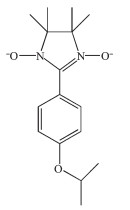
 Figure Scheme 1.
Molecule structure of NIT-Ph-4-OCHCH3CH3
Figure Scheme 1.
Molecule structure of NIT-Ph-4-OCHCH3CH3

含氮氧自由基的Gd,Tb,Dy配合物的合成、结构及磁性
English
Gd (Ⅲ), Tb (Ⅲ) and Dy (Ⅲ) Complexes Based on a Nitronyl Nitroxide Radical: Syntheses, Structures and Magnetic Properties
-
Key words:
- nitronyl nitroxide radical
- / lanthanides
- / crystal structure
- / magnetic properties
-
0 Introduction
Single-molecule magnets (SMMs) have attracted many scientists attention in the past two decades[1-4]. This type of materials are characterized as slow magnetization relaxation caused by the association of large ground state spin (ST) value with a significant uniaxial (Ising-like) magnetic anisotropy (D), which leads to a significant energy barrier to magnetization reversal (U)[5-7]. The SMMs have been found potential applications for the uses of high-density magnetic memories, magnetic refrigeration, quantum computing devices and spintronics at the molecular level[8-11]. Recently 4f metal ions were considered to be good candidates for the construction of SMMs due to their significant magnetic anisotropy arising from the large, unquenched orbital angular momentum. Up to now, a variety of 4f metal ions based SMMs have been reported[12-17].
Apart from the choice of the metal ions, the ligand design also plays an important role. The use of organic radical ligands in the creation of new magnetic molecular compounds have attracted much attention since the discovery of the first radical-4f SMM by Gatteschis group[18]. As is well known, the stable radical ligands can generate typically stronger intramolecular magnetic exchange coupling. The strong exchange coupling between lanthanides and radicals generally leads to superior SMMs. Recently, a binuclear Tb (Ⅲ) complex bridged by a N23- radical has been reported with a record blocking temperature of 13.9 K[19-20]. So far various organic radicals such as nitronyl nitroxide, verdazyl and semiquinone radicals have been reported[21-25]. However, researches are focused in particular on the nitronyl nitroxide (NIT) family of radicals, because these type of radicals are relatively stable and easy to obtain derivatives with substituents containing donor atoms. Nitronyl nitroxide radicals can act as bidentate ligands through their identical N-O coordination groups and give rise to complexes with different structures. Unfortunately, NIT radicals are poorly donating ligands, thus utilization of strong electron-withdrawing coligands such as hexafluoroace-tylacetonate (hfac) and trifluoroacetylacetonate (tfac) are necessary. However, the steric demand of these coligands restrict the dimensionality of the resulting metal-radical compounds. So, it is easier to get zero-and one-dimensional compounds by this strategy.
To further study the magnetic properties of NIT radical-lanthanide compounds, in this paper we report a novel nitronyl nitroxide radical (Scheme 1) and its corresponding Ln-nitronyl nitroxide compounds Ln (hfac)3(NIT-Ph-4-OCHCH3CH3)2(Ln=Gd (1), Tb (2), Dy (3); hfac=hexafluoroacetylacetonate; NIT-Ph-4-OCHCH3CH3=2-(4-isopropoxyphenyl)-4, 4, 5, 5-tetramet-hyl-imidazoline-1-oxyl-3-oxide), their crystal structures and magnetic properties were described in detail.
1 Experimental
1.1 Materials and physical measurements
All the starting chemicals were obtained from Aldrich and used without further purification. The radical ligand NIT-Ph-4-OCHCH3CH3 was prepared according to literature method[26]. Elemental analyses (C, H, N) were determined by Perkin-Elmer 240 elemental analyzer. The infrared spectra was recorded from KBr pellets in the range of 4 000~400 cm-1 with a Bruker Tensor 27 IR spectrometer. The magnetic measurements were carried out with MPMSXL-7 SQUID magnetometer. Diamagnetic corrections were made with Pascals constants for all the constituent atoms.
1.2 Synthesis of Complex 1
A solution of Gd (hfac)3·2H2O (0.05 mmol) in 25 mL dry heptane was heated to reflux for 2 h. Then the solution was cooled to about 60 ℃, a solution of NIT-Ph-4-OCHCH3CH3 (0.1 mmol) in 2 mL of CH2Cl2 was added. The resulting solution was stirred for about 3 min and then cooled to room temperature. The filtrate was allowed to stand at room temperature for slow evaporation. After three days, some blue crystals were collected. Yield: 31.4 mg (45.5% based on Gd). Elemental analysis calculated for C47H49F18N4O12Gd (%): C: 41.47; H: 3.63; N: 4.12. Found (%): C: 41.88; H: 3.69; N: 4.22.
1.3 Synthesis of Complex 2
Complex 2 was synthesized with the same procedure for complex 1 using Tb (hfac)3·2H2O instead of Gd (hfac)3·2H2O. Yield: 32.7 mg (47.9%). Elemental analysis calculated for C47H49F18N4O12Tb (%): C: 41.42; H: 3.62; N: 4.11. Found (%): C: 40.69; H: 3.57; N: 4.22.
1.4 Synthesis of Complex 3
Complex 3 was synthesized with the same procedure for complex 1 using Dy (hfac)3·2H2O instead of Gd (hfac)3·2H2O. Yield: 29.3 mg (42.9%). Elemental analysis calculated for C47H49F18N4O12Dy (%): C: 41.31; H: 3.61; N: 4.10. Found (%): C: 40.91; H: 3.77; N: 4.17.
1.5 Crystal Structure Determination and Refine-ment
X-ray single-crystal diffraction data for complexes 1, 2 and 3 were collected using a Rigaku Saturn CCD diffractometer at 113(2) K with Mo Kα radiation (λ=0.071 073 nm). The structure was solved by direct methods by utilizing the program SHELXS-97[27] and refined by full-matrix least-squares methods on F2 with the use of the SHELXL-97 program package[28]. Anisotropic thermal parameters were assigned to all non-hydrogen atoms. The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter. Disordered carbon atoms were observed in the hfac ligands for both compounds and disorders were also observed for some fluorine atoms. Pertinent crystallographic data and structure refinement parameters for these complexes were listed in Table 1. Selected bond lengths and bond angles of complexes 1, 2 and 3 are listed in Table 2.
Complex 1 2 3 Empirical formula C47H49F18Gd1N4O12 C47H49F18Tb1N4O12 C47H49F18Dy1N4O12 Formula weight 1 361.15 1 362.82 1 366.40 Crystal system Triclinic Monoclinic Triclinic Space group P1 P21/c P1 a/nm 1.219 5(6) 1.987 2(4) 1.214 6(10) b/nm 1.491 9(7) 1.255 6(3) 1.489 1(12) c/nm 1.738 6(7) 2.282 1(5) 1.727 6(13) α/(°) 98.890(5) 90 98.842(6) β/(°) 103.057(5) 98.865(5) 102.753(6) γ/(°) 111.943(3) 90 111.987(14) V/nm3 2.756(2) 2.731(4) 2.728(4) Z 2 4 2 Dc/(g•cm-3) 1.64 1.609 1.664 μ/mm-1 1.325 1.377 1.493 Rint 0.062 2 0.073 6 0.027 2 F (000) 1 362 2 728 1 366 Reflections collected 22 857 46 848 23 418 Independent reflections 9 649 9 876 9 822 GOF on F2 1.017 1.032 1.021 R1a [I > 2σ(I)] 0.026 8 0.053 2 0.037 8 wR2b [I > 2σ(I)] 0.062 5 0.144 5 0.091 1 aR1=Σ(‖Fo|-|Fc‖)/Σ|Fo|; bwR2=[Σw (Fo2-Fc2)2/Σw (Fo)2]1/2. 1 Gd (1)-O (6) 0.233 0(8) Gd (1)-O (7) 0.233 3(8) O (2)-N (l) 0.127 1(3) Gd (1)-O (3) 0.234 5(7) Gd (1)-O (9) 0.235 0(8) O (6)-N (3) 0.130 7(3) Gd (1)-O (8) 0.252 9(8) Gd⑴-O (12) 0.239 97(18) O (3)-N (2) 0.130 4(2) O (6)-Gd (1)-O (7) 93.24(7) O (7)-Gd (1)-O (3) 105.83(7) O (11)-Gd (1)-O (9) 148.80(6) O (6)-Gd (1)-O (11) 102.93(7) O (11)-Gd (1)-O (3) 88.67(7) O (3)-Gd (1)-O (9) 73.27(6) 0(7)-Gd (l)-0(ll) l36.79(6) 0(6)-Gd (l)-0(9) 76.52(6) 0(6)-Gd (l)-0(l0) 69.66(7) 0(6)-Gd (l)-0(3) l37.62(6) 0(7)-Gd (l)-0(9) 73.74(6) 0(7)-Gd (l)-0(l0) l45.23(6) 0(ll)-Gd (l)-0(l0) 77.69(6) 0(3)-Gd (l)-0(l0) 73.46(6) 2 Tb (l)-0(4) 0.234 0(3) Tb (l)-0(ll) 0.236 0(3) 0(5)-N (4) 0.l27 4(5) Tb (l)-0(2) 0.234 5(3) Tb (l)-0(8) 0.237 4(3) 0(2)-N (2) 0.l3l 7(5) Tb (l)-0(7) 0.234 6(3) Tb (l)-0(l2) 0.238 l (4) N (3)-0(4) 0.l3l 7(5) 0(4)-Tb (l)-0(2) l37.l0(l2) 0(2)-Tb (l)-0(ll) l03.35(l2) 0(7)-Tb (l)-0(8) 72.69(l3) 0(4)-Tb (l)-0(7) l03.62(l3) 0(7)-Tb (l)-0(ll) l37.94(l2) 0(ll)-Tb (l)-0(8) 74.0l (l2) 0(2)-Tb (l)-0(7) 90.96(l3) 0(4)-Tb (l)-0(8) 75.98(l2) 0(4)-Tb (l)-0(l2) l49.6l (l2) 0(4)-Tb (l)-0(ll) 92.38(l3) 0(2)-Tb (l)-0(8) l46.60(l2) 0(2)-Tb (l)-0(l2) 73.00(l2) 0(7)-Tb (l)-0(l2) 74.68(l3) 0(ll)-Tb (l)-0(l2) 72.28(l3) 3 Dy (l)-0(2) 0.232 l (3) Dy (l)-0(8) 0.235 0(3) 0(4)-N (4) 0.l26 9(4) Dy (l)-0(9) 0.233 5(3) Dy (l)-0(ll) 0.235 l (3) 0(5)-N (3) 0.l29 7(4) Dy (l)-0(l2) 0.234 6(3) Dy (l)-0(5) 0.235 4(3) 0(2)-N (2) 0.l30 4(5) 0(2)-Dy (l)-0(9) 92.77(ll) 0(9)-Dy (l)-0(8) l36.97(ll) 0(l2)-Dy (l)-0(ll) 74.09(ll) 0(2)-Dy (l)-0(l2) 69.99(l2) 0(l2)-Dy (l)-0(8) 76.9l (l2) 0(8)-Dy (l)-0(ll) l49.09(ll) 0(9)-Dy (l)-0(l2) l45.80(ll) 0(2)-Dy (l)-0(ll) 76.58(l2) 0(2)-Dy (l)-0(5) l37.83(ll) 0(2)-Dy (l)-0(8) l03.30(l2) 0(9)-Dy (l)-0(ll) 73.22(ll) 0(9)-Dy (l)-0(5) l05.9l (l2) 0(l2)-Dy (l)-0(5) 73.72(ll) 0(8)-Dy (l)-0(5) 88.47(l2) CCDC: 985443, 1; 1043098, 2; 985444, 3.
2 Results and discussion
2.1 Crystal Structure of Complex 1
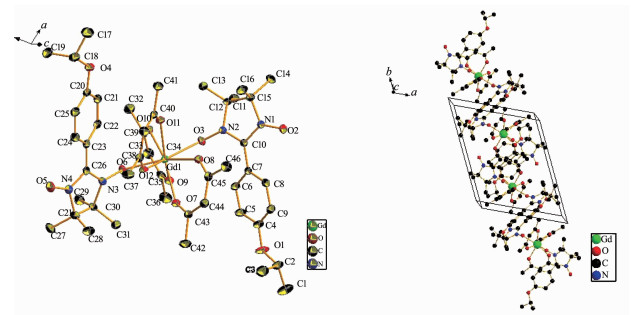
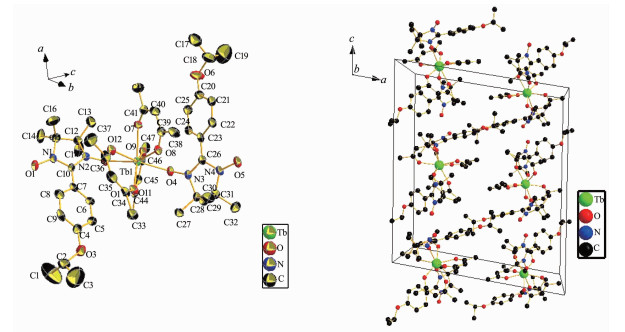
Single-crystal X-ray diffraction analyses reveal that all these three compounds show similar radical-Ln (Ⅲ)-radical structures, which are composed of one Ln (hfac)3 unit and two NIT-Ph-4-OCHCH3CH3 radicals. Compounds 1 and 3 are isostructural and crystallize in the P1 space group, while compound 2 crystallizes in the P21/c space group.
In complex 1, the central Gd (Ⅲ) ions are eight-coordinate with eight oxygen atoms. Two radical ligands bond to one Gd (Ⅲ) ion via the oxygen atoms of N-O coordination groups. The bond length of Gd (1)-O (6) is 0.234 8 nm while the bond length of Gd (1)-O (3) is 0.237 8 nm. The N (2)-O (3) and N (3)-O (6) bond lengths of nitronyl nitroxide radicals are 0.130 4 nm and 0.130 7 nm, respectively. The uncoordinated N (1)-O (2) and N (4)-O (5) bond lengths are 0.127 1 nm and 0.127 2 nm, respectively, which are comparable to those of reported tri-spin radical-Ln (Ⅲ)-radical complexes[29-35]. The other six oxygen atoms are from three hfacs with the Gd-O bond lengths in the range of 0.236 0~0.242 9 nm. The nearest Gd…Gd distance between the adjacent molecules is 1.024 9 nm (Fig. 1).
2.2 Crystal structure of complex 2
The crystal structure of complex 2 shows that the central Tb (Ⅲ) ions are eight-coordinated with eight oxygen atoms. Two radical ligands bond to one Tb (Ⅲ) ion via the oxygen atoms of N-O coordination groups. The bond length of Tb (1)-O (4) is 0.234 1 nm while the bond length of Tb (1)-O (2) is 0.234 5 nm. The N (3)-O (4) and N (2)-O (2) bond lengths of nitronyl nitroxide radicals are 0.131 6 nm and 0.131 8 nm respectively. The uncoordinated N (1)-O (1) and N (4)-O (5) bond lengths are 0.128 3 nm and 0.127 3 nm, respectively, which are comparable to those of reported tri-spin radical-Ln (Ⅲ)-radical complexes[29-35]. The other six oxygen atoms are from three hfacs with the Tb-O bond lengths in the range of 0.234 6~0.241 7 nm. The nearest Tb…Tb distance between the adjacent molecules is 1.080 8 nm, which is a little bit longer than that for complex 1 (Fig. 2).
2.3 Crystal Structure of Complex 3
Compound 3 is isostructural to compound 1 and the bond lengths of Dy-O are in the range of 0.232 1~0.239 3 nm, which are a little shorter than the bond lenths of Gd-O.
2.4 Magnetic Properties of Complex 1
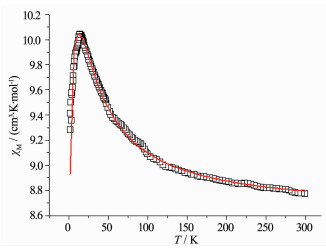
Variable-temperature magnetic susceptibilities of complexes 1, 2, and 3 were measured from 300 to 2.0 K in an applied field of 1 kOe. The χMT vs T plot for 1 are shown in Fig. 3. At 300 K, the χMT value is 8.77 cm3·K·mol-1, close to the theoretical value of 8.63 cm3·K·mol-1 (Uncoupled one Gd (Ⅲ) ion, f7 electron configuration, χMT=7.88 cm3·K·mol-1) plus two organic radicals (S=1/2, χMT=0.375 cm3·K·mol-1)). Upon cooling, the χMT value of complex 1 increases steadily to a maximum of 10.04 cm3·K·mol-1 at 15 K, afterward decreases to 9.28 cm3·K·mol-1 at 2.0 K.
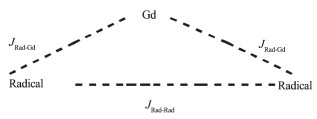
As shown in Scheme 2, there are two kinds of magnetic interactions in this radical-Gd (Ⅲ)-radical complex at the same time. The first one is Gd (Ⅲ)-radical interaction and the second one is radical-radical interaction.
The magnetic interactions between Gd (Ⅲ) and the radicals can be described by isotropic exchange interaction. Thus the experimental data for complex 1 can be analyzed with an expression derived from a spin Hamiltonian. Considering the g value range of the radical and Gd (Ⅲ) ion, we assume that the radical and Gd (Ⅲ) ion have the same g value. Thus the variable-temperature magnetic susceptibility data for complex 1 can be analyzed by a theoretical expression (Eq.2) deduced from a spin Hamiltonian (Eq.1)[30-35].
The best fitting leads to g=2.00, JRad-Gd=2.57 cm-1, JRad-Rad=-9.98 cm-1 for complex 1. The positive value of JRad-Gd indicates that there is a weak ferromagnetic interaction between the Gd (Ⅲ) and the radicals in the molecule. The negative JRad-Rad indicates the antiferromagnetic interaction between the two intramolecular radicals. The obtained J value is comparable with the previously reported GdⅢ-radicals compounds[30-35].
2.5 Magnetic properties of complex 2
While for complex 2 (Fig. 4), at 300 K, the χMT value is 13.08 cm3·K·mol-1, close to the theoretical value 12.57 cm3·K·mol-1 in uncoupled system of one Tb (Ⅲ) ion (f9 electron configuration, χMT=11.82 cm3·K·mol-1) plus two organic radical (S=1/2, χMT=0.375 cm3·K·mol-1). Upon cooling, the χMT value of complex 2 increases steadily to a maximum of 28.62 cm3·K·mol-1 at 3.0 K, afterward the value decreases to 28.54 cm3·K·mol-1 at 2.0 K. The increase of χMT suggests the presence of ferromagnetic interaction between the Tb (Ⅲ) and the organic radical. The decrease of χMT at low temperature indicates the antiferromagnetic interaction between the two intramolecular radicals. The magnetic properties of complex 2 are similar to those of previously reported[29-35].
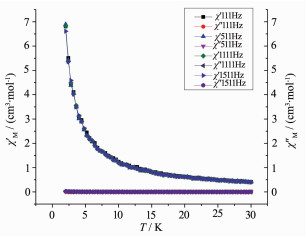
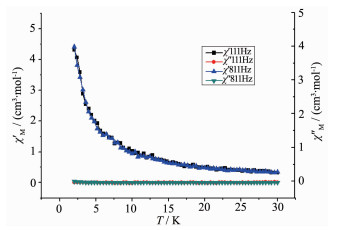
Alternating current (ac) susceptibility measure-ments for complex 2 were carried out in low temperature regime under a zero dc field to investigate the dynamics of the magnetization. As shown in Fig. 5, there are no obvious frequency dependent out-of-phase signals. We do not think that complex 2 express SMM behavior at low temperature. This may due to the small energy barrier which could not prevent the inversion of spin.
2.6 Magnetic properties of complex 3
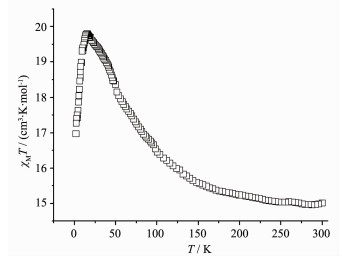
Complex 3 shows similar magnetic properties with complex 1 (Fig. 6). At 300 K, the χMT value is 15.01 cm3·K·mol-1, close to the theoretical value of 14.92 cm3·K·mol-1. Upon cooling, the χMT value of complex 3 increases steadily to a maximum of 19.79 cm3·K·mol-1 at 15.4 K, afterward decreases to 16.97 cm3·K·mol-1 at 2.0 K. The plot also suggests the presence of ferromagnetic interaction between the Dy (Ⅲ) and the coordinated N-O groups of the organic radicals and the antiferromagnetic interaction between the two intramolecular radicals.
Alternating current (ac) susceptibility measure-ments for complex 3 were also carried out in low temperature regime under a zero dc field. The result (Fig. 7) shows that there are no obvious frequency dependent out-of-phase signals. Like complex 2, complex 3 doesnt express SMMs behavior at low temperature.
3 Conclusions
A novel nitronyl nitroxide radical and its three corresponding mononuclear tri-spin compounds Ln (hfac)3(NIT-Ph-4-OCHCH3CH3)2 (Ln=Gd (1), Tb (2), Dy (3).) have been synthesized and characterized. The magnetic studies reveal that ferromagnetic interactions (between the intramolecular Ln and radical) and antiferromagnetic interactions (between the intramole-cular radicals) are coexist in these complexes. Complexes 2 and 3 do not have SMMs behavior at low temperature, this may due to the small energy barrier which could not prevent the inversion of spin.
-
-
[1]
Kahn O. Molecular Magnetism. New York: Wiley-VCH, 1993.
-
[2]
Moller S, Perlov C, Jackson W, et al. Nature, 2003, 426:166-169 doi: 10.1038/nature02070
-
[3]
Kahn M L, Sutter J P, Golhen S, et al. J. Am. Chem. Soc., 2000, 122:3413-3421 doi: 10.1021/ja994175o
-
[4]
Zhang P, Guo Y N, Tang J K. Coord. Chem. Rev., 2013, 257: 1728-1737 doi: 10.1016/j.ccr.2013.01.012
-
[5]
Ruiz-Molina D, Mas-Torrent M, Gómez J, et al. Adv. Mater., 2003, 15:42-49 doi: 10.1002/(ISSN)1521-4095
-
[6]
Clemente-León M, Soyer H, Coronado E, et al. Angew. Chem. Int. Ed., 1998, 37:2842-2845 doi: 10.1002/(ISSN)1521-3773
-
[7]
Cornia A, Fabretti A C, Pacchioni M, L, et al. Angew. Chem. Int. Ed., 2003, 42:1645-1651 doi: 10.1002/anie.200350981
-
[8]
Gatteschi D, Caneschi A, Pardi L, et al. Science, 1994, 265: 1054-1058 doi: 10.1126/science.265.5175.1054
-
[9]
Bar A K, Pichon C, Gogoi N, et al. Chem. Commun., 2015, 51:3616-3619 doi: 10.1039/C4CC10182K
-
[10]
Liu R N, Li L C, Wang X L, et al. Chem. Commun., 2010, 46:2566-2570 doi: 10.1039/b918554b
-
[11]
Bogani L, Wernsdorfer W. Nat. Mater., 2008, 7:179-183 doi: 10.1038/nmat2133
-
[12]
Caneschi A, Gatteschi D, Lalioti N, et al. Angew. Chem. Int. Ed., 2001, 40:1760-1793 doi: 10.1002/(ISSN)1521-3773
-
[13]
Bogani L, Sangregorio C, Sessoli R, et al. Angew. Chem. Int. Ed., 2005, 44:5817-5821 doi: 10.1002/(ISSN)1521-3773
-
[14]
Bernot K, Luzon J, Bogani L, et al. J. Am. Chem. Soc., 2009, 131:5573-5579 doi: 10.1021/ja8100038
-
[15]
Liu J L, Wu J Y, Chen Y C, et al. Angew. Chem. Int. Ed., 2014, 53:12966-12970 doi: 10.1002/anie.201407799
-
[16]
Chatelain L, Walsh J P S, Pecaut J, et al. Angew. Chem. Int. Ed., 2014, 53:13434-13439 doi: 10.1002/anie.201407334
-
[17]
Zhang P, Zhang L, Wang C. J. Am. Chem. Soc., 2014, 136: 4484-4489 doi: 10.1021/ja500793x
-
[18]
Poneti G, Bernot K, Bogani L, et al. Chem. Commun., 2007 1807-1809
-
[19]
Rinehart J D, Fang M, Evans W J, et al. J. Am. Chem. Soc., 2011, 133:14236-14239 doi: 10.1021/ja206286h
-
[20]
Rinehart J D, Fang M, Evans W J, et al. Nat. Chem., 2011, 3:538-542 doi: 10.1038/nchem.1063
-
[21]
Chernick E T, Casillas R, Zirzlmeier J, et al. J. Am. Chem. Soc., 2015, 137:857-863 doi: 10.1021/ja510958k
-
[22]
Mailman A, Winter S M, Wong J W L, et al. J. Am. Chem. Soc., 2015, 137:1044-1049 doi: 10.1021/ja512235h
-
[23]
Wang X F, Hu P, Li Y G, et al. Chem. Asian J., 2015, 10: 325-330 doi: 10.1002/asia.v10.2
-
[24]
Zhu M, Hu P, Li Y, et al. Chem. Eur. J., 2014, 20:13356-13364 doi: 10.1002/chem.201403581
-
[25]
Wang Z X, Zhang X, Zhang Y Z, et al. Angew. Chem. Int. Ed., 2014, 53:11567-11570 doi: 10.1002/anie.201407628
-
[26]
Ullman E F, Osiecki J H, Boocock D G B, et al. J. Am. Chem. Soc., 1972, 94:7049-7059 doi: 10.1021/ja00775a031
-
[27]
Sheldrick G M. SHELXS-97: Program for the Solution of Crystal Structures, University of Göttingen, Germany, 1997. http://www.oalib.com/references/16733729
-
[28]
Sheldrick G M. SHELXL-97: Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1997. http://www.oalib.com/references/13283787
-
[29]
Hu P, Zhang C, Gao Y, et al. Inorg. Chim. Acta, 2013, 398: 136-140 doi: 10.1016/j.ica.2012.12.025
-
[30]
Zhou N, Ma Y, Wang C, et al. Dalton Trans., 2009:8489-8492
-
[31]
Wang C, Wang Y L, Qin Z X, et al. Inorg. Chem. Commun., 2012, 20:112-117 doi: 10.1016/j.inoche.2012.02.030
-
[32]
Zhang C X, Chen H W, Wang W M, et al. Inorg. Chem. Commun., 2012, 24:177-181 doi: 10.1016/j.inoche.2012.07.003
-
[33]
Du F X, Hu P, Gao Y Y, et al. Inorg. Chem. Commun., 2014, 48:166-170 doi: 10.1016/j.inoche.2014.08.035
-
[34]
Zhang C X, Qiao X M, Kong Y K, et al. J. Mol. Struct., 2015, 108:1348-1354
-
[35]
Li L L, Liu S, Zhang Y, et al. Dalton Trans., 2015, 44:6118-6125 doi: 10.1039/C4DT03941F
-
[1]
-
Table 1. Crystal data and structure refinement for 1, 2 and 3
Complex 1 2 3 Empirical formula C47H49F18Gd1N4O12 C47H49F18Tb1N4O12 C47H49F18Dy1N4O12 Formula weight 1 361.15 1 362.82 1 366.40 Crystal system Triclinic Monoclinic Triclinic Space group P1 P21/c P1 a/nm 1.219 5(6) 1.987 2(4) 1.214 6(10) b/nm 1.491 9(7) 1.255 6(3) 1.489 1(12) c/nm 1.738 6(7) 2.282 1(5) 1.727 6(13) α/(°) 98.890(5) 90 98.842(6) β/(°) 103.057(5) 98.865(5) 102.753(6) γ/(°) 111.943(3) 90 111.987(14) V/nm3 2.756(2) 2.731(4) 2.728(4) Z 2 4 2 Dc/(g•cm-3) 1.64 1.609 1.664 μ/mm-1 1.325 1.377 1.493 Rint 0.062 2 0.073 6 0.027 2 F (000) 1 362 2 728 1 366 Reflections collected 22 857 46 848 23 418 Independent reflections 9 649 9 876 9 822 GOF on F2 1.017 1.032 1.021 R1a [I > 2σ(I)] 0.026 8 0.053 2 0.037 8 wR2b [I > 2σ(I)] 0.062 5 0.144 5 0.091 1 aR1=Σ(‖Fo|-|Fc‖)/Σ|Fo|; bwR2=[Σw (Fo2-Fc2)2/Σw (Fo)2]1/2. Table 2. Selected bond distances (nm) and Angles (°) for 1, 2 and 3
1 Gd (1)-O (6) 0.233 0(8) Gd (1)-O (7) 0.233 3(8) O (2)-N (l) 0.127 1(3) Gd (1)-O (3) 0.234 5(7) Gd (1)-O (9) 0.235 0(8) O (6)-N (3) 0.130 7(3) Gd (1)-O (8) 0.252 9(8) Gd⑴-O (12) 0.239 97(18) O (3)-N (2) 0.130 4(2) O (6)-Gd (1)-O (7) 93.24(7) O (7)-Gd (1)-O (3) 105.83(7) O (11)-Gd (1)-O (9) 148.80(6) O (6)-Gd (1)-O (11) 102.93(7) O (11)-Gd (1)-O (3) 88.67(7) O (3)-Gd (1)-O (9) 73.27(6) 0(7)-Gd (l)-0(ll) l36.79(6) 0(6)-Gd (l)-0(9) 76.52(6) 0(6)-Gd (l)-0(l0) 69.66(7) 0(6)-Gd (l)-0(3) l37.62(6) 0(7)-Gd (l)-0(9) 73.74(6) 0(7)-Gd (l)-0(l0) l45.23(6) 0(ll)-Gd (l)-0(l0) 77.69(6) 0(3)-Gd (l)-0(l0) 73.46(6) 2 Tb (l)-0(4) 0.234 0(3) Tb (l)-0(ll) 0.236 0(3) 0(5)-N (4) 0.l27 4(5) Tb (l)-0(2) 0.234 5(3) Tb (l)-0(8) 0.237 4(3) 0(2)-N (2) 0.l3l 7(5) Tb (l)-0(7) 0.234 6(3) Tb (l)-0(l2) 0.238 l (4) N (3)-0(4) 0.l3l 7(5) 0(4)-Tb (l)-0(2) l37.l0(l2) 0(2)-Tb (l)-0(ll) l03.35(l2) 0(7)-Tb (l)-0(8) 72.69(l3) 0(4)-Tb (l)-0(7) l03.62(l3) 0(7)-Tb (l)-0(ll) l37.94(l2) 0(ll)-Tb (l)-0(8) 74.0l (l2) 0(2)-Tb (l)-0(7) 90.96(l3) 0(4)-Tb (l)-0(8) 75.98(l2) 0(4)-Tb (l)-0(l2) l49.6l (l2) 0(4)-Tb (l)-0(ll) 92.38(l3) 0(2)-Tb (l)-0(8) l46.60(l2) 0(2)-Tb (l)-0(l2) 73.00(l2) 0(7)-Tb (l)-0(l2) 74.68(l3) 0(ll)-Tb (l)-0(l2) 72.28(l3) 3 Dy (l)-0(2) 0.232 l (3) Dy (l)-0(8) 0.235 0(3) 0(4)-N (4) 0.l26 9(4) Dy (l)-0(9) 0.233 5(3) Dy (l)-0(ll) 0.235 l (3) 0(5)-N (3) 0.l29 7(4) Dy (l)-0(l2) 0.234 6(3) Dy (l)-0(5) 0.235 4(3) 0(2)-N (2) 0.l30 4(5) 0(2)-Dy (l)-0(9) 92.77(ll) 0(9)-Dy (l)-0(8) l36.97(ll) 0(l2)-Dy (l)-0(ll) 74.09(ll) 0(2)-Dy (l)-0(l2) 69.99(l2) 0(l2)-Dy (l)-0(8) 76.9l (l2) 0(8)-Dy (l)-0(ll) l49.09(ll) 0(9)-Dy (l)-0(l2) l45.80(ll) 0(2)-Dy (l)-0(ll) 76.58(l2) 0(2)-Dy (l)-0(5) l37.83(ll) 0(2)-Dy (l)-0(8) l03.30(l2) 0(9)-Dy (l)-0(ll) 73.22(ll) 0(9)-Dy (l)-0(5) l05.9l (l2) 0(l2)-Dy (l)-0(5) 73.72(ll) 0(8)-Dy (l)-0(5) 88.47(l2) -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 0
- 文章访问数: 521
- HTML全文浏览量: 24



 下载:
下载:








 下载:
下载:

