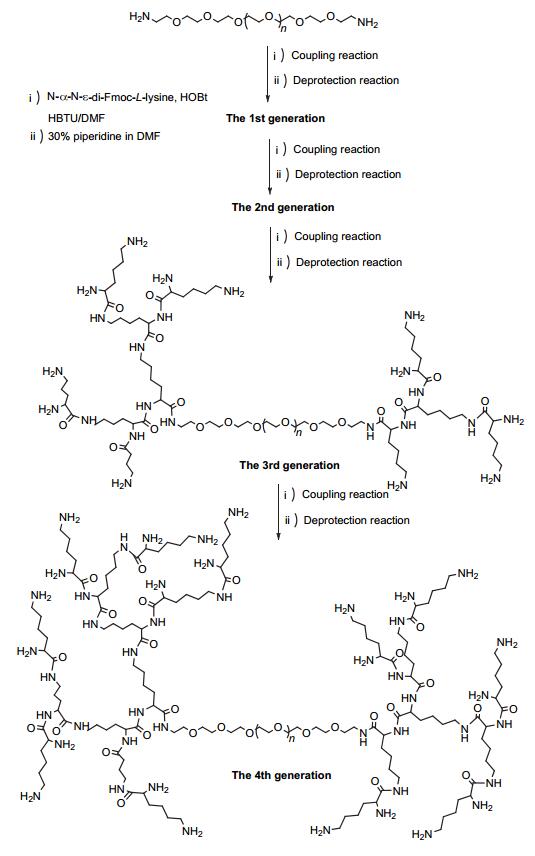
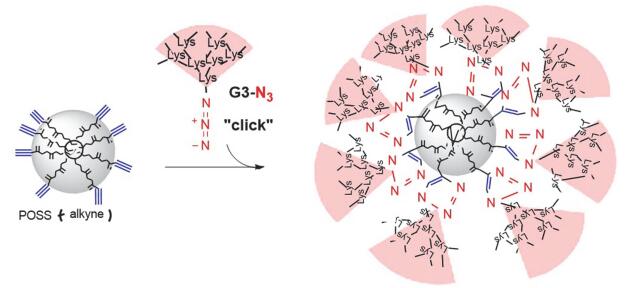
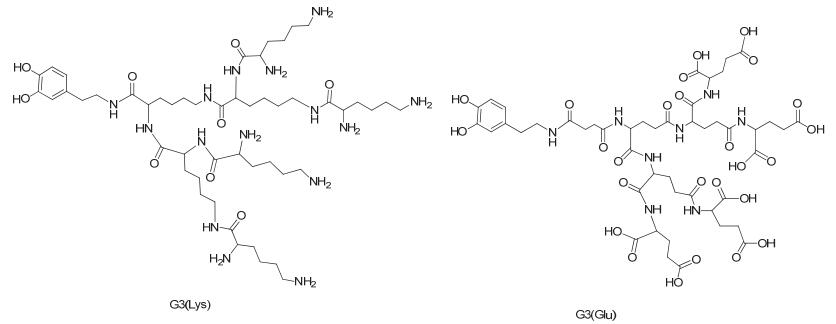
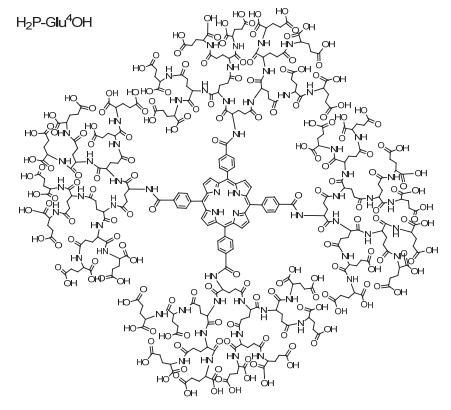
Citation:
Zhang Shaofei, Yang Jiandong, Liu Mingzhu, Lü Shaoyu, Gao Chunmei, Wu Can, Zhu Zhaoyan. Synthesis of Peptide Dendrimers and Their Application in the Drug Delivery System[J]. Acta Chimica Sinica,
;2016, 74(5): 401-409.
doi:
10.6023/A16020096

-
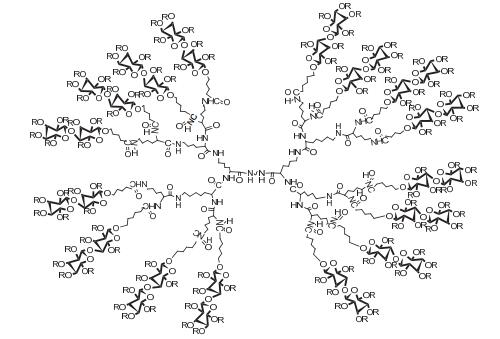
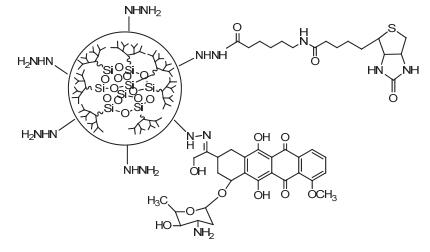
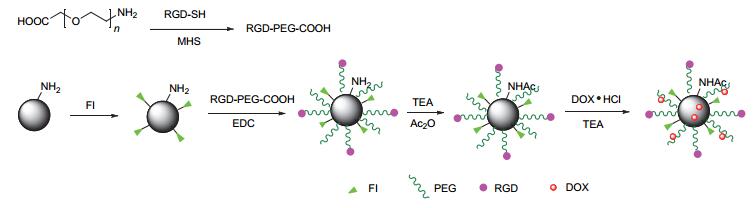
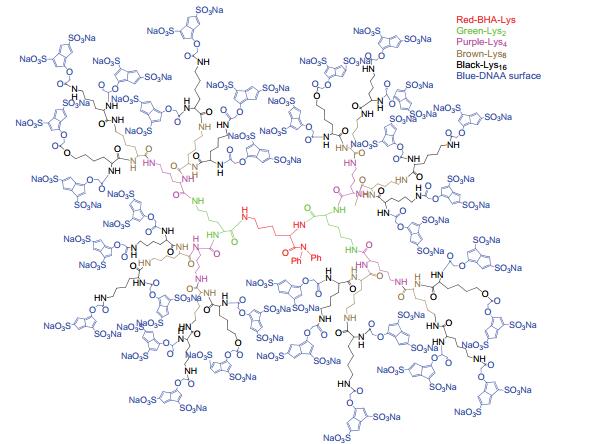
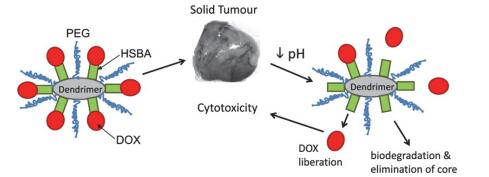
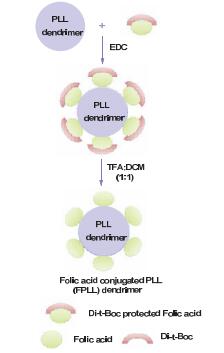
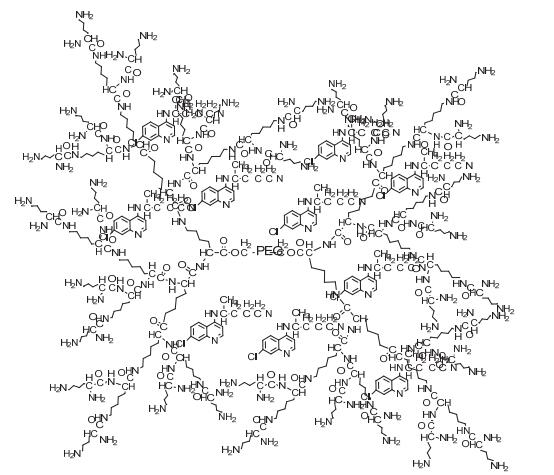
Dendrimers are a novel polymer material, which have received more and more attention due to the functional groups on their surface, hydrophobic cavity and adjustable sizes. Thus, dendrimers have been widely used in many fields. Peptide dendrimer is a sort of dendritic polymer, which contains peptide bonds in the structure. Owing to the globular structure similar to the protein, excellent water solubility, biocompatibility, biodegradability and low toxicity, peptide dendrimer could be used as drug delivery carrier. In addition, hydrophobic cavity can be used to solubilize hydrophobic drugs, in which the drugs can be released slowly. The present review highlights the current status of synthesis of peptide dendrimers, and it also summarizes and forecasts the interaction mechanism between drug molecules and peptide dendrimers, and the application of peptide dendrimers in drug delivery system.
-
Keywords:
- polymer material,
- peptide dendrimers,
- globular structure,
- drug delivery,
- carrier
-

-
-
[1]
Tomalia, D.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Polym. J. 1985, 17, 117. doi: 10.1295/polymj.17.117
-
[2]
Newkome, G. R.; Yao, Z.; Baker, G. R.; Gupta, V. K. J. Org. Chem. 1985, 50, 2003. doi: 10.1021/jo00211a052
-
[3]
Tian, W.; Ma, Y. Chem. Soc. Rev. 2013, 42, 707.
-
[4]
Nanjwade, B. K.; Bechra, H. M.; Derkar, G. K.; Manvi, F. V.; Nanjwade, V. K. Eur. J. Pharm. Sci. 2009, 38, 189.
-
[5]
Surendra, T.; Malay, K. D. J. Appl. Pharm. Sci. 2013, 3, 143.
-
[6]
Klajnert, B.; Bryszewskar, M. Acta Biochim. Pol. 2001, 48, 203.
-
[7]
Prashant, K.; Keerti, J.; Narendra, K. J. Prog. Polym. Sci. 2014, 39, 276.
-
[8]
Li, J.; Zeng, Y.; Zhang, X.; Yu, T.; Chen, J.; Li, Y. Acta Chim. Sinica 2014, 72, 1158.
-
[9]
Li, J.; Zeng, Y.; Zhang, X.; Yu, T.; Chen, J.; Li, Y. Acta Chim. Sinica 2015, 73, 827.
-
[10]
Elizabeth, R.; Gillies, J.; Fréchet, M. J. Drug Discov. Today 2005, 10, 38.
-
[11]
Marie, V.; Walter; Michael, M. Chem. Soc. Rev. 2012, 41, 4593. doi: 10.1039/c2cs35062a
-
[12]
Elham, A.; Sedigheh, F. A.; Abolfazl, A.; Morteza, M.; Hamid, T. N.; Sang, W. J.; Younes, H.; Kazem, N.-K.; Roghiyeh, P.-A. Nanoscale Res. Lett. 2014, 9, 248. doi: 10.1186/1556-276X-9-248
-
[13]
Laia, C.; Glòria, S.; Miquel, P.; Ernest, G.; Miriam, R.; Fernando, A. Chem. Rev. 2005, 105, 1670.
-
[14]
She, W. C.; Xu, X. H.; Wang, G.; Luo, K.; Gu, Z. W. Mater. China 2012, 31, 21.
-
[15]
Gu, Z. W.; Luo, K.; She, W. C.; Wu, Y.; He, B. Scientia Sinica Chimica 2010, 40, 210.
-
[16]
Xu, X.; Yuan, H.; Chang, J.; He, B.; Gu, Z. Angew. Chem. Int. Ed. 2012, 124, 3185.
-
[17]
Wang, F.; Xu, L.; Chu, G.; Shi, J.; Guo, Q. Chin. J. Org. Chem. 2016, 36, 218. doi: 10.6023/cjoc201505014
-
[18]
Merrifield, R. B. J. Am. Chem. Soc. 1964, 3, 1385.
-
[19]
Daniel, K. S.; Sahar, M.; Ulrik, B. Tetrahedron Lett. 2014, 55, 3942. doi: 10.1016/j.tetlet.2014.04.127
-
[20]
Laia, C.; Glòria, S.; Beatriz, M.; Ricardo, P. T.; Miriam, R.; Miquel, P.; Fernando, A.; Ernest, G. J. Am. Chem. Soc. 2002, 124, 8878.
-
[21]
Kitamatsu, M.; Kitabatake, M.; Noutoshi, Y.; Ohtsuki, T. Biopolymers 2013, 100, 65.
-
[22]
Lin, X. F.; Wang, Y. G. J. Org. Chem. 2005, 25, 1157.
-
[23]
Denkewalter, R. G.; Kole, J.; Lukasavage, W. J. US 4289872, 1981 [Chem. Abstr. 1981, 102, 79324].
-
[24]
Feng, Y.; He, Y. M.; Zhao, L. W.; Huang, Y. Y.; Fan, Q. H. Org. Lett. 2007, 9, 2261. doi: 10.1021/ol0705393
-
[25]
Joon, S. C.; Dong, K. J.; Chang, H. K.; Kwan, K.; Jong, S. P. J. Am. Chem. Soc. 2000, 122, 475.
-
[26]
Hu, J.; He, J.; Zhang, M.; Ni, P. Acta Polymerica Sinica 2013, (3), 300.
-
[27]
John, E. M.; Adam, D. M. Chem. Soc. Rev. 2007, 36, 1250.
-
[28]
Mehmet, A. T.; Baris, K.; Yusuf, Y. Prog. Polym. Sci. 2016, 52, 19. doi: 10.1016/j.progpolymsci.2015.09.003
-
[29]
Dirk, T. S. R.; Wilma, E. G.; Remco, M.; Arwin, J. B.; Hans, J. F. J.; Roland, J. P.; Rob, M. J. L. Chem. Commun. 2005, 36, 4582.
-
[30]
Yim, C. B.; Boerman, O. C.; Visser, M.; Jong, M.; Dechesne, A. C.; Rijkers, D. T. S.; Liskamp, R. M. J. Bioconjugate Chem. 2009, 20, 1323. doi: 10.1021/bc900052n
-
[31]
Pu, Y. J.; Yuan, H.; Yang, M.; He, B.; Gu, Z. W. Chin. Chem. Lett. 2013, 24, 917. doi: 10.1016/j.cclet.2013.06.015
-
[32]
Li, N.; Li, N.; Yi, Q.; Luo, K.; Guo, C.; Pan, D.; Gu, Z. Biomaterials 2014, 35, 9533.
-
[33]
Pan, D.; She, W.; Guo, C.; Luo, K.; Yi, Q.; Gu, Z. Biomaterials 2014, 35, 10081.
-
[34]
Zhang, C.; Pan, D.; Luo, K.; Li, N.; Guo, C.; Zheng, X.; Gu, Z. 2014, 5, 5228.
-
[35]
Reddy, N.; Reddy, R.; Jiang, Q. Trends Biotechnol. 2015, 33, 362. doi: 10.1016/j.tibtech.2015.03.008
-
[36]
Domeradzka, N.; Werten, M.; Wolf, F.; Vries, R. Curr. Opin. Biotechnol. 2016, 39, 61.
-
[37]
Li, C. Y.; Wang, H. J.; Cao, J. M.; Zhang, J.; Yu, X. Q. Eur. J. Med. Chem. 2014, 87, 414.
-
[38]
Buhleier, E.; Wehner, W.; Vögtle, F. Synthesis 1978, 2, 155.
-
[39]
Lin, Y.; Weng, L.; Qi, Q. The Scientific World J. 2015, 2015, 5.
-
[40]
Hawker, C. J.; Frechet, J. M. J. Am. Chem. Soc. 1990, 112, 7638. doi: 10.1021/ja00177a027
-
[41]
Scott, M. G.; Jean, M. J. F. Chem. Rev. 2001, 101, 3819. doi: 10.1021/cr990116h
-
[42]
Zhu, R.; Jiang, W.; Pu, Y.; Luo, K.; Wu, Y.; He, B.; Gu, Z. J. Mater. Chem. 2011, 21, 5466.
-
[43]
Pierre, M.; Gilles, Q.; Ling, P. Tetrahedron Lett. 2015, 56, 4043. doi: 10.1016/j.tetlet.2015.05.036
-
[44]
Olga, F.; Alexander, G.; Vladimir, R. J. Am. Chem. Soc. 2003, 125, 4885.
-
[45]
Dykes, M. G.; Brierley, J. L.; Smith, K. D.; McGrail, P. T.; Seeley, G. J. Chem. Eur. J, 2001, 7, 4731.
-
[46]
Al-Jamal, K. T.; Al-Jamal, W.; Wang, J. T.; Rubio, N.; Buddle, J.; Gathercole, D.; Zloh, M.; Kostarelos, K. ACS Nano 2013, 7, 1905. doi: 10.1021/nn305860k
-
[47]
Li, Y.; Han, S.; Toshiyuki, U. Sen-i Gakkaishi 2015, 71, 13.
-
[48]
Yuan, H.; Luo, K.; Lai, Y.; Pu, Y.; He, B.; Wang, G.; Wu, Y.; Gu, Z. Mol. Pharm. 2010, 7, 957.
-
[49]
Pu, Y.; Chang, S.; Yuan, H.; Wang, G.; He, B.; Gu, Z. Biomaterials 2013, 34, 3659.
-
[50]
Glòria, S.; Laia, C.; Ernest, G. M. R.; Fernando, A. Pept. Sci. 2004, 76, 284.
-
[51]
Torres, Á.; Albericio, F.; Royo, M. Eur. J. Org. Chem. 2013, 36, 8280.
-
[52]
Emanuele, A.; Attwood, D. Adv. Drug Delivery Rev. 2005, 57, 2147. doi: 10.1016/j.addr.2005.09.012
-
[53]
He, X.; Alves, S. C.; Oliveira, N.; Rodrigues, J.; Zhu, J.; BÁnyai, I.; TomÁs, H.; Shi, X. Colloids Surf. B: Biointerfaces 2015, 125, 83.
-
[54]
Gillies, E.; Fréchet, J. Drug Discov. Today 2005, 10, 35. doi: 10.1016/S1359-6446(04)03276-3
-
[55]
Boas, U.; Karlsson, A.; Waal, B. F. M.; Meijer, E. W. J. Org. Chem. 2001, 66, 2136. doi: 10.1021/jo001573x
-
[56]
Aulenta, F.; Hayes, W. S. Eur. Polym. J. 2003, 39, 1741. doi: 10.1016/S0014-3057(03)00100-9
-
[57]
Tyssen, D.; Henderson, S. A.; Johnson, A. PLoS One 2010, 5, 5.
-
[58]
Fox, M. E.; Guillaudeu, S.; Fréchet, J. M. J.; Jerger, K.; Macaraeg, N.; Szoka, F. C. Mol. Pharm. 2009, 6, 1563.
-
[59]
Craik, D. J.; Fairlie, D.; Liras, P. S.; Price, D. Chem. Biol. Drug Des. 2013, 81, 136. doi: 10.1111/cbdd.2012.81.issue-1
-
[60]
Zhang, X.; Zhang, Z.; Xu, X.; Li, Y.; Li, Y.; Jian, Y.; Gu, Z. Angew. Chem. Int. Ed. 2015, 54, 4289. doi: 10.1002/anie.201500683
-
[61]
Zhang, C.; Pan, D.; Luo, K.; She, W.; Guo, C.; Yang, Y.; Gu, Z. Adv. Healthcare Mater. 2014, 3, 1299. doi: 10.1002/adhm.v3.8
-
[62]
Kaminskas, L. M.; Kelly, B. D.; McLeod, V. M.; Sberna, G.; Owen, D. J.; Boyd, B. J.; Porter, C. J. H. J. Control. Release 2011, 152, 338.
-
[63]
Kaminskas, L. M.; Kelly, B. D.; McLeod, V. M.; Boyd, B. J.; Krippne, G. Y.; Williams, E. D.; Porter, C. J. H. Mol. Pharmaceutics 2009, 6, 1190. doi: 10.1021/mp900049a
-
[64]
Kaminskas, L. M.; Kelly, B. D.; McLeod, V. M.; Sberna, G.; Boyd, B. J.; Owen, D. J.; Porter, C. J. H. Mol. Pharmaceutics 2011, 8, 338. doi: 10.1021/mp1001872
-
[65]
Jain, K.; Gupta, U.; Jain, N. K. Eur. J. Pharm. Biopharm. 2014, 87, 503.
-
[66]
Bhadra, D.; Bhadra, S.; Jain, N. K. Pharm. Res. 2006, 23, 628.
-
[67]
Agrawal, P.; Gupta, U.; Jain, N. K. Biomaterials 2007, 28, 3349. doi: 10.1016/j.biomaterials.2007.04.004
-
[1]
-

-
-
[1]
Peng YUE , Liyao SHI , Jinglei CUI , Huirong ZHANG , Yanxia GUO . Effects of Ce and Mn promoters on the selective oxidation of ammonia over V2O5/TiO2 catalyst. Chinese Journal of Inorganic Chemistry, 2025, 41(2): 293-307. doi: 10.11862/CJIC.20240210
-
[2]
Haoxiang Zhang , Zhihan Zhao , Yongchen Jin , Zhiqiang Niu , Jinlei Tian . Synthesis of an Efficient Absorbent Gel: A Recommended Comprehensive Chemistry Experiment. University Chemistry, 2024, 39(11): 251-258. doi: 10.12461/PKU.DXHX202401084
-
[3]
Wenjian Zhang , Mengxin Fan , Wenwen Fei , Wei Bai . Cultivation of Critical Thinking Ability: Based on RAFT Polymerization-Induced Self-Assembly. University Chemistry, 2025, 40(4): 108-112. doi: 10.12461/PKU.DXHX202406099
-
[4]
Rui Xu , Wei Li , Tianyi Li . Exploration of Teaching Reform in the Course of “Principles of Chemical Engineering” in the Polymer Materials and Engineering Major. University Chemistry, 2025, 40(4): 54-58. doi: 10.12461/PKU.DXHX202404081
-
[5]
Jiamin Zhang , Zhen Fan , Jianzhong Du . Integrated Teaching Method Combining Domestic and International Perspectives: A Case Study on Cultivating Innovative Talents in Polymeric Biomaterials. University Chemistry, 2025, 40(7): 156-160. doi: 10.12461/PKU.DXHX202409131
-
[6]
Chenfei Li , Xu Han , Qimeng Zhang , Ben Zhang , Xinyao Huang , Mingxiao Deng , Caixia Zheng , Haizhu Sun . Measurement of Stress-Strain Curves of Polymeric Materials Using a Non-Contact Displacement Detector. University Chemistry, 2026, 41(1): 179-187. doi: 10.12461/PKU.DXHX202505101
-
[7]
Wen-Bing Hu . Systematic Introduction of Polymer Chain Structures. University Chemistry, 2025, 40(4): 15-19. doi: 10.3866/PKU.DXHX202401014
-
[8]
Yan Wang , Haolong Li , Chengji Zhao , Zheng Chen , Quan Lin , Yupeng Guo , Jianxin Mu , Kun Liu , Zhong-Yuan Lu , Junqi Sun . Construction Practice of the National First-Class Undergraduate Major in Polymer Materials and Engineering at Jilin University. University Chemistry, 2025, 40(4): 46-53. doi: 10.12461/PKU.DXHX202403083
-
[9]
Xuejun Lai , Anqiang Zhang , Tao Wang , Shuizhu Wu , Guangzhao Zhang . Construction and Practice of the First-Class Undergraduate Education Program for Polymer Materials and Engineering Major Students with “Solid Foundation, Strong Capability and High Potential”. University Chemistry, 2025, 40(4): 119-125. doi: 10.12461/PKU.DXHX202407012
-
[10]
Ruonan Li , Shijie Liang , Yunhua Xu , Cuifen Zhang , Zheng Tang , Baiqiao Liu , Weiwei Li . Chlorine-Substituted Double-Cable Conjugated Polymers with Near-Infrared Absorption for Low Energy Loss Single-Component Organic Solar Cells. Acta Physico-Chimica Sinica, 2024, 40(8): 2307037-0. doi: 10.3866/PKU.WHXB202307037
-
[11]
Wen Tang , Luyu Sui , Qian Chen , Jun Shao , Xinwen Peng , Jianwen Jiang , Shuiliang Chen . Project-based Teaching of “the Condensed State of Polymers”: Unveiling the Lithium-Ion Battery Separator. University Chemistry, 2025, 40(11): 115-126. doi: 10.12461/PKU.DXHX202412108
-
[12]
Songmei Ma , Ying Zhang , Gang Liu , Wenlong Xu . Comprehensive Experiment Teaching Exploration and Practice in Polymeric Materials Integrating Research-Driven Learning, Creativity-Enhanced Competency, and Science-Education Synergy: A Case Study of Machine Learning-Assisted Intelligent Handwriting Recognition System. University Chemistry, 2026, 41(1): 289-297. doi: 10.12461/PKU.DXHX202509083
-
[13]
Weijie Yang , Mansheng Chen , Chen Xu , Fujian Xu . Hydroxyl-Rich Polycations: Innovative Materials Empowering Life and Health. University Chemistry, 2025, 40(9): 332-343. doi: 10.12461/PKU.DXHX202410072
-
[14]
Jianchuan Wang , Wei Wu , Cunpu Li , Zhaohong Zuo , Luxi Tan . Exploration on the Construction of Polymer Course Groups in Non-Polymer-Related Majors. University Chemistry, 2026, 41(2): 154-160. doi: 10.12461/PKU.DXHX202502095
-
[15]
Zhenhua Wang , Haoyang Feng , Xiaoyang Shao , Wenru Fan . Vitamins in Solid Propellants: Controlled Synthesis of Neutral Macromolecular Bonding Agents. University Chemistry, 2025, 40(4): 1-9. doi: 10.3866/PKU.DXHX202401007
-
[16]
Kai Yang , Gehua Bi , Yong Zhang , Delin Jin , Ziwei Xu , Qian Wang , Lingbao Xing . Comprehensive Polymer Chemistry Experiment Design: Preparation and Characterization of Rigid Polyurethane Foam Materials. University Chemistry, 2024, 39(4): 206-212. doi: 10.3866/PKU.DXHX202308045
-
[17]
Laiying Zhang , Yinghuan Wu , Yazi Yu , Yecheng Xu , Haojie Zhang , Weitai Wu . Innovation and Practice of Polymer Chemistry Experiment Teaching for Non-Polymer Major Students of Chemistry: Taking the Synthesis, Solution Property, Optical Performance and Application of Thermo-Sensitive Polymers as an Example. University Chemistry, 2024, 39(4): 213-220. doi: 10.3866/PKU.DXHX202310126
-
[18]
Yuhui Yang , Jintian Luo , Biao Zuo . A Teaching Approach to Polymer Surface and Interface in Undergraduate Polymer Physics Courses. University Chemistry, 2025, 40(4): 126-130. doi: 10.12461/PKU.DXHX202408056
-
[19]
Bei Liu , Heng Li , Mei Yang , Yijiang Liu . Teaching Reform and Exploration in Polymer Chemistry with an “Experiment-Intensified” Approach for Masters in Materials and Chemical Engineering. University Chemistry, 2025, 40(4): 10-14. doi: 10.3866/PKU.DXHX202401010
-
[20]
Changjie Yin , Boyu Wang , Dantong Qiao , Huimin Li . Polymer Comprehensive Experimental Design: Preparation and Properties of Repeatable Processing Styrene Butadiene Rubber Materials under the “Dual Carbon” Strategy. University Chemistry, 2025, 40(11): 221-232. doi: 10.12461/PKU.DXHX202412046
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(3380)
- HTML views(540)

 Login In
Login In




 DownLoad:
DownLoad: