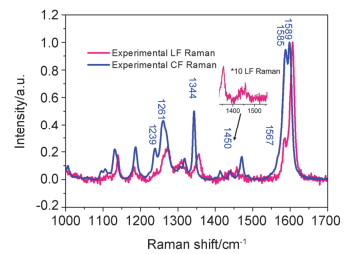
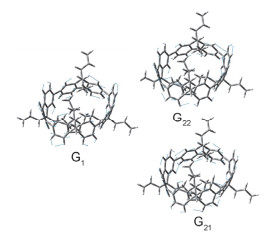
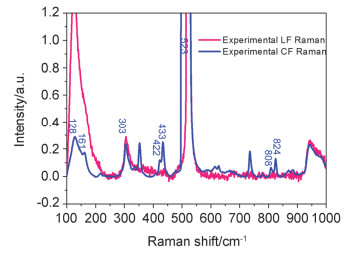
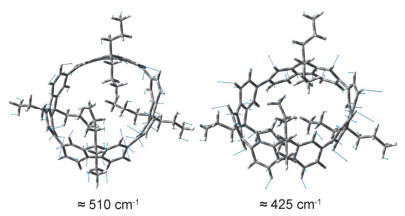
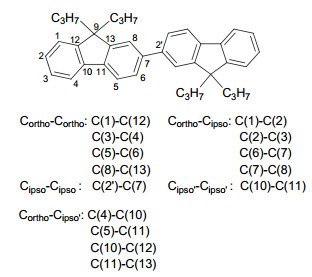
-
[1]
Xie, L. H.; Yin, C. R.; Lai, W. Y.; Fan, Q. L.; Huang, W. Prog. Polym. Sci. 2012, 37, 1192.
doi: 10.1016/j.progpolymsci.2012.02.003
-
[2]
Sun, M. L.; Xu, R. C.; Xie, L. H.; Wei, Y.; Huang, W. Chinese J. Chem. 2015, 33, 815.
doi: 10.1002/cjoc.v33.8
-
[3]
Qian, Y.; Zhang, X.; Xie, L. H.; Qi, D.; Chandran, B. K.; Chen, X.; Huang, W. Adv. Mater. 2016, 28, 9243.
doi: 10.1002/adma.201601278
-
[4]
Bao, Z. N.; Rogers, J. A.; Katz, H. E. J. Mater. Chem. 1999, 9, 1895.
doi: 10.1039/a902652e
-
[5]
Park, S.; Lee, M. H.; Ahn, K. S.; Choi, H. H.; Shin, J.; Xu, J.; Mei, J. G.; Cho, K.; Bao, Z. A.; Lee, D. R.; Kang, M. S.; Kim, D. H. Adv. Funct. Mater. 2016, 26, 4627.
doi: 10.1002/adfm.v26.26
-
[6]
Liu, Y.; Yuan, J.; Zou, Y. P.; Li, Y. F. Acta Chim. Sinica 2017, 75, 257.
doi: 10.3969/j.issn.0253-2409.2017.03.001
-
[7]
Songbuer; Li, M. H.; Imerhasan, M. Chin. J. Org. Chem. 2018, 38, 594.
-
[8]
Yamago, S.; Kayahara, E.; Iwamoto, T. Chem. Rec. 2014, 14, 84.
doi: 10.1002/tcr.v14.1
-
[9]
Lewis, S. E. Chem. Soc. Rev. 2015, 44, 2221.
doi: 10.1039/C4CS00366G
-
[10]
Darzi, E. R.; Jasti, R. Chem. Soc. Rev. 2015, 44, 6401.
doi: 10.1039/C5CS00143A
-
[11]
Segawa, Y.; Yagi, A.; Itami, K. Phys. Sci. Rev. 2017, 2, 20160102.
-
[12]
Kayahara, E.; Kouyama, T.; Kato, T.; Yamago, S. J. Am. Chem. Soc. 2016, 138, 338.
doi: 10.1021/jacs.5b10855
-
[13]
Liu, Y. Y.; Lin, J. Y.; Bo, Y. F.; Xie, L. H.; Yi, M. D.; Zhang, X. W.; Zhang, H. M.; Loh, T. P.; Huang, W. Org. Lett. 2016, 18, 172.
doi: 10.1021/acs.orglett.5b03038
-
[14]
Fujitsuka, M.; Cho, D. W.; Iwamoto, T.; Yamago, S.; Majima, T. Phys. Chem. Chem. Phys. 2012, 14, 14585.
doi: 10.1039/c2cp42712e
-
[15]
Segawa, Y.; Fukazawa, A.; Matsuura, S.; Omachi, H.; Yamaguchi, S.; Irle, S.; Itami, K. Org. Biomol. Chem. 2012, 10, 5979.
doi: 10.1039/c2ob25199j
-
[16]
Camacho, C.; Niehaus, T. A.; Itami, K.; Irle, S. Chem. Sci. 2013, 4, 187.
doi: 10.1039/C2SC20878D
-
[17]
Castiglioni, C.; Delzoppo, M.; Zerbi, G. J. Raman Spectrosc. 1993, 24, 485.
doi: 10.1002/jrs.v24:8
-
[18]
Rebelo, S. L.; Guedes, A.; Szefczyk, M. E.; Pereira, A. M.; Araujo, J. P.; Freire, C. Phys. Chem. Chem. Phys. 2016, 18, 12784.
doi: 10.1039/C5CP06519D
-
[19]
Moura, L. G.; Moutinho, M. V. O.; Venezuela, P.; Mauri, F.; Righi, A.; Strano, M. S.; Fantini, C.; Pimenta, M. A. Carbon 2017, 117, 41.
doi: 10.1016/j.carbon.2017.02.048
-
[20]
Piao, Y.; Simpson, J. R.; Streit, J. K.; Ao, G.; Zheng, M.; Fagan, J. A.; Hight Walker, A. R. ACS Nano 2016, 10, 5252.
doi: 10.1021/acsnano.6b01031
-
[21]
Fujitsuka, M.; Iwamoto, T.; Kayahara, E.; Yamago, S.; Majima, T. ChemPhysChem 2013, 14, 1570.
doi: 10.1002/cphc.v14.8
-
[22]
Chen, H.; Golder, M. R.; Wang, F.; Jasti, R.; Swan, A. K. Carbon 2014, 67, 203.
doi: 10.1016/j.carbon.2013.09.082
-
[23]
Alvarez, M. P.; Burrezo, P. M.; Kertesz, M.; Iwamoto, T.; Yamago, S.; Xia, J.; Jasti, R.; Navarrete, J. T. L.; Taravillo, M.; Baonza, V. G. Angew. Chem. Int. Ed. 2014, 53, 7033.
doi: 10.1002/anie.201400719
-
[24]
Chen, H.; Golder, M. R.; Wang, F.; Doorn, S. K.; Jasti, R.; Tretiak, S.; Swan, A. K. J. Phys. Chem. C 2015, 119, 2879.
doi: 10.1021/jp5117195
-
[25]
Pena-Alvarez, M.; Qiu, L.; Taravillo, M.; Baonza, V. G.; Delgado, M. C.; Yamago, S.; Jasti, R.; Navarrete, J. T.; Casado, J.; Kertesz, M. Phys. Chem. Chem. Phys. 2016, 18, 11683.
doi: 10.1039/C5CP05500H
-
[26]
Liu, Y. Y.; Li, J. W.; Bo, Y. F.; Yang, L.; Zhang, X. F.; Xie, L. H.; Yi, M. D.; Huang, W. Acta Phys.-Chim. Sin. 2017, 33, 1803.
-
[27]
Ariu, M.; Lidzey, D. G.; Bradley, D. D. C. Synthetic Met. 2000, 111, 607.
-
[28]
Arif, M.; Volz, C.; Guha, S. Phys. Rev. Lett. 2006, 96, 025503.
doi: 10.1103/PhysRevLett.96.025503
-
[29]
Volz, C.; Arif, M.; Guha, S. J. Chem. Phys. 2007, 126, 064905.
-
[30]
Tsoi, W. C.; Lidzey, D. G. J. Phys. Condens. Mat. 2008, 20, 125213.
doi: 10.1088/0953-8984/20/12/125213
-
[31]
Liu, B.; Lin, J. Y.; Liu, F.; Yu, M. N.; Zhang, X. W.; Xia, R. D.; Yang, T.; Fang, Y. T.; Xie, L. H.; Huang, W. ACS Appl. Mater. Inter. 2016, 8, 21648.
doi: 10.1021/acsami.6b05247
-
[32]
Irle, S.; Lischka, H. J. Mol. Struc.-Theochem 1996, 364, 15.
doi: 10.1016/0166-1280(95)04465-5

 Login In
Login In

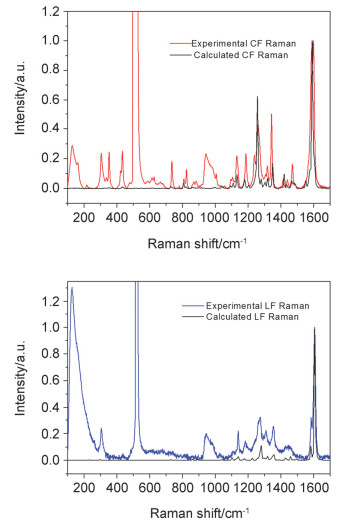





 DownLoad:
DownLoad: