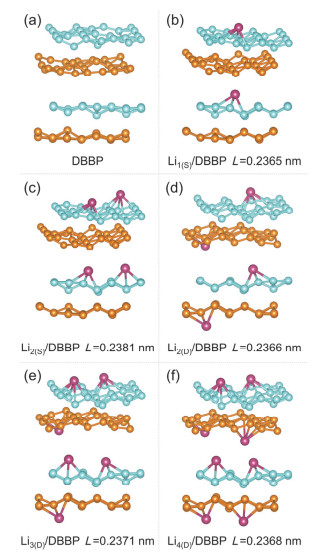
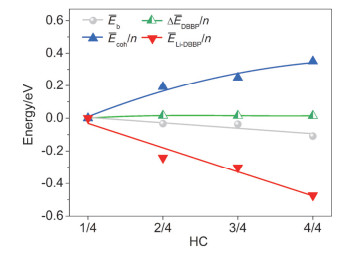
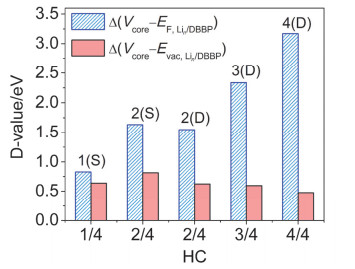
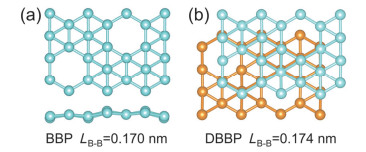
-
[1]
Mannix, A. J.; Zhou, X. F.; Kiraly, B.; Wood, J. D.; Alducin, D.; Myers, B. D.; Liu, X. L.; Fisher, B. L.; Santiago, U.; Guest, J. R.; Yacaman, M. J.; Ponce, A.; Oganov, A. R.; Hersam, M. C.; Guisinger, N. P. Science 2015, 350, 1513.
doi: 10.1126/science.aad1080
-
[2]
Feng, B.; Zhang, J.; Zhong, Q.; Li, W.; Li, S.; Li, H.; Cheng, P.; Meng, S.; Chen, L.; Wu, K. Nat. Chem. 2016, 8, 563.
doi: 10.1038/nchem.2491
-
[3]
Zhang, D.; Yuan, Z.; Zhang, G.; Tian, N.; Liu, D.; Zhang, Y. Acta Chim. Sinica 2018, 76, 537(in Chinese).
-
[4]
Yuan, Z.; Liu, D.; Tian, N.; Zhang, G.; Zhang, Y. Acta Chim. Sinica 2016, 74, 488(in Chinese).
-
[5]
Han, Y.; Geng, Z.; Wang, Y.; Liang, J.; Yan, P. Acta Chim. Sinica 2009, 67, 773(in Chinese).
-
[6]
Zhang, L.; Gao, S.; Liu, W.; Tang, R.; Shang, N.; Wang, C.; Wang, Z. Chin. J. Org. Chem. 2014, 34, 1542(in Chinese).
-
[7]
Chang, Z.-W.; Meng, F.-L.; Zhong, H.-X.; Zhang, X.-B. Chin. J. Chem. 2018, 36, 287.
doi: 10.1002/cjoc.201700752
-
[8]
Xu, Z.; Li, Y.; Shi, P.; Wang, B.; Huang, X. Chin. J. Org. Chem. 2013, 33, 2162(in Chinese).
-
[9]
Galeev, T. R.; Chen, Q.; Guo, J.-C.; Bai, H.; Miao, C.-Q.; Lu, H.-G.; Sergeeva, A. P.; Li, S.-D.; Boldyrev, A. I. Phys. Chem. Chem. Phys. 2011, 13, 11575.
doi: 10.1039/c1cp20439d
-
[10]
Sun, X.; Liu, X.; Yin, J.; Yu, J.; Li, Y.; Hang, Y.; Zhou, X.; Yu, M.; Li, J.; Tai, G.; Guo, W. Adv. Funct. Mater. 2016, 27, 1603300.
-
[11]
Zheng, B.; Yu, H.-T.; Lian, Y.-F.; Xie, Y. Chem. Phys. Lett. 2016, 648, 81.
doi: 10.1016/j.cplett.2016.01.074
-
[12]
Wu, X.; Dai, J.; Zhao, Y.; Zhuo, Z.; Yang, J.; Zeng, X. C. ACS Nano 2012, 6, 7443.
doi: 10.1021/nn302696v
-
[13]
Mannix, A. J.; Zhang, Z.; Guisinger, N. P.; Yakobson, B. I.; Hersam, M. C. Nat. Nanotechnol. 2018, 13, 444.
doi: 10.1038/s41565-018-0157-4
-
[14]
Zhong, Q.; Kong, L.; Gou, J.; Li, W.; Sheng, S.; Yang, S.; Cheng, P.; Li, H.; Wu, K.; Chen, L. Phys. Rev. Mater. 2017, 1, 021001.
doi: 10.1103/PhysRevMaterials.1.021001
-
[15]
Xie, S.-Y.; Wang, Y.; Li, X.-B. Adv. Mater. 2019, 31, 1900392.
doi: 10.1002/adma.201900392
-
[16]
Wang, Q.; Xue, M.; Zhang, Z. Acta Phys. Chim. Sin. 2019, 35, 565(in Chinese).
doi: 10.3866/PKU.WHXB201805080
-
[17]
Zhong, Q.; Zhang, J.; Cheng, P.; Feng, B.; Li, W.; Sheng, S.; Li, H.; Meng, S.; Chen, L.; Wu, K. J. Phys.:Condens. Matter 2017, 29, 095002.
doi: 10.1088/1361-648X/aa5165
-
[18]
Li, W.; Kong, L.; Chen, C.; Gou, J.; Sheng, S.; Zhang, W.; Li, H.; Chen, L.; Cheng, P.; Wu, K. Sci. Bull. 2018, 63, 282.
doi: 10.1016/j.scib.2018.02.006
-
[19]
Kiraly, B.; Liu, X.; Wang, L.; Zhang, Z.; Mannix, A. J.; Fisher, B. L.; Yakobson, B. I.; Hersam, M. C.; Guisinger, N. P. ACS Nano 2019, 13, 3816.
doi: 10.1021/acsnano.8b09339
-
[20]
Wu, R.; Drozdov, I. K.; Eltinge, S.; Zahl, P.; Ismail-Beigi, S.; Bozovic, I.; Gozar, A. Nat. Nanotechnol. 2019, 14, 44.
doi: 10.1038/s41565-018-0317-6
-
[21]
Ranjan, P.; Sahu, T. K.; Bhushan, R.; Yamijala, S. S. R. K. C.; Late, D. J.; Kumar, P.; Vinu, A. Adv. Mater. 2019, 31, 1900353.
doi: 10.1002/adma.201900353
-
[22]
Wang, Z.-Q.; Lu, T.-Y.; Wang, H.-Q.; Feng, Y. P.; Zheng, J.-C. Front. Phys. 2019, 14, 33403.
doi: 10.1007/s11467-019-0884-5
-
[23]
Zhang, Z.; Yang, Y.; Gao, G.; Yakobson, B. I. Angew. Chem. Int. Ed. 2015, 54, 13022.
doi: 10.1002/anie.201505425
-
[24]
Zhang, Z.; Mannix, A. J.; Hu, Z.; Kiraly, B.; Guisinger, N. P.; Hersam, M. C.; Yakobson, B. I. Nano Lett. 2016, 16, 6622.
doi: 10.1021/acs.nanolett.6b03349
-
[25]
Penev, E. S.; Bhowmick, S.; Sadrzadeh, A.; Yakobson, B. I. Nano Lett. 2012, 12, 2441.
doi: 10.1021/nl3004754
-
[26]
Xiao, H.; Cao, W.; Ouyang, T.; Guo, S.; He, C.; Zhong, J. Sci. Rep. 2017, 7, 45986.
doi: 10.1038/srep45986
-
[27]
Adamska, L.; Sadasiyam, S.; Foley, J. J.; Darancet, P.; Sharifzadeh, S. J. Phys. Chem. C 2018, 122, 4037.
doi: 10.1021/acs.jpcc.7b10197
-
[28]
Penev, E. S.; Kutana, A.; Yakobson, B. I. Nano Lett. 2016, 16, 2522.
doi: 10.1021/acs.nanolett.6b00070
-
[29]
Jiang, H. R.; Lu, Z. H.; Wu, M. C.; Ciucci, F.; Zhao, T. S. Nano Energy 2016, 23, 97.
doi: 10.1016/j.nanoen.2016.03.013
-
[30]
Lebon, A.; Aguilera-del-Toro, R. H.; Gallego, L. J.; Vega, A. Int. J. Hydrogen Energy 2019, 44, 1021.
doi: 10.1016/j.ijhydene.2018.10.241
-
[31]
Shukla, V.; Warna, J.; Jena, N. K.; Grigoriev, A.; Ahuja, R. J. Phys. Chem. C 2017, 121, 26869.
doi: 10.1021/acs.jpcc.7b09552
-
[32]
Singh, Y.; Back, S.; Jung, Y. Phys. Chem. Chem. Phys. 2018, 20, 21095.
doi: 10.1039/C8CP03130D
-
[33]
Chen, Y.; Yu, G.; Chen, W.; Liu, Y.; Li, G.-D.; Zhu, P.; Tao, Q.; Li, Q.; Liu, J.; Shen, X.; Li, H.; Huang, X.; Wang, D.; Asefa, T.; Zou, X. J. Am. Chem. Soc. 2017, 139, 12370.
doi: 10.1021/jacs.7b06337
-
[34]
Shen, H.; Li, Y.; Sun, Q. Nanoscale 2018, 10, 11064.
doi: 10.1039/C8NR01855C
-
[35]
Rao, D.; Zhang, L.; Meng, Z.; Zhang, X.; Wang, Y.; Qiao, G.; Shen, X.; Xia, H.; Liu, J.; Lu, R. J. Mater. Chem. A 2017, 5, 2328.
doi: 10.1039/C6TA09730H
-
[36]
Leng, S.; Sun, X.; Yang, Y.; Zhang, R. Mater. Res. Express 2019, 6, 085504.
doi: 10.1088/2053-1591/ab1a88
-
[37]
Jiang, H. R.; Shyy, W.; Liu, M.; Ren, Y. X.; Zhao, T. S. J. Mater. Chem. A 2018, 6, 2107.
doi: 10.1039/C7TA09244J
-
[38]
Xu, S.-G.; Li, X.-T.; Zhao, Y.-J.; Liao, J.-H.; Xu, W.-P.; Yang, X.-B.; Xu, H. J. Am. Chem. Soc. 2017, 139, 17233.
doi: 10.1021/jacs.7b08680
-
[39]
Kistanov, A. A.; Cai, Y.; Zhou, K.; Srikanth, N.; Dmitriev, S. V.; Zhang, Y.-W. Nanoscale 2018, 10, 1403.
doi: 10.1039/C7NR06537J
-
[40]
Garcia-Fuente, A.; Carrete, J.; Vega, A.; Gallego, L. J. Phys. Chem. Chem. Phys. 2017, 19, 1054.
doi: 10.1039/C6CP07432D
-
[41]
Zhou, X.-F.; Oganov, A. R.; Wang, Z.; Popov, I. A.; Boldyrev, A. I.; Wang, H.-T. Phys. Rev. B 2016, 93, 085406.
doi: 10.1103/PhysRevB.93.085406
-
[42]
Gao, M.; Li, Q.-Z.; Yan, X.-W.; Wang, J. Phys. Rev. B 2017, 95, 024505.
doi: 10.1103/PhysRevB.95.024505
-
[43]
Zhong, H.; Huang, K.; Yu, G.; Yuan, S. Phys. Rev. B 2018, 98, 054104.
doi: 10.1103/PhysRevB.98.054104
-
[44]
Li, H.; Jing, L.; Liu, W.; Lin, J.; Tay, R. Y.; Tsang, S. H.; Teo, E. H. T. ACS Nano 2018, 12, 1262.
doi: 10.1021/acsnano.7b07444
-
[45]
Zheng, B.; Qiao, L.; Yu, H.-T.; Wang, Q.-Y.; Xie, Y.; Qu, C.-Q. Phys. Chem. Chem. Phys. 2018, 20, 15139.
doi: 10.1039/C8CP01048J
-
[46]
Zhang, Z.; Penev, E. S.; Yakobson, B. I. Chem. Soc. Rev. 2017, 46, 6746.
doi: 10.1039/C7CS00261K
-
[47]
Kwon, K. C.; Choi, K. S.; Kim, S. Y. Adv. Funct. Mater. 2012, 22, 4724.
doi: 10.1002/adfm.201200997
-
[48]
Jia, T.; Zheng, N.; Cai, W.; Ying, L.; Huang, F. Acta Chim. Sinica 2017, 75, 808(in Chinese).
-
[49]
Zhang, K.; Guan, X.; Huang, F.; Cao, Y. Acta Chim. Sinica 2012, 70, 2489(in Chinese).
-
[50]
Xu, J.; Chang, Y.; Gan, L.; Ma, Y.; Zhai, T. Adv. Sci. 2015, 2, 1500023.
doi: 10.1002/advs.201500023
-
[51]
Bezugly, V.; Kunstmann, J.; Grundkötter-Stock, B.; Frauenheim, T.; Niehaus, T.; Cuniberti, G. ACS Nano 2011, 5, 4997.
doi: 10.1021/nn201099a
-
[52]
Zheng, B.; Yu, H.-T.; Xie, Y.; Lian, Y.-F. ACS Appl. Mater. Interfaces 2014, 6, 19690.
doi: 10.1021/am504674p
-
[53]
Kumar, P. V.; Bernardi, M.; Grossman, J. C. ACS Nano 2013, 7, 1638.
doi: 10.1021/nn305507p
-
[54]
He, C.; Yu, Z.; Sun, L. Z.; Zhong, J. X. J. Comput. Theor. Nanosci. 2012, 9, 16.
doi: 10.1166/jctn.2012.1990
-
[55]
Xie, Y.; Yu, H.; Zhang, H.; Fu, H. Phys. Chem. Chem. Phys. 2012, 14, 4391.
doi: 10.1039/c2cp23964g
-
[56]
Kwon, K. C.; Choi, K. S.; Kim, B. J.; Lee, J. L.; Kim, S. Y. J. Phys. Chem. C 2012, 116, 26586.
doi: 10.1021/jp3069927
-
[57]
Huang, J. H.; Fang, J. H.; Liu, C. C.; Chu, C. W. ACS Nano 2011, 5, 6262.
doi: 10.1021/nn201253w
-
[58]
Hao, J.-H.; Wang, Z.-J.; Wang, Y.-F.; Yin, Y.-H.; Jiang, R.; Jin, Q.-H. Solid State Sci. 2015, 50, 69.
doi: 10.1016/j.solidstatesciences.2015.10.015
-
[59]
Yi, T.; Zheng, B.; Yu, H.; Xie, Y. Chem. Res. Chin. Univ. 2017, 33, 631.
doi: 10.1007/s40242-017-7038-5
-
[60]
Zheng, B.; Xie, Y.; Deng, Y.-Y.; Wang, Z.-Q.; Lou, Y.-Q.; Qian, Y.-Y.; He, J.; Yu, H.-T. Adv. Theory Simul. 2020, 1900249.
-
[61]
Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.
doi: 10.1103/PhysRevLett.77.3865
-
[62]
Grimme, S. J. Comput. Chem. 2006, 27, 1787.
doi: 10.1002/jcc.20495
-
[63]
Monkhorst, H. J.; Pack, J. D. Phys. Rev. B 1976, 13, 5188.
doi: 10.1103/PhysRevB.13.5188
-
[64]
Olsen, R. A.; Kroes, G. J.; Henkelman, G.; Arnaldsson, A.; Jónsson, H. J. Chem. Phys. 2004, 121, 9776.
doi: 10.1063/1.1809574
-
[65]
Henkelman, G.; Jonsson, H. J. Chem. Phys. 2000, 113, 9978.
doi: 10.1063/1.1323224
-
[66]
Delley, B. J. Chem. Phys. 1990, 92, 508.
doi: 10.1063/1.458452
-
[67]
Tang, H.; Ismail-Beigi, S. Phys. Rev. Lett. 2007, 99, 115501.
doi: 10.1103/PhysRevLett.99.115501
-
[68]
Banerjee, S.; Periyasamy, G.; Pati, S. K. J. Mater. Chem. A 2014, 2, 3856.
doi: 10.1039/c3ta14041e
-
[69]
Zhang, X.; Hu, J.; Cheng, Y.; Yang, H. Y.; Yao, Y.; Yang, S. A. Nanoscale 2016, 8, 15340.
doi: 10.1039/C6NR04186H
-
[70]
Jiang, H. R.; Lu, Z. H.; Wu, M. C.; Ciucci, F.; Zhao, T. S. Nano Energy 2016, 23, 97.
doi: 10.1016/j.nanoen.2016.03.013
-
[71]
Jin, K. H.; Choi, S. M.; Jhi, S. H. Phys. Rev. B 2010, 82, 033414.
-
[72]
Zhang, H. ACS Nano 2015, 9, 9451.
doi: 10.1021/acsnano.5b05040
-
[73]
An, H.; Liu, C.-S.; Zeng, Z. Phys. Rev. B 2011, 83, 115456.
doi: 10.1103/PhysRevB.83.115456
-
[74]
Li, Y.; Zhou, G.; Li, J.; Gu, B.-L.; Duan, W. J. Phys. Chem. C 2008, 112, 19268.
doi: 10.1021/jp807156g
-
[75]
Wang, Y. S.; Wang, F.; Li, M.; Xu, B.; Sun, Q.; Jia, Y. Appl. Surf. Sci. 2012, 258, 8874.
doi: 10.1016/j.apsusc.2012.05.107
-
[76]
Liu, F.; Shen, C.; Su, Z.; Ding, X.; Deng, S.; Chen, J.; Xu, N.; Gao, H. J. Mater. Chem. 2010, 20, 2197.
doi: 10.1039/b919260c
-
[77]
Bae, G.; Cha, J.; Lee, H.; Park, W.; Park, N. Carbon 2012, 50, 851.
doi: 10.1016/j.carbon.2011.09.044
-
[78]
Michaelson, H. B. J. Appl. Phys. 1977, 48, 5.
doi: 10.1063/1.323361
-
[79]
Lorenzo, M.; Escher, C.; Latychevskaia, T.; Fink, H.-W. Nano Lett. 2018, 18, 3421.
doi: 10.1021/acs.nanolett.8b00359
-
[80]
Wang, G.; Shen, X.; Yao, J.; Park, J. Carbon 2009, 47, 2049.
doi: 10.1016/j.carbon.2009.03.053
-
[81]
Pan, D.; Wang, S.; Zhao, B.; Wu, M.; Zhang, H.; Wang, Y.; Jiao, Z. Chem. Mater. 2009, 21, 3136.
doi: 10.1021/cm900395k
-
[82]
Bhardwaj, T.; Antic, A.; Pavan, B.; Barone, V.; Fahlman, B. D. J. Am. Chem. Soc. 2010, 132, 12556.
doi: 10.1021/ja106162f
-
[83]
Fan, X.; Zheng, W. T.; Kuo, J.-L. ACS Appl. Mater. Interfaces 2012, 4, 2432.
doi: 10.1021/am3000962
-
[84]
Er, S.; de Wijs, G. A.; Brocks, G. J. Phys. Chem. C 2009, 113, 18962.
doi: 10.1021/jp9077079
-
[85]
Peng, X.; Tang, F.; Copple, A. J. Phys.:Condens. Matter 2012, 24, 075501.
doi: 10.1088/0953-8984/24/7/075501
-
[86]
Shan, B.; Cho, K. Phys. Rev. Lett. 2005, 94, 236602.
doi: 10.1103/PhysRevLett.94.236602
-
[87]
Leung, T. C.; Kao, C. L.; Su, W. S.; Feng, Y. J.; Chan, C. T. Phys. Rev. B 2003, 68, 195408.
doi: 10.1103/PhysRevB.68.195408

 Login In
Login In






 DownLoad:
DownLoad: