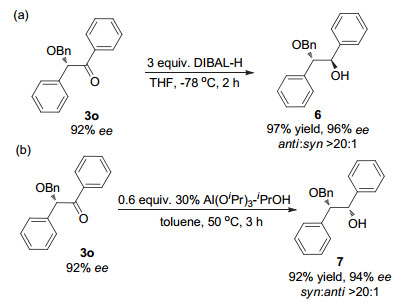
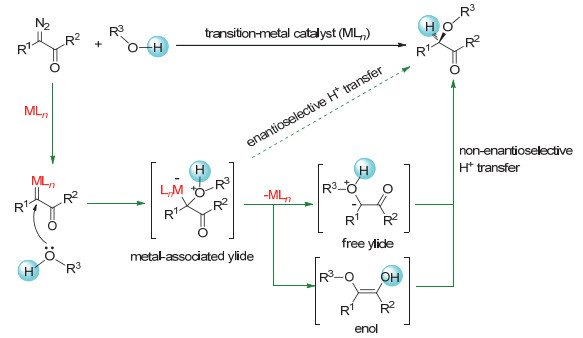
-
[1]
-
[2]
For selected reviews, see: (a) Ye, T.; Mckervey, M. A. Chem.Rev. 1994, 94, 1091. (b) Zhang, Z.-H.; Wang, J.-B. Tetrahedron 2008, 64, 6577. (c) Zhu, S.-F.; Zhou, Q.-L. Acc.Chem.Res. 2012, 45, 1365. (d) Fei, N.; Gillingham, D. Chem.Soc.Rev. 2013, 42, 4918. (e) Zhu, S.-F.; Zhou, Q.-L. Nat.Sci.Rev. 2014, 1, 580. (f) Maguire, A. R.; McKervey, M. A. Chem.Rev. 2015, 115, 9981.
-
[3]
For selected examples, see: (a) Maier, T. C.; Fu, G. C. J.Am.Chem.Soc. 2006, 128, 4594. (b) Chen, C.; Zhu, S.-F.; Liu, B.; Wang, L.-X.; Zhou, Q.-L. J.Am. Chem.Soc. 2007, 129, 12616. (c) Zhu, S.-F.; Chen, C.; Cai, Y.; Zhou, Q.-L. Angew.Chem., Int.Ed. 2008, 47, 932. (d) Zhu, S.-F.; Song, X.-G.; Li, Y.; Cai, Y.; Zhou, Q.-L. J. Am.Chem.Soc. 2010, 132, 16374. (e) Zhu, S.-F.; Cai, Y.; Mao, H.-X.; Xie, J.-H.; Zhou, Q.-L. Nat.Chem. 2010, 2, 546. (f) Osako, T.; Panichakul, D.; Uozumi, Y. Org.Lett. 2012, 14, 194. (g) Song, X.-G.; Zhu, S.-F.; Xie, X.-L.; Zhou, Q.-L. Angew.Chem., Int.Ed. 2013, 52, 2555. (h) Xie, X.-L.; Zhu, S.-F.; Guo, J.-X.; Cai, Y.; Zhou, Q.-L. Angew.Chem., Int.Ed. 2014, 53, 2978. (i) Tan, F.; Liu, X.-H.; Hao, X.-Y.; Tang, Y.; Lin, L.-L.; Feng, X.-M. ACS Catal. 2016, 6, 6930. (j) Zhang, Y.-L.; Yao, Y.; He, L.; Liu, Y.; Shi, L. Adv.Synth. Catal. 2017, 359, 2754. (k) Huang, D.-R.; Xu, G.-Y.; Peng, S.-Y.; Sun, J.-T. Chem.Commun. 2017, 53, 3197.
-
[4]
For preparation and applications of α-diazoketones in catalytic asymmetric reactions, see: (a) Doyle, M. P.; McKervey, M. A.; Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998. (b) Doyle, M. P.; Eismont, M. Y.; Zhou, Q.-L. Russ. Chem.Bull. 1997, 46, 955. (c) Kitagaki, S.; Anada, M.; Kataoka, O.; Matsuno, K.; Umeda, C.; Watanabe, N.; Hashimoto, S. J.Am. Chem.Soc. 1999, 121, 1417. (d) Barberis, M.; Pérez-Prieto, J.; Stiriba, S.-E.; Lahuerta, P. Org.Lett. 2001, 3, 3317. (e) Hwang, C. H.; Chong, Y. H.; Song, S. Y.; Kwak, H. S.; Lee, E. Chem. Commun. 2004, 816. (f) Suga, H.; Ishimoto, D.; Higuchi, S.; Ohtsuka, M.; Arikawa, T.; Tsuchida, T.; Kakehi, A.; Baba, T. Org.Lett. 2007, 9, 4359. (g) Taber, D. F.; Tian, W. J.Org.Chem. 2008, 73, 7560. (h) Denton, J. R.; Davies, H. M. L. Org.Lett. 2009, 11, 787. (i) Xu, X.; Qian, Y.; Yang, L.; Hu, W. Chem.Commun. 2011, 47, 797. (j) Qian, Y.; Jing, C.; Liu, S.; Hu, W. Chem. Commun. 2013, 49, 2700. (k) Taber, D. F.; Paquette, C. M.; Gu, P.; Tian, W. J.Org.Chem. 2013, 78, 9772.
-
[5]
For non-enantioselective O—H insertion using α-diazoketones as carbene precursors, see: (a) Yates, P. J.Am.Chem.Soc. 1952, 74, 5376. (b) Shinada, T.; Kawakami, T.; Sakai, H.; Takada, I.; Ohfune, Y. Tetrahedron Lett. 1998, 39, 3757. (c) Nelson, T. D.; Song, Z. J.; Thompson, A. S.; Zhao, M.; DeMarco, A.; Reamer, R. A.; Huntington, M. F.; Grabowsk, E. J.; Reider, P. J. Tetrahedron Lett. 2000, 41, 1877. (d) Muthusamy, S.; Babu, S. A.; Gunanathan, C. Tetrahedron Lett. 2002, 43, 3133. (e) Ronan, B.; Bacqué, E.; Barrière, J. C. Tetrahedron 2004, 60, 3819. (f) Muthusamy, S.; Gnanaprakasam, B.; Suresh, E. Org.Lett. 2005, 7, 4577. (g) Jung, J. C.; Avery, M. A. Tetrahedron Lett. 2006, 47, 7969.
-
[6]
-
[7]
-
[8]
Franklin, A. D.; Haque, M. S.; Robert, M. P. J. Org.Chem. 1989, 54, 2021.
doi: 10.1021/jo00269a054
-
[9]
Yin, J.-J.; Mark, A. H.; Karen, M. C.; Joseph, D. A. J.Org.Chem. 2006, 71, 840.
doi: 10.1021/jo052121t
-
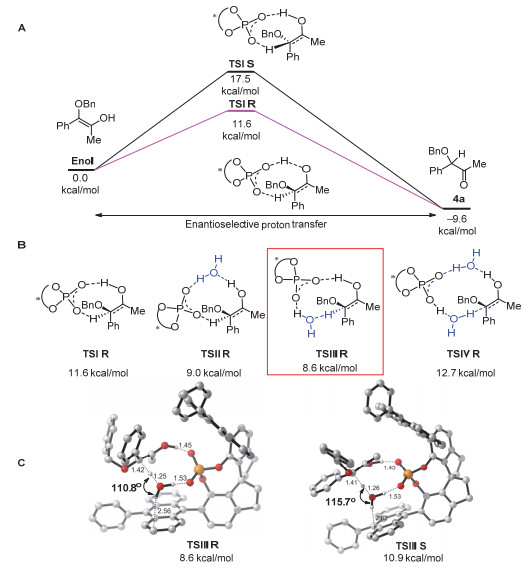
[10]
For details of DFT calculation, see supporting information and relevant literature ref. 6c.
-
[11]
Liang, Y.; Zhou, H.-L.; Yu, Z.-X. J.Am.Chem.Soc. 2009, 131, 17783.
doi: 10.1021/ja9086566

 Login In
Login In






 DownLoad:
DownLoad: