5±32.9 1t 3, 4-OCH2O O (CH2CH2)2 66.6±6.8 6.4±2.2 NA 1u 3, 4-OCH2O (CH2)5 256.9±47.4 31.3±5.4 NA 5-FU[20] 26.0±6.0 56.5±3.4 79.3±5.1 aNA: IC50>500 μmol/L. 表 1 化合物1a~1u的抗肿瘤活性a
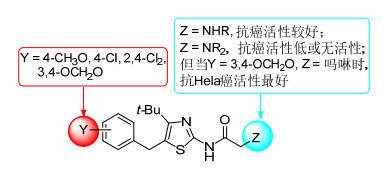
Table 1. Data of antitumor activity of compounds 1a~1u由表 1可知, 对A549细胞的抑制活性, 化合物1e和1f的活性优于阳性对照物5-FU (IC50=26.0±6.0 μmol/L), 其余化合物活性较低或没有活性.对Hela细胞的抑制活性, N-(4-叔丁基-5-苄基噻唑-2-基) 单烷氨基乙酰胺 (1a~1i)、化合物1r、1t和1u的活性优于阳性对照物5-FU [IC50=(56.5±3.4) μmol/L], 其中N-[4-叔丁基-5-(胡椒基) 噻唑-2-基]吗啉基乙酰胺 (1t) 的活性最好 (IC50=6.4±2.2 μmol/L).其余化合物活性较低或没有活性.对MCF-7细胞的抑制活性, N-(4-叔丁基-5-苄基噻唑-2-基) 单烷氨基乙酰胺 (1a~1i) 的活性优于阳性对照物5-FU (IC50=79.3±5.1 μmol/L), 其余化合物对MCF-7无抑制活性. N-(4-叔丁基-5-苄基噻唑-2-基) 二烷氨基乙酰胺 (1j~1s) 对三种癌细胞的抑制活性较低或无活性.化合物1的构效关系见图 1.
1.3 优选化合物抗肿瘤活性评价
1.3.1 AO-EB双染实验
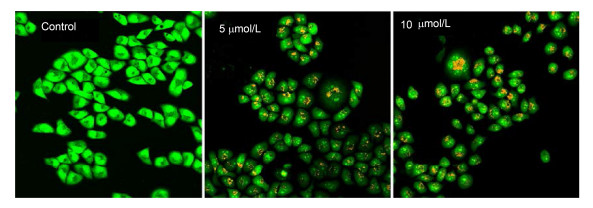
AO-EB双染实验的原理:吖啶橙 (AO) 可以进入细胞膜完整的细胞, 与细胞核内DNA形成发绿色荧光的复合物; 溴乙锭 (EB) 只能透过胞膜受损的细胞, 嵌入胞核DNA发出橘红色荧光.优选化合物1t对Hela细胞AO/EB双染结果见图 2.
从图 2可看出, 化合物1t可显著诱导Hela细胞凋亡.较阴性对照组而言, 化合物1t以5 μmol/L作用Hela细胞24 h时, 细胞形态变为圆状, 核染色质出现固缩, 细胞开始出现凋亡状态; 随着化合物1t作用浓度增大到10 μmol/L时, 细胞核染色质固缩明显, 核质橘红色染色范围明显增多, 且荧光增强, 细胞显现出晚期凋亡状态.
1.3.2 细胞周期实验
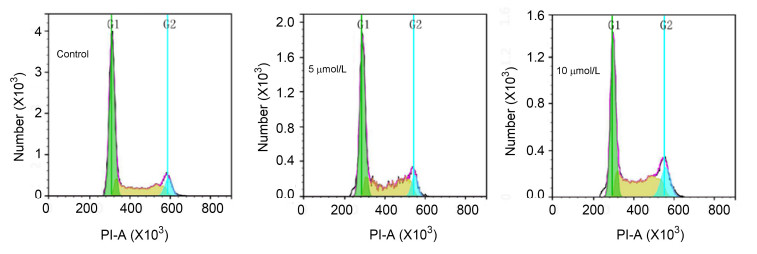
流式细胞仪是根据细胞中DNA的含量分析细胞所处周期.碘化丙啶 (PI) 可穿过细胞膜与细胞内DNA结合产生荧光, 而且DNA的含量与荧光的强度成正相关.优选化合物1t对Hela细胞有丝分裂周期影响结果见图 3和图 4.
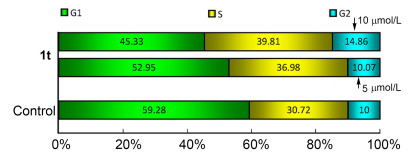
由图 3和图 4分析比较可看出, 化合物1t可阻滞宫颈癌细胞 (Hela) 的有丝分裂.较阴性对照组而言, 当化合物1t以5 μmol/L浓度作用Hela细胞24 h时, S期细胞比例由30.72%上升至36.98%, G2期细胞比例由10.00%上升到10.07%, 而G1期细胞比例由59.28%下降为52.95%, 说明化合物1t可阻滞Hela细胞有丝分裂的S期; 当化合物1t作用浓度增大至10 μmol/L时, 对比5 μmol/L, S期细胞比例由36.98%上升为39.81%, 说明随着浓度增大, 化合物1t对Hela细胞S期阻滞作用增强.
2 结论
设计合成21个N-(4-叔丁基-5-苄基噻唑-2-基) 氨基乙酰胺, 经1H NMR、13C NMR和元素分析确证其结构.抗肿瘤测试结果表明, 化合物1对3种肿瘤细胞有较好的抑制活性:化合物1e和1f对A549细胞的抑制活性较高, 化合物1对Hela细胞表现出优良的抑制活性. N-(4-叔丁基-5-苄基噻唑-2-基) 单烷氨基乙酰胺 (1a~1i)、1r, 1t和1u的活性优于阳性对照物5-FU, 其中N-[4-叔丁基-5-(胡椒基) 噻唑-2-基]吗啉基乙酰胺 (1t) 的活性最好.化合物1对MCF-7细胞抑制活性好, N-(4-叔丁基-5-苄基噻唑-2-基) 单烷氨基乙酰胺 (1a~1i) 的活性优于阳性对照物5-FU.选择对Hela细胞抑制活性最好的化合物1t做了AO/EB双染和细胞周期实验, 化合物1t可显著诱导肿瘤细胞凋亡, 抑制Hela细胞有丝分裂在S期, 具有进一步开发研究的价值.
3 实验部分
3.1 仪器与试剂
ZF-2型三用紫外仪 (上海安亭电子仪器厂); 薄层层析硅胶板 (烟台江友硅胶开发有限公司); R-1002N型旋转蒸发仪、SHB-Ⅲ型循环水式多用真空泵 (郑州长城科工贸有限公司); RY-1G型熔点仪 (天津天光光学仪器有限公司); VARIAN INOVA-400核磁共振仪 (美国Varian公司), 1H NMR核磁共振频率为399.970 MHz, 13C NMR核磁共振频率为100 MHz, TMS为内标; 溶剂和试剂均为市售分析纯或化学纯; VARIO EL Ⅲ元素分析仪 (德国).化合物3的合成见文献[19].
3.2 化合物的合成
3.3 抗肿瘤活性测试
测试肿瘤细胞株:非小细胞肺癌细胞株A549、宫颈癌细胞株Hela和乳腺癌细胞株MCF-7(中南大学湘雅医学院细胞库提供).测试方法及IC50值计算方法见文献[21].
辅助材料 (Supporting Information)中间体4a~4c和化合物1a~1u的1H NMR、13C NMR谱图.这些材料可以免费从本刊网站 (http://sioc-journal.cn/) 上下载.
3.2.4 化合物1j~1m的合成
1.0 mmol化合物4a~4c与2.0 mmol二级胺和1.0 mmol吡啶, 在5 mL四氢呋喃中, 室温反应10 h, 反应毕, 减压脱溶, 加二氯甲烷, 饱和食盐水洗, 无水硫酸钠干燥, 脱溶, 柱层析, 乙醇重结晶, 抽滤, 乙醇洗, 干燥得化合物1j~1m.
N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]-2-二乙氨基乙酰胺 (1j):淡黄色固体, 收率76.7%. m.p. 109~111 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.10 (t, J=7.2 Hz, 6H, 2×CH3), 1.34 (s, 9H, 3×CH3), 2.66 (q, J=7.2 Hz, 4H, 2×CH2), 3.22 (s, 2H, COCH2), 4.26 (s, 2H, ArCH2), 7.02 (d, J=8.4 Hz, 1H, C6H3-6-H), 7.16 (dd, J=8.4, 2.0 Hz, 1H, C6H3-5-H), 7.39 (d, J=2.0 Hz, 1H, C6H3-3-H). Anal. calcd for C20H27Cl2N3OS: C 56.07, H 6.35, Cl 16.55, N 9.81, S 7.48; found C 56.01, H 6.23, Cl 16.45, N 9.75, S 7.39.
N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]-2-二丁氨基乙酰胺 (1k):淡黄色固体, 收率86.8%, m.p. 84~86 ℃; 1H NMR (400 MHz, CDCl3) δ: 0.93 (t, J=8.0 Hz, 6H, 2×CH3), 1.34 (s, 9H, 3×CH3), 1.35~1.41 (m, 4H, 2×CH2CH3), 1.44~1.49 (m, 4H, 2×CH2), 2.54 (t, J=7.2 Hz, 4H, 2×NCH2), 3.22 (s, 2H, COCH2), 4.26 (s, 2H, ArCH2), 7.03 (d, J=8.4 Hz, 1H, C6H3-6-H), 7.16 (dd, J=8.4, 2.0 Hz, 1H, C6H3-5-H), 7.39 (d, J=2.0 Hz, 1H, C6H3-3-H). Anal. calcd for C24H35Cl2N3OS: C 59.49, H 7.28, N 8.67, S 6.62; found C 59.35, H 7.15, N 8.61, S 6.58.
N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]-2-二乙氨基乙酰胺 (1l):白色固体, 收率33.0%. m.p. 101~103 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.09 (t, J=7.2 Hz, 6H, 2×CH3), 1.35 (s, 9H, 3×CH3), 2.66(q, J=7.2 Hz, 4H, 2×CH2), 3.22 (s, 2H, COCH2), 4.21 (s, 2H, ArCH2), 7.12 (d, J=8.4 Hz, 2H, C6H4-2, 6-H), 7.25 (d, J=8.4 Hz, 2H, C6H4 3, 5-H). Anal. calcd for C20H28ClN3OS: C 60.97, H 7.16, N 10.67, S 8.14; found C 60.90, H 7.11, N 10.60, S 8.09.
N-[4-叔丁基-5-(4-甲氧基苄基) 噻唑-2-基]-2-二乙氨基乙酰胺 (1m):白色固体, 收率14.4%. m.p. 88~90 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.08 (t, J=6.8 Hz, 6H, 2×CH3), 1.37 (s, 9H, 3×CH3), 2.65 (q, J=6.8 Hz, 4H, 2×CH2), 3.21 (s, 2H, COCH2), 3.78 (s, 3H, OCH3), 4.18 (s, 2H, ArCH2), 6.82 (d, J=8.8 Hz, 2H, C6H4-3, 5-H), 7.11(d, J=8.8 Hz, 2H, C6H4-2, 6-H). Anal. calcd for C21H31N3O2S: C 64.75, H 8.02, N 10.79, S 8.23; found C 64.70, H 7.92, N 10.71, S 8.18.
3.2.1 中间体4a~4d的合成
20.0 mmol化合物3a、20.0 mmol三乙胺和催化量的DMAP溶于10 mL二氯甲烷中, 冰浴下缓慢滴加20 mmol氯乙酰氯, 反应0.5 h.反应毕, 饱和碳酸氢钠溶液洗至中性, 无水硫酸钠干燥, 减压脱溶, 柱层析[V(石油醚):V(乙酸乙酯)=16:1]得白色固体N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]-2-氯乙酰胺 (4a), 收率85.6%. m.p. 123~125 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.35 (s, 9H, 3×CH3), 4.23 (s, 2H, COCH2), 4.27 (s, 2H, CH2), 7.01 (d, J=8.4 Hz, 1H, C6H3-6-H), 7.17 (dd, J=8.4, J=2.0 Hz, 1H, C6H3-5-H), 7.40 (d, J=2.0 Hz, 1H, C6H3-3-H); 13C NMR (100 MHz, CDCl3) δ: 30.38, 30.77, 36.07, 42.12, 122.31, 127.44, 129.43, 130.93, 133.31, 134.42, 136.69, 152.11, 154.68, 163.79. Anal. calcd for C16H17Cl3N2OS: C 49.06, H 4.37, N 7.15, S 8.19; found C 49.26, H 4.56, N 7.24, S 8.11.
4b~4d的合成方法同4a.
N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]-2-氯乙酰胺 (4b):反应0.5 h, 白色固体, 收率92.7%. m.p. 153~155 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.35 (s, 9H, 3×CH3), 4.21 (s, 2H, COCH2), 4.22 (s, 2H, CH2), 7.11 (d, J=8.4 Hz, 2H, C6H4-2, 6-H), 7.26 (d, J=8.4 Hz, 2H, C6H4-3, 5-H); 13C NMR (100 MHz, CDCl3) δ: 30.98, 32.25, 35.93, 42.11, 124.05, 128.83, 129.67, 132.47, 138.89, 152.11, 154.15, 163.89. Anal. calcd for C16H18Cl2N2OS: C 53.78, H 5.08, N 7.84, S 8.97; found C 53.90, H 5.06, N 7.71, S 8.96.
N-[4-叔丁基-5-(4-甲氧基苄基) 噻唑-2-基]-2-氯乙酰胺 (4c):反应0.5 h, 白色固体, 收率44.6%. m.p. 103~105 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.37 (s, 9H, 3×CH3), 3.79 (s, 3H, OCH3), 4.18 (s, 2H, COCH2), 4.20 (s, 2H, CH2), 6.82 (d, J=8.4 Hz, 2H, C6H4-3, 5-H), 7.11 (d, J=8.4 Hz, 2H, C6H4-2, 6-H); 13C NMR (100 MHz, CDCl3) δ: 31.03, 32.25, 35.87, 42.15, 55.41, 114.11, 125.89, 129.46, 132.54, 151.95, 153.53, 158.40, 163.69. Anal. calcd for C17H21ClN2O2S: C 57.86, H 6.00, N 7.94, S 9.09; found C 58.12, H 6.24, N 7.80, S 9.13.
N-[4-叔丁基-5-(胡椒基) 噻唑-2-基]-2-氯乙酰胺 (4d):反应0.5 h, 白色固体, 收率82%. m.p. 127~129 ℃, 1H NMR (400 MHz, CDCl3) δ:1.38 (s, 9H, 3×CH3), 4.15 (s, 2H, OCH2), 4.22 (s, 2H, ClCH2), 5.94 (s, 2H, CH2), 6.64 (d, J=8.0 Hz, 1H, C6H3), 6.66 (s, 1H, C6H3), 6.74 (d, J=8.0 Hz, 1H, C6H3); 13C NMR (100 MHz, CDCl3) δ: 30.80, 32.54, 35.65, 41.91, 100.89, 108.18, 108.75, 121.20, 125.27, 134.03, 146.17, 147.74, 151.86, 153.48, 163.58. Anal. calcd for C18H21ClN2O2S: C 59.25, H 5.80, N 7.68, S 8.79; found C 59.39, H 6.01, N 7.52, S 8.72.
3.2.2 化合物1a~1i的合成
1.0 mmol化合物4a~4c、2.0 mmol一级胺和1.0 mmol吡啶溶于5 mL四氢呋喃, 室温反应8~14 h, 减压脱溶, 加二氯甲烷, 饱和食盐水洗, 无水硫酸钠干燥, 脱溶, 加石油醚析出固体, 抽滤, 干燥得淡黄色固体1a~1i.
N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]-2-甲氨基乙酰胺 (1a):反应8 h, 淡黄色固体, 收率88.1%. m.p. 136~137 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.33 (s, 9H, 3×CH3), 2.53 (s, 3H, CH3), 3.47 (s, 2H, COCH2), 4.26 (s, 2H, CH2), 7.02 (d, J=8.4 Hz, 1H, C6H3-6-H), 7.16 (dd, J=8.4, 2.0 Hz, 1H, C6H3-5-H), 7.39 (d, J=2.0 Hz, 1H, C6H3-3-H); 13C NMR (100 MHz, CDCl3)δ: 30.29, 30.82, 35.96, 37.00, 54.25, 120.99, 127.38, 129.33, 130.95, 133.13, 134.36, 136.90, 153.31, 154.20, 169.94. Anal. calcd for C17H21Cl2N3OS: C 52.85, H 5.48, N 10.88, S 8.30; found C 52.65, H 5.46, N 10.73, S 8.24.
N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]-2-乙氨基乙酰胺 (1b):反应14 h, 淡黄色固体, 收率75.0%. m.p. 115~117 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.18 (t, J=7.2 Hz, 3H, CH3), 1.34 (s, 9H, 3×CH3), 2.75 (q, J=7.2 Hz, 2H, CH2), 3.46 (s, 2H, COCH2), 4.26 (s, 2H, ArCH2), 7.02 (d, J=8.4 Hz, 1H, C6H3-6-H), 7.16 (dd, J=8.4, 2.0 Hz, 1H, C6H3-5-H), 7.39 (d, J=2.0 Hz, 1H, C6H3-3-H); 13C NMR (100 MHz, CDCl3) δ: 15.26, 30.27, 30.81, 35.97, 44.79, 51.96, 120.92, 127.35, 129.29, 130.92, 133.08, 134.33, 136.90, 153.21, 154.25, 170.22. Anal. calcd for C18H23Cl2N3OS: C 54.00, H 5.79, N 10.50, S 8.01; found C 53.97, H 5.68, N 10.45, S 8.00.
N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]-2-丁氨基乙酰胺 (1c):反应14 h, 淡黄色固体, 收率46.7%, m.p. 95~97 ℃; 1H NMR (400 MHz, CDCl3) δ: 0.94 (t, J=7.2 Hz, 3H, CH3), 1.34 (s, 9H, 3×CH3), 1.38~1.41 (m, 2H, CH2CH3), 1.52~1.59 (m, 2H, CH2C2H5), 2.72 (t, J=7.2 Hz, 2H, NHCH2)3.51 (s, 2H, COCH2), 4.25 (s, 2H, CH2), 7.02 (d, J=8.4 Hz, 1H, C6H3-6-H), 7.16 (dd, J=8.4, 2.0 Hz, 1H, C6H3-5-H), 7.39 (d, J=2.0 Hz, 1H, C6H3-3-H); 13C NMR (100 MHz, CDCl3) δ: 14.11, 20.41, 30.31, 30.83, 32.14, 36.02, 50.27, 52.17, 120.93, 127.37, 129.31, 130.95, 133.10, 134.35, 136.96, 153.03, 154.39, 170.12. Anal. calcd for C20H27Cl2N3OS: C 56.07, H 6.35, N 9.81, S 7.48; found C 56.00, H 6.23, N 9.72, S 7.45.
N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]-2-甲氨基乙酰胺 (1d):反应8 h, 淡黄色固体, 收率68.2%. m.p. 119~121 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.34 (s, 9H, 3×CH3), 2.51 (s, 3H, CH3), 3.45 (s, 2H, COCH2), 4.21 (s, 2H, CH2), 7.11 (d, J=8.4 Hz, 2H, C6H4-2, 6-H), 7.25 (d, J=8.4 Hz, 2H, C6H4-3, 5-H); 13C NMR (100 MHz, CDCl3) δ: 31.06, 32.24, 35.86, 36.91, 54.26, 122.83, 128.79, 129.70, 132.35, 139.17, 153.34, 153.73, 169.97. Anal. calcd for C17H22ClN3OS: C 58.02, H 6.30, N 11.94, S 9.11; found C 57.92, H 6.23, N 11.83, S 9.07.
N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]-2-乙氨基乙酰胺 (1e):反应14 h, 淡黄色固体, 收率79.2%. m.p. 118~120 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.17 (t, J=7.2 Hz, 3H, CH3), 1.35 (s, 9H, 3×CH3), 2.74 (q, J=7.2 Hz, 2H, CH2), 3.45 (s, 2H, COCH2), 4.21 (s, 2H, ArCH2), 7.11 (d, J=8.4 Hz, 2H, C6H4-2, 6-H), 7.25 (d, J=8.4 Hz, 2H, C6H4-3, 5-H); 13C NMR (100 MHz, CDCl3) δ: 15.40, 31.10, 32.29, 35.94, 44.85, 52.04, 122.83, 128.80, 129.72, 132.36, 139.24, 152.95, 153.91, 170.11. Anal. calcd for C18H24ClN3OS: C 59.08, H 6.61, N 11.48, S 8.76; found C 59.00, H 6.56, N 11.39, S 8.72.
N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]-2-丁氨基乙酰胺 (1f):反应14 h, 白色固体, 收率59.6%. m.p. 109~111 ℃; 1H NMR (400 MHz, CDCl3) δ: 0.94 (t, J=7.2 Hz, 3H, CH3), 1.35 (s, 9H, 3×CH3), 1.36~1.41 (m, 2H, CH2CH3), 1.49~1.54 (m, 2H, CH2C2H5), 2.68 (t, J=7.2 Hz, 2H, NHCH2), 3.45 (s, 2H, COCH2), 4.20 (s, 2H, ArCH2), 7.11 (d, J=8.0 Hz, 2H, C6H4-2, 6-H), 7.25 (d, J=8.0 Hz, 2H, C6H4-3, 5-H); 13C NMR (100 MHz, CDCl3) δ: 14.09, 20.40, 31.09, 32.14, 32.30, 35.95, 50.26, 52.19, 122.82, 128.81, 129.73, 132.36, 139.25, 152.92, 153.92, 170.02. Anal. calcd for C20H28ClN3OS: C 60.97, H 7.16, N 10.67, S 8.14; found C 60.87, H 7.09, N 10.52, S 8.06.
N-[4-叔丁基-5-(4-甲氧基苄基) 噻唑-2-基]-2-甲氨基乙酰胺 (1g):反应8 h, 乳白色固体, 收率89.3%. m.p. 145~147 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.36 (s, 9H, 3×CH3), 2.50 (s, 3H, CH3), 3.43 (d, J=4.0 Hz, 2H, COCH2), 3.78 (s, 3H, OCH3), 4.17 (s, 2H, CH2), 6.82 (d, J=8.8 Hz, 2H, C6H4-3, 5-H), 7.11 (d, J=8.8 Hz, 2H, C6H4-2, 6-H); 13C NMR (100 MHz, CDCl3)δ: 31.08, 32.20, 35.79, 36.93, 54.28, 55.41, 114.07, 124.57, 129.43, 132.80, 153.09, 153.15, 158.33, 169.80. Anal. calcd for C18H25-N3O2S: C 62.22, H 7.25, N 12.09, S 9.23; found C 62.13, H 7.16, N 12.01, S 9.15.
N-[4-叔丁基-5-(4-甲氧基苄基) 噻唑-2-基]-2-乙氨基乙酰胺 (1h):反应14 h, 淡黄色固体, 收率74.8%. m.p. 125~127 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.16 (t, J=7.2 Hz, 3H, CH3), 1.36 (s, 9H, 3×CH3), 2.73 (q, J=7.2 Hz, 2H, CH2), 3.44 (s, 2H, COCH2), 3.78 (s, 3H, OCH3), 4.17 (s, 2H, ArCH2), 6.82 (d, J=8.8 Hz, 2H, C6H4-3, 5-H), 7.11 (d, J=8.8 Hz, 2H, C6H4-2, 6-H); 13C NMR (100 MHz, CDCl3) δ: 15.30, 31.09, 32.19, 35.81, 44.79, 52.04, 55.40, 114.05, 124.53, 129.42, 132.82, 152.89, 153.22, 158.30, 170.06. Anal. calcd for C19H27N3O2S: C 63.13, H 7.53, N 11.62, S 8.87; found C 63.02, H 7.45, N 11.53, S 8.73.
N-[4-叔丁基-5-(4-甲氧基苄基) 噻唑-2-基]-2-丁氨基乙酰胺 (1i):反应14 h, 白色固体, 收率21.4%. m.p. 73~75 ℃; 1H NMR (400 MHz, CDCl3) δ: 0.93 (t, J=7.2 Hz, 3H, CH3), 1.36 (s, 9H, 3×CH3), 1.35~1.40 (m, 2H, CH2CH3), 1.49~1.54 (m, 2H, CH2C2H5), 2.68 (t, J=7.2 Hz, NHCH2), 3.45 (s, 2H, COCH2), 3.78 (s, 3H, OCH3), 4.17 (s, 2H, ArCH2), 6.82 (d, J=8.4 Hz, 2H, C6H4-3, 5-H), 7.10 (d, J=8.4 Hz, 2H, C6H4-2, 6-H); 13C NMR (100 MHz, CDCl3) δ: 14.09, 20.41, 31.10, 32.12, 32.22, 35.85, 50.22, 52.21, 55.42, 114.06, 124.53, 129.43, 132.84, 152.89, 153.21, 158.31, 169.96. Anal. calcd for C21H31N3O2S: C 64.75, H 8.02, N 10.79, S 8.23; found C 64.68, H 7.96, N 10.70, S 8.12.
3.2.5 化合物1n~1u的合成
1.0 mmol化合物4a~4d与2.0 mmol哌啶/吗啉和1.0 mmol吡啶或三乙胺, 在5 mL四氢呋喃中, 室温反应10 h, 反应结束后, 减压脱溶, 加二氯甲烷, 饱和食盐水洗, 无水硫酸钠干燥, 脱溶, 加石油醚析出固体, 抽滤, 石油醚洗, 干燥得化合物1n~1u.
N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]哌啶基乙酰胺 (1n):白色固体, 收率68.2%. m.p. 184~185 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.34 (s, 9H, 3×CH3), 1.50 (s, 2H, 哌啶环4-H), 1.70 (s, 4H, 哌啶环3, 5-H), 2.55 (s, 4H, 哌啶环2, 6-H), 3.16 (s, 2H, COCH2), 4.26 (s, 2H, CH2), 7.02 (d, J=8.4 Hz, 1H, C6H3-6-H), 7.16 (dd, J=8.4, 2.4 Hz, 1H, C6H3-5-H), 7.39 (d, J=2.4 Hz, 1H, C6H3-3-H). Anal. calcd for C21H27Cl2N3OS: C 55.38, H 6.20, N 12.30, S 7.04; found C 55.32, H 6.17, N 12.26, S 7.01.
N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]哌啶基乙酰胺 (1o):淡黄色固体, 收率39.4%. m.p. 143~145 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.36 (s, 9H, 3×CH3), 1.49 (s, 2H, 哌啶环4-H), 1.68 (s, 4H, 哌啶环3, 5-H), 2.55 (s, 4H, 哌啶环2, 6-H), 3.16 (s, 2H, COCH2), 4.21 (s, 2H, CH2), 7.11 (d, J=8.0 Hz, 2H, C6H4-2, 6-H), 7.25 (d, J=8.0 Hz, 2H, C6H4-3, 5-H). Anal. calcd for C21H28ClN3OS: C 59.91, H 6.94, N 13.31, S 7.62; found C 59.82, H 6.87, N 13.26, S 7.56.
N-[4-叔丁基-5-(4-甲氧基苄基) 噻唑-2-基]哌啶基乙酰胺 (1p):淡黄色固体, 收率82.6%. m.p. 130~132 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.38 (s, 9H, 3×CH3), 1.49 (s, 2H, 哌啶环4-H), 1.69 (s, 4H, 哌啶环3, 5-H), 2.56 (s, 4H, 哌啶环2, 6-H), 3.17 (s, 2H, COCH2), 3.78 (s, 3H, OCH3), 4.17 (s, 2H, CH2), 6.82 (d, J=8.4 Hz, 2H, C6H4-3, 5-H), 7.10 (d, J=8.4 Hz, 2H, C6H4-2, 6-H). Anal. calcd for C22H31N3O2S: C 63.43, H 7.74, N 13.45, S 7.70; found C 63.36, H 7.67, N 13.37, S 7.68.
N-[4-叔丁基-5-(2, 4-二氯苄基) 噻唑-2-基]吗啉基乙酰胺 (1q):灰色固体, 收率79.2%. m.p. 188~190 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.35 (s, 9H, 3×CH3), 2.61 (t, J=4.4 Hz, 4H, CH2NCH2), 3.20 (s, 2H, COCH2), 3.81 (t, J=4.4 Hz, 4H, CH2OCH2), 4.26 (s, 2H, CH2), 7.02 (d, J=8.4 Hz, 1H, C6H3 6-H), 7.16 (dd, J=8.4, 1.6 Hz, 1H, C6H3-5-H), 7.39 (d, J=1.6 Hz, 1H, C6H3-3-H), 9.97 (s, 1H, NH). Anal. calcd for C20H25Cl2N3O2S: C 52.52, H 5.73, N 12.25, S 7.01; found C 52.47, H 5.68, N 12.18, S 6.95.
N-[4-叔丁基-5-(4-氯苄基) 噻唑-2-基]吗啉基乙酰胺 (1r):淡黄色固体, 收率73.5%, m.p. 115~116 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.36 (s, 9H, 3×CH3), 2.61 (t, J=4.4 Hz, 4H, CH2NCH2), 3.20 (s, 2H, COCH2), 3.81 (t, J=4.4 Hz, 4H, CH2OCH2), 4.21 (s, 2H, CH2), 7.11 (d, J=8.4 Hz, 2H, C6H4-2, 6-H), 7.25 (d, J=8.4 Hz, 2H, C6H4-3, 5-H). Anal. calcd for C20H26ClN3O2S: C 56.79, H 6.43, N, 13.25, S 7.58; found C 56.71, H 6.37, N 13.17, S 7.53.
N-[4-叔丁基-5-(4-甲氧基苄基) 噻唑-2-基]吗啉基乙酰胺 (1s):乳白色固体, 收率95.5%, m.p. 143~144 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.38 (s, 9H, 3×CH3), 2.60 (t, J=4.0 Hz, 4H, CH2NCH2), 3.18 (s, 2H, COCH2), 3.79 (s, 3H, OCH3), 3.80 (t, J=4.0 Hz, 4H, CH2OCH2), 4.18 (s, 2H, CH2), 6.82 (d, J=8.0 Hz, 2H, C6H4-3, 5-H), 7.10 (d, J=8.0 Hz, 2H, C6H4-2, 6-H). Anal. calcd for C21H29N3O3S: C 60.26, H 7.22, N 13.39, S 7.66; found C 60.17, H 7.17, N 13.35, S 7.61.
N-[4-叔丁基-5-(胡椒基) 噻唑-2-基]吗啉基乙酰胺 (1t):白色固体, 收率90%. m.p. 160~162 ℃; 1H NMR (400 MHz, CDCl3) δ:1.40 (s, 9H, 3×CH3), 2.59~2.63 (m, 4H, morpholine ring), 3.21 (s, 2H, COCH2), 3.83 (s, 4H, morpholine ring), 4.15 (s, 2H, CH2), 5.94 (s, 2H, OCH2O), 6.64~6.66 (m, 2H, C6H3), 6.73 (d, J=8.0 Hz, 1H, C6H3), 9.91(s, 1H, NH); 13C NMR (100 MHz, CDCl3) δ:30.89, 32.55, 35.65, 53.83, 61.60, 66.70, 100.95, 108.24, 108.81, 121.26, 124.45, 134.08, 146.21, 147.79, 152.30, 152.95, 167.89. Anal. calcd for C22H29N3O3S: C 60.41, H 6.52, N 10.06, S 7.68; found C 60.25, H 6.41, N 9.89, S 7.55.
N-[4-叔丁基-5-(胡椒基) 噻唑-2-基]哌啶基乙酰胺 (1u):白色固体, 收率88%. m.p. 126~128 ℃; 1H NMR (400 MHz, CDCl3) δ:1.38 (s, 9H, 3×CH3), 1.50 (s, 2H, piperidine ring 4-CH2), 1.70 (s, 4H, piperidine ring), 2.54 (s, 4H, piperidine ring), 3.15 (s, 2H, COCH2), 4.15 (s, 2H, CH2), 5.93 (s, 2H, OCH2O), 6.63~6.67 (m, 2H, C6H3), 6.72 (d, J=8.0 Hz, 1H, C6H3), 10.12 (s, 1H, NH); 13C NMR (100 MHz, CDCl3) δ: 22.49, 28.21, 30.93, 32.60, 35.66, 43.24, 54.70, 100.95, 108.23, 108.86, 121.28, 124.11, 134.31, 146.19, 147.80, 152.73, 153.40, 168.25. Anal. calcd for C23H31N3O2S: C 63.59, H 7.03, N 10.11, S 7.72; found C 63.43, H 6.88, N 9.92, S 7.58.
-
-
[1]
Pokhodylo, N.; Shyyka, O.; Matiychuk, V. Med. Chem. Res. 2014, 23, 2426. doi: 10.1007/s00044-013-0841-8
-
[2]
Lee, Y. S. E.; Chuang, S. H.; Huang, L. Y. L.; Lai, C. L.; Lin, Y. H.; Yang, J. Y.; Liu, C. W.; Yang, S. C.; Lin, H. S.; Chang, C. C.; Lai, J. Y.; Jian, P. S.; Lam, K.; Chang, J. M.; Lau, J. Y. N.; Huang, J. J. J. Med. Chem. 2014, 57, 4098. doi: 10.1021/jm401990s
-
[3]
Gorczynski, M. J.; Leal, R. M.; Mooberry, S. L.; Bushweller, J. H.; Brown, M. L. Bioorg. Med. Chem. 2004, 12, 1029. doi: 10.1016/j.bmc.2003.12.003
-
[4]
姜凤超, 成冲云, 药学学报, 2006, 41, 727.Jiang, F. C.; Cheng, C. Y. Acta Pharm. Sin. 2006, 41, 727 (in Chinese).
-
[5]
Bjoern, E.; Guido, K.; Christian, H. CN 101277692, 2007[Chem. Abstr. 2007, 146, 163101].
-
[6]
程宇, 王辉, Dinesh Addla, 周成合, 有机化学, 2016, 36, 1. doi: 10.6023/cjoc201509006Chen, Y.; Wang, H.; Dinesh, A.; Zhou, C. H. Chin. J. Org. Chem. 2016, 36, 1 (in Chinese). doi: 10.6023/cjoc201509006
-
[7]
廖启华, 林森, 邓瑞红, 黄志强, 邓柯玉, 严兆华, 有机化学, 2015, 35, 1923. doi: 10.6023/cjoc201503002Liao, Q. H.; Lin, S.; Deng, R. H.; Huang, Z. Q.; Deng, K. Y.; Yan, Z. H. Chin. J. Org. Chem. 2015, 35, 1923 (in Chinese). doi: 10.6023/cjoc201503002
-
[8]
(a) Hu, A. X.; Huo, S. F.; Xia, S.; Li, W.; Ye, J.; Peng, J. M.; Xiang, J. N. CN 102070556, 2011[Chem. Abstr. 2011, 154, 615156].
(b) Hu, A. X.; Li, W.; Xia, S.; Ye, J.; Huo, S. F.; Zou, S. S.; Peng, J. M.; Xiang, J. N. CN 102319244, 2012 [Chem. Abstr. 2012, 156, 194844]. -
[9]
Makam, P.; Kannan, T. Eur. J. Med. Chem. 2014, 87, 643. doi: 10.1016/j.ejmech.2014.09.086
-
[10]
Turan-Zitouni, G.; Özdemir, A.; Kaplancıkli, Z. A. Phosphorus, Sulfur, Silicon Relat. Elem. 2011, 186, 233. doi: 10.1080/10426507.2010.494643
-
[11]
Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Eur. J. Med. Chem. 2009, 44, 1198. doi: 10.1016/j.ejmech.2008.05.029
-
[12]
Siddiqui, N.; Ahsan, W. Eur. J. Med. Chem. 2010, 45, 1536. doi: 10.1016/j.ejmech.2009.12.062
-
[13]
Iino, T.; Tsukahara, D.; Kamata, K.; Sasaki, K.; Ohyama, S.; Hosaka, H.; Hasegawa, T.; Chiba, M.; Nagata, Y.; Eiki, J.; Nishimura, T. Bioorg. Med. Chem. 2009, 17, 2733. doi: 10.1016/j.bmc.2009.02.038
-
[14]
Abdel-Wahab, B. F.; Mohamed, S. F.; Amr, A. E. G. E.; Abdalla, M. Monatsh. Chem. 2008, 139, 1083. doi: 10.1007/s00706-008-0896-2
-
[15]
Rostom, S. A. F.; Faidallah, H. M.; Radwan, M. F.; Badr, M. H. Eur. J. Med. Chem. 2014, 76, 170. doi: 10.1016/j.ejmech.2014.02.027
-
[16]
Gurdal, E. E.; Durmaz, I.; Cetin-Atalay, R.; Yarim, M. J. Enzyme Inhib. Med. Chem. 2015, 30, 649. doi: 10.3109/14756366.2014.959513
-
[17]
崔建国, 赵丹丹, 何冬梅, 黄燕敏, 刘志平, 林啟福, 石海信, 甘春芳, 有机化学, 2016, 36, 630. doi: 10.6023/cjoc201509021Cui, J. G.; Zhao, D. D.; He, D. M.; Huang, Y. M.; Liu, Z. P.; Lin, Q. F.; Shi, H. X.; Gan, C. F. Chin. J. Org. Chem. 2016, 36, 630 (in Chinese). doi: 10.6023/cjoc201509021
-
[18]
(a) Hu, A. X.; Peng, J. M.; Fang, Y. L.; Shen, K.; Li, W.; Yan, X. W. CN 103333132, 2013 [Chem. Abstr. 2013, 158, 359731].
(b) Hu, A. X.; Peng, J. M.; Shen, K.; Li, W.; Yan, X. W.; Fang, Y. L. CN 103601697, 2013 [Chem. Abstr. 2013, 158, 446959]. -
[19]
彭俊梅, 博士论文, 湖南大学, 长沙, 2013.Peng, J. M. Ph.D. Dissertation, Hunan University, Changsha, 2013, (in Chinese).
-
[20]
Wu, Z. L.; Fang, Y. L.; Tang, Y. T.; Xiao, M. W.; Ye, J.; Li, G. X. A.; Hu, X. Med. Chem. Commun. 2016, 7, 1768. doi: 10.1039/C6MD00234J
-
[21]
彭俊梅, 李婉, 申坤, 霍素芳, 叶姣, 胡艾希, 高等学校化学学报, 2013, 34, 1646.Peng, J. M.; Li, W.; Shen, K.; Huo, S. F.; Ye, J.; Hu, A. X. Chem. J. Chin. Univ. 2013, 34, 1646 (in Chinese).
-
[1]
-
表 1 化合物1a~1u的抗肿瘤活性a
Table 1. Data of antitumor activity of compounds 1a~1u
Compd. Y R/R2 IC50/(μmol•L-1) A549 Hela MCF-7 1a 2, 4-Cl2 CH3 55.6±3.4 38.2±5.3 36.2±2.2 1b 2, 4-Cl2 Et 45.6±2.0 46.5±8.3 28.9±0.5 1c 2, 4-Cl2 n-Bu 89.3±1.9 49.7±4.2 25.2±1.2 1d 4-Cl CH3 41.2±7.2 51.0±7.4 35.0±2.6 1e 4-Cl Et 19.2±8.4 28.0±0.9 30.0±1.1 1f 4-Cl n-Bu 18.1±5.8 47.6±18.1 11.8±2.0 1g 4-OCH3 CH3 45.4±10.0 35.7±4.7 33.6±10.0 1h 4-OCH3 Et 55.9±6.5 42.1±6.2 52.3±8.9 1i 4-OCH3 n-Bu 26.4±3.4 12.9±1.3 32.2±1.2 1j 2, 4-Cl2 Et2 192.6±8.2 397.2±61.1 205.4±0.8 1k 2, 4-Cl2 n-Bu2 NA NA 456.2±14.8 1l 4-Cl Et2 261.5±114.4 274.9±10.2 139.4±26.8 1m 4-OCH3 Et2 NA 196.5±0.8 NA 1n 2, 4-Cl2 (CH2)5 333.8±68.1 NA NA 1o 4-Cl (CH2)5 379.5±170.1 136.7±42.9 NA 1p 4-OCH3 (CH2)5 NA 243.6±26.4 484.8±47.1 1q 2, 4-Cl2 O (CH2CH2)2 188.5±6.1 NA NA 1r 4-Cl O (CH2CH2)2 343.2±93.4 51.5±9.8 176.6±13.2 1s 4-OCH3 O (CH2CH2)2 441.7±7.6 265.1±45.3 379.5±32.9 1t 3, 4-OCH2O O (CH2CH2)2 66.6±6.8 6.4±2.2 NA 1u 3, 4-OCH2O (CH2)5 256.9±47.4 31.3±5.4 NA 5-FU[20] 26.0±6.0 56.5±3.4 79.3±5.1 aNA: IC50>500 μmol/L. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 2
- 文章访问数: 1509
- HTML全文浏览量: 145


 下载:
下载:





 下载:
下载:

