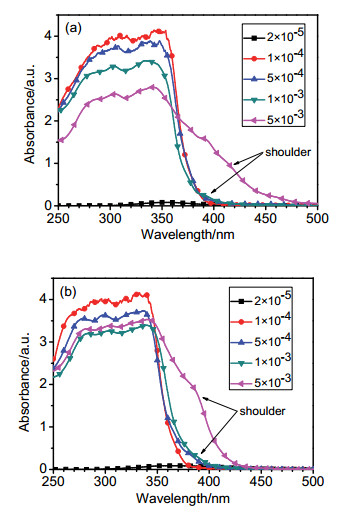
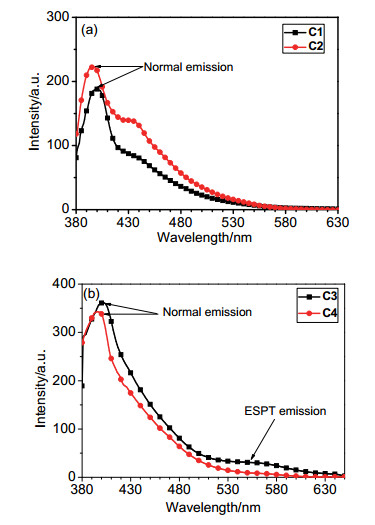
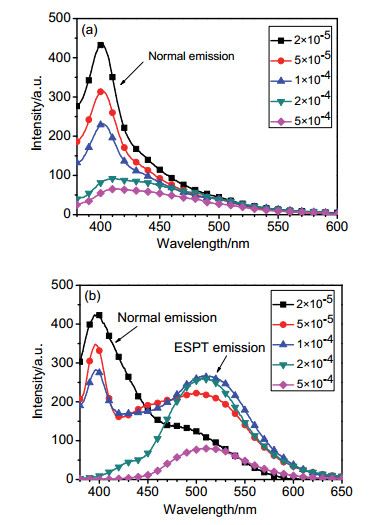
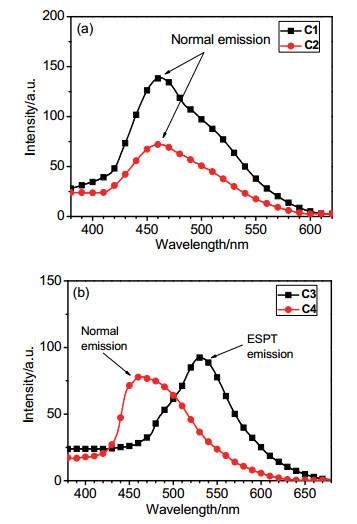
-
[1]
Ayad, S.; Posey, V.; Das, A.; Montgomery, J. M.; Hanson, K. Chem. Commun. 2019, 55(9), 1263.
doi: 10.1039/C8CC07949H
-
[2]
Li, P.; Zeng, Y.; Chen, J. P.; Li, Y. Y.; Li, Y. Acta Chim. Sinica 2012, 70, 1611.
-
[3]
Zhang, P.; Zhang, Y. M.; Lin, Q.; Yao, H.; Wei, T. B. Chin. J. Org. Chem. 2014, 34, 1300.
-
[4]
Sedgwick, A. C.; Wu, L. L.; Han, H. H.; Bull, S. D.; He, X. P.; James, T. D.; Sessler, J. L.; Tang, B. Z.; Tian, H.; Yoon, J. Y. Chem. Soc. Rev. 2018, 47(23), 8842.
doi: 10.1039/C8CS00185E
-
[5]
Wang, M.; Cheng, C.; Song, J.; Wang, J.; Zhou, X.; Xiang, H.; Liu, J. Chinese J. Chem. 2018, 36, 698.
doi: 10.1002/cjoc.201800115
-
[6]
Pan, S. N.; Tang, H. Y.; Song, Z. K.; Li, J.; Guo, Y. Chinese J. Chem. 2017, 35, 1263.
doi: 10.1002/cjoc.201600923
-
[7]
Wu, K.; Zhang, T.; Wang, Z.; Wang, L.; Zhan, L.; Zhan, L. S.; Gong, S. L.; Zhong, C.; Lu, Z. H.; Zhang, S.; Yang, C. L. J. Am. Chem. Soc. 2018, 140, 8877.
doi: 10.1021/jacs.8b04795
-
[8]
Serdiuk, I. E.; Roshal, A. D. Dyes Pigments 2017, 138, 223.
doi: 10.1016/j.dyepig.2016.11.028
-
[9]
Simkovitch, R.; Kisin-Finfer, E.; Shomer, S.; Gepshtein, R.; Shabat, D.; Huppert, D. J. Photoch. Photobio. A 2013, 254, 45.
doi: 10.1016/j.jphotochem.2013.01.004
-
[10]
Fernandez-Ramos, A.; Martinez-Nunez, E.; Vazquez, S. A.; Rios, M. A.; Estevez, C. M.; Merchan, M.; Serrano-Andres, L. J. Phys. Chem. A 2007, 111(26), 5907.
-
[11]
Hsu, S. C.; Wang, T. P.; Kao, C. L.; Chen, H. F.; Yang, P. Y.; Chen, H. Y. J. Phys. Chem. B 2013, 117(7), 2096.
doi: 10.1021/jp400299v
-
[12]
Yokoyama, H.; Watanabe, H.; Omi, T.; Ishiuchi, S. I.; Fujii, M. J. Phys. Chem. A 2001, 105(41), 9366.
doi: 10.1021/jp011245g
-
[13]
Zhao, L.; Liu, J.; Zhou, P. J. Phys. Chem. A 2016, 120, 7419.
doi: 10.1021/acs.jpca.6b05719
-
[14]
Yokoyama, H.; Watanabe, H.; Omi, T.; Ishiuchi, S. I.; Fujii, M. J. Phys. Chem. A 2001, 105(41), 9366.
doi: 10.1021/jp011245g
-
[15]
Fang, H. Spectrochim. Acta A 2019, 214, 152.
doi: 10.1016/j.saa.2019.02.016
-
[16]
Wang, D. J.; Xu, Y. Q.; Sun, S. G.; Li, H. J. Chin. J. Appl. Chem. 2018, 35(1), 1.
-
[17]
Qin, X. Z.; Li, G.; Wang, Z. Q.; Ding, G..; Luo, Z. P.; Li, H. R.; Chen, L. Y.; Gao, F. Dyes Pigments 2017, 145, 538.
doi: 10.1016/j.dyepig.2017.06.055
-
[18]
Wang, Y.; Li, M.; Zhang, Y.; Yang, J.; Zhu, S.; Sheng, L.; Zhang, S. X. A. Chem. Commun. 2013, 49(59), 6587.
doi: 10.1039/C3CC42747A
-
[19]
Casanovas, J.; Namba, A. M.; León, S.; Aquino, G. L.; da Silva, G. V. J.; Alemán, C. J. Org. Chem. 2001, 66, 3775.
doi: 10.1021/jo0016982
-
[20]
Yi, P. G.; Yang, X. C.; Liu, J.; Hou, B.; Yu, X. Y.; Li, X. F.; Wang, Z. X.; Zheng, B. S. Chin. J. Org. Chem. 2013, 33, 1451.
-
[21]
Ingham, K.; El-Bayoumi, M. A. J. Am. Chem. Soc. 1974, 96(6), 1674.
doi: 10.1021/ja00813a006
-
[22]
Parada, G. A.; Markle, T. F.; Glover, S. D.; Hammarström, L.; Ott, S.; Zietz, B. Chem.-Eur. J. 2015, 21, 6362.
doi: 10.1002/chem.201500244
-
[23]
Qin, T. Y.; Zeng, Y.; Chen, J. P.; Yu, T. J.; Li, Y. Acta Chim. Sinica 2017, 75, 1164.
doi: 10.3969/j.issn.0253-2409.2017.10.002
-
[24]
Doroshenko, A. O.; Posokhov, E. A.; Verezubova, A. A.; Ptyagina, L. M. J. Phys. Org. Chem. 2000, 13, 253.
doi: 10.1002/1099-1395(200005)13:5<253::AID-POC238>3.0.CO;2-D
-
[25]
Mehata, M. S.; Joshi, H. C.; Tripathi, H. B. Chem. Phys. Lett. 2002, 359(3-4), 314.
doi: 10.1016/S0009-2614(02)00716-9
-
[26]
Bulska, H.; Chodkowska, A. J. Am. Chem. Soc. 1980, 102(9), 3259.
doi: 10.1021/ja00529a069

 Login In
Login In






 DownLoad:
DownLoad: