Citation:
Sun Guofeng, Su Min, Fang Jie, Borzov Maxim, Nie Wanli. Research of the Stereoselectivity and Mechanism of the Hydroboration Reaction Between B(C6F5)3/Ammonium Chloride Systems with Terminal Alkyne[J]. Acta Chimica Sinica,
;2017, 75(8): 824-830.
doi:
10.6023/A17040141

-
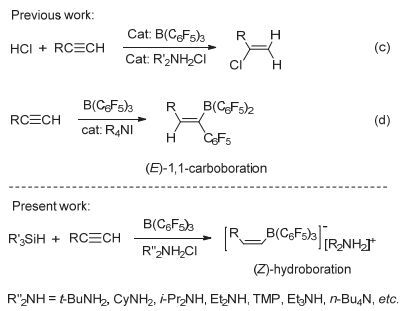
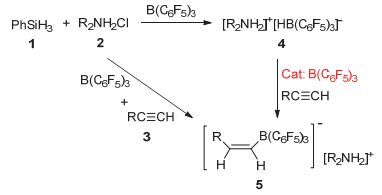
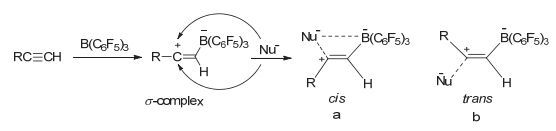
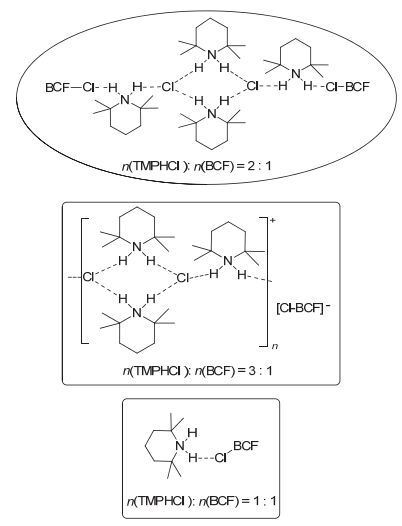
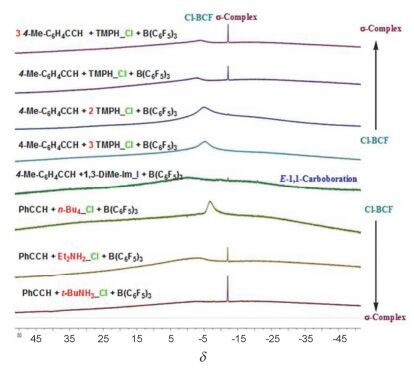
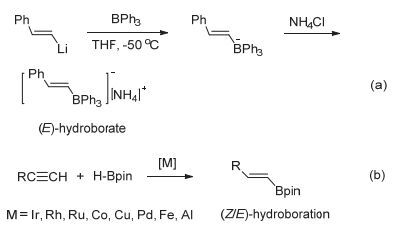
Stereoselective hydroboration reaction of alkynes has been considered as one of the most important organic reaction. To date a handful of metal-catalyzed systems have been demonstrated to achieve trans-hydroboration of alkynes. This paper describes the first non-metal-catalyzed systems which could stereoselectively hydroborate the terminal alkynes in a trans-configuration. The Lewis acid B(C6F5)3 and ammonium chloride have been used as the reaction substrates, and phenylsilane as the hydride source. The hydroboration reaction could be performed in a one-pot procedure by mixing of B(C6F5)3, ammonium chloride and silane together in an equivalent amount. But this one-pot reaction is not so nice since there is always mixed with the ammonium hydroborate[R2NH2]+[H-B(C6F5)3]- intermediates products. A series of ammonium hydroborates prepared from the corresponding primary, secondary, tertiary and quaternary amine hydrochlorides have been isolated, and used in the directly hydroboration with terminal alkynes. To our surprise the ammonium hydroborate[R2NH2]+[H-B(C6F5)3]- could not react with the alkynes alone. When using[R2NH2]+[H-B(C6F5)3]- to react with alkynes, trace amount of catalytic Lewis acid B(C6F5)3 is necessary to firstly activate the carbon-carbon triple bonds and form the crucial zwitterionic σ-complexes. The mechanism study has shown that different from the typical Lewis acid/Lewis base FLPs system reacted with alkynes, in this B(C6F5)3/ammonium chloride system the ammonium chloride plays an important role on the stereoselective control of the reaction. The week interaction between the Cl ion and B(C6F5)3 in the σ-complexes has not only slowed down the unfavorite 1, 1-carboboration reaction, but also stabilized the σ-complexes which has offer the chance for the nucleophilic reagent to attack the reaction center in a cis-or trans-mode. In our experiment the bulky ion[H-B(C6F5)3]-could only attach the active alkynes from the trans-side and form the Z-hydroboration product. This work demonstrates that the combination of the ammonium halides with the Lewis acid B(C6F5)3 could act as a novel "frustrated Lewis pair" to activate alkynes, and will enable the development of even more sophisticated FLP and related catalyzed reactions.
-
Keywords:
- ammonium chloride,
- B(C6F5)3,
- silane,
- alkyne,
- hydroboration
-

-
-
[1]
Kropp, M. A.; Baillargeon, M.; Park, K. M.; Ahamidapaty, K.; Schuster, G. B. J. Am. Chem. Soc. 1991, 113, 2155. doi: 10.1021/ja00006a038
-
[2]
Ohmura, T.; Yamamoto, Y.; Miyaura, N. J. Am. Chem. Soc. 2000, 122, 4990. doi: 10.1021/ja0002823
-
[3]
Gunanathan, C.; Hoelscher, M.; Pan, F.; Leitner, W. J. Am. Chem. Soc. 2012, 134, 14349. doi: 10.1021/ja307233p
-
[4]
Obligacion, J. V.; Neely, J. M.; Yazdani, A. N.; Pappas, I.; Chirik, P. J. J. Am. Chem. Soc. 2015, 137, 5855. doi: 10.1021/jacs.5b00936
-
[5]
-
[6]
Xu, S.-M.; Zhang, Y.-Z.; Li, B.; Liu, S. H.-Y. J. Am. Chem. Soc. 2016, 138(44), 14566. doi: 10.1021/jacs.6b09759
-
[7]
-
[8]
Bismuto, A.; Thomas, S. P.; Cowley, M. J. Angew. Chem., Int. Ed. 2016, 55, 15356. doi: 10.1002/anie.v55.49
-
[9]
Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124. doi: 10.1126/science.1134230
-
[10]
Welch, G. C.; Stephan, D. W. J. Am. Chem. Soc. 2007, 129, 1880. doi: 10.1021/ja067961j
-
[11]
Chen, D.-J.; Wang, Y.; Klankermayer, J. Angew. Chem., Int. Ed. 2010, 49, 9475. doi: 10.1002/anie.201004525
-
[12]
Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46. doi: 10.1002/anie.200903708
-
[13]
Stephan, D. W. Acc. Chem. Res. 2015, 48, 306. doi: 10.1021/ar500375j
-
[14]
Chen, C.; Kehr, G..; Fröhlich, R.; Erker, G. J. Am. Chem. Soc. 2010, 132, 13594. doi: 10.1021/ja106365j
-
[15]
Chen, C.; Voss, T.; Fröhlich, R.; Kehr, G.; Erker, G. Org. Lett. 2011, 13, 62. doi: 10.1021/ol102544x
-
[16]
Jiang, C.; Blacque, O.; Berke, H. Organometallics 2010, 29, 125. doi: 10.1021/om9008636
-
[17]
Reddy, J. S.; Xu, B.-H.; Mahdi, T.; Fröhlich, R.; Kehr, G.; Stephan, D. W.; Erker, G. Organometallics 2012, 31, 5638. doi: 10.1021/om3006068
-
[18]
Nie, W.-L.; Klare, H. F. T.; Oestreich, M.; FrÖhlich, R.; Kehr, G.; Erker, G. Z. Naturforsch. 2012, 67b, 987.
-
[19]
Xu, Y.-Y.; Li, Z.; Borzov, M.; Nie, W.-L. Chem. Prog. 2012, 24(8), 1526.
-
[20]
Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1203.
-
[21]
Hu, X.; Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1025. doi: 10.3866/PKU.WHXB201504141
-
[22]
Wen, Z.-G.; Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2016, 74, 498
-
[23]
-
[24]
Nie, W.-L.; Sun, G.-F.; Tian, C.; Borzov, M. Naturforsch. 2016, 71(10) b, 1029.
-
[25]
-
[1]
-

-
-
[1]
Jiamin Li , Wenyue Zhong , Kin Shing Chan . “烯”君入瓮又入学——据元素周期表与酸碱理论谈烯烃教学. University Chemistry, 2025, 40(6): 177-182. doi: 10.12461/PKU.DXHX202408040
-
[2]
Daojuan Cheng , Fang Fang . Exploration and Implementation of Science-Education Integration in Organic Chemistry Teaching for Pharmacy Majors: A Case Study on Nucleophilic Substitution Reactions of Alkyl Halides. University Chemistry, 2024, 39(11): 72-78. doi: 10.12461/PKU.DXHX202403105
-
[3]
Yujie WANG , Laobang WANG , Zheng ZHANG , Qi LIU , Jianping LANG . Construction of W/Cu/S cluster-based supramolecular compounds via alkynyl/sulfur cycloaddition and their third-order nonlinear optical properties. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 2069-2077. doi: 10.11862/CJIC.20250129
-
[4]
Yinuo Wang , Siran Wang , Yilong Zhao , Dazhen Xu . Selective Synthesis of Diarylmethyl Anilines and Triarylmethanes via Multicomponent Reactions: Introduce a Comprehensive Experiment of Organic Chemistry. University Chemistry, 2024, 39(8): 324-330. doi: 10.3866/PKU.DXHX202401063
-
[5]
Bolin Sun , Jie Chen , Ling Zhou . 乙烯型卤代烃的亲核取代反应. University Chemistry, 2025, 40(8): 152-157. doi: 10.12461/PKU.DXHX202410032
-
[6]
Guowen Xing , Guangjian Liu , Le Chang . Five Types of Reactions of Carbonyl Oxonium Intermediates in University Organic Chemistry Teaching. University Chemistry, 2025, 40(4): 282-290. doi: 10.12461/PKU.DXHX202407058
-
[7]
Yue Zhao , Yanfei Li , Tao Xiong . Copper Hydride-Catalyzed Nucleophilic Additions of Unsaturated Hydrocarbons to Aldehydes and Ketones. University Chemistry, 2024, 39(4): 280-285. doi: 10.3866/PKU.DXHX202309001
-
[8]
Hui-Ying Chen , Hao-Lin Zhu , Pei-Qin Liao , Xiao-Ming Chen . Integration of Ru(Ⅱ)-Bipyridyl and Zinc(Ⅱ)-Porphyrin Moieties in a Metal-Organic Framework for Efficient Overall CO2 Photoreduction. Acta Physico-Chimica Sinica, 2024, 40(4): 2306046-0. doi: 10.3866/PKU.WHXB202306046
-
[9]
Shiyi WANG , Chaolong CHEN , Xiangjian KONG , Lansun ZHENG , Lasheng LONG . Polynuclear lanthanide compound [Ce4ⅢCe6Ⅳ(μ3-O)4(μ4-O)4(acac)14(CH3O)6]·2CH3OH for the hydroboration of amides to amine. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 88-96. doi: 10.11862/CJIC.20240342
-
[10]
Jiajie Li , Xiaocong Ma , Jufang Zheng , Qiang Wan , Xiaoshun Zhou , Yahao Wang . Recent Advances in In-Situ Raman Spectroscopy for Investigating Electrocatalytic Organic Reaction Mechanisms. University Chemistry, 2025, 40(4): 261-276. doi: 10.12461/PKU.DXHX202406117
-
[11]
Yan Qi , Yueqin Yu , Weisi Guo , Yongjun Liu . 过渡金属参与的有机反应案例教学与实践探索. University Chemistry, 2025, 40(6): 111-117. doi: 10.12461/PKU.DXHX202411021
-
[12]
Yansong Xiao , Yi Huang , Xingxing Ma , Qiuling Song . The Matteson Reaction in Organic Synthesis: From Fundamentals to Frontiers. University Chemistry, 2025, 40(10): 114-120. doi: 10.12461/PKU.DXHX202411023
-
[13]
Ruige ZHANG , Zhe ZHANG , He ZHENG , Zhan SHI . Recent advances of metal-organic frameworks for alkaline electrocatalytic oxygen evolution reaction. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 2011-2028. doi: 10.11862/CJIC.20250185
-
[14]
Zhengyu Zhou , Huiqin Yao , Youlin Wu , Teng Li , Noritatsu Tsubaki , Zhiliang Jin . Synergistic Effect of Cu-Graphdiyne/Transition Bimetallic Tungstate Formed S-Scheme Heterojunction for Enhanced Photocatalytic Hydrogen Evolution. Acta Physico-Chimica Sinica, 2024, 40(10): 2312010-0. doi: 10.3866/PKU.WHXB202312010
-
[15]
Xiaogang YANG , Xinya ZHANG , Jing LI , Huilin WANG , Min LI , Xiaotian WEI , Xinci WU , Lufang MA . Synthesis, structure, and photoelectric properties of Zinc(Ⅱ)-triphenylamine based metal-organic framework. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 2078-2086. doi: 10.11862/CJIC.20250167
-
[16]
Baitong Wei , Jinxin Guo , Xigong Liu , Rongxiu Zhu , Lei Liu . Theoretical Study on the Structure, Stability of Hydrocarbon Free Radicals and Selectivity of Alkane Chlorination Reaction. University Chemistry, 2025, 40(3): 402-407. doi: 10.12461/PKU.DXHX202406003
-
[17]
Peiran ZHAO , Yuqian LIU , Cheng HE , Chunying DUAN . A functionalized Eu3+ metal-organic framework for selective fluorescent detection of pyrene. Chinese Journal of Inorganic Chemistry, 2024, 40(4): 713-724. doi: 10.11862/CJIC.20230355
-
[18]
Weina Wang , Lixia Feng , Fengyi Liu , Wenliang Wang . Computational Chemistry Experiments in Facilitating the Study of Organic Reaction Mechanism: A Case Study of Electrophilic Addition of HCl to Asymmetric Alkenes. University Chemistry, 2025, 40(3): 206-214. doi: 10.12461/PKU.DXHX202407022
-
[19]
Dan Liu . 可见光-有机小分子协同催化的不对称自由基反应研究进展. University Chemistry, 2025, 40(6): 118-128. doi: 10.12461/PKU.DXHX202408101
-
[20]
Yan Kong , Wei Wei , Lekai Xu , Chen Chen . Electrochemical Synthesis of Organonitrogen Compounds from N-integrated CO2 Reduction Reaction. Acta Physico-Chimica Sinica, 2024, 40(8): 2307049-0. doi: 10.3866/PKU.WHXB202307049
-
[1]
Metrics
- PDF Downloads(11)
- Abstract views(3737)
- HTML views(813)

 Login In
Login In




 DownLoad:
DownLoad: