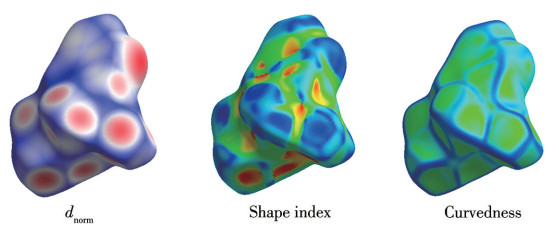
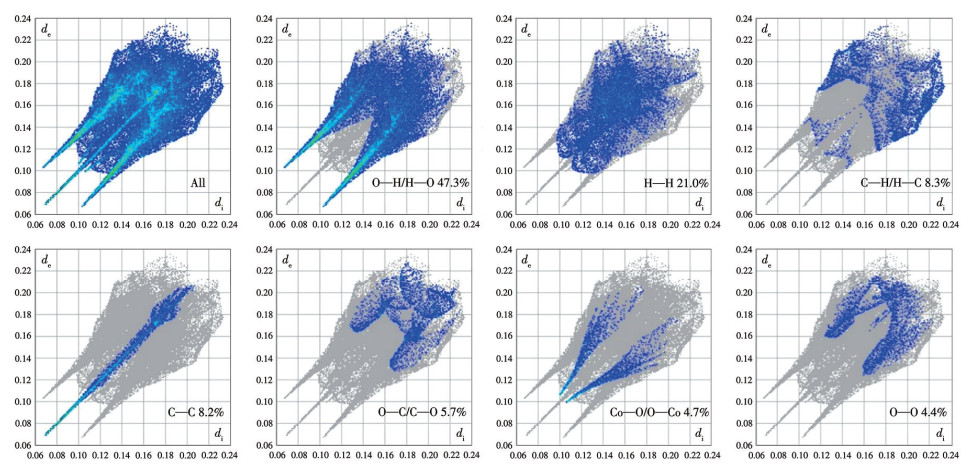
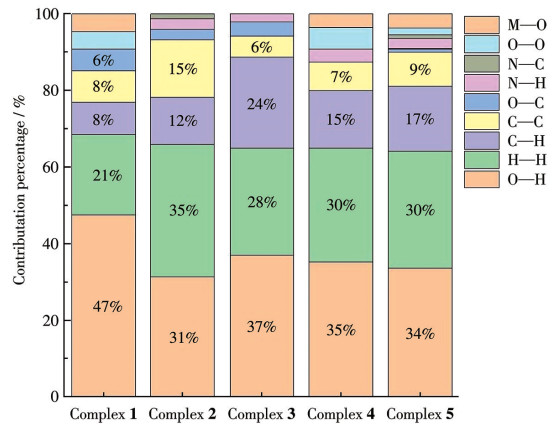
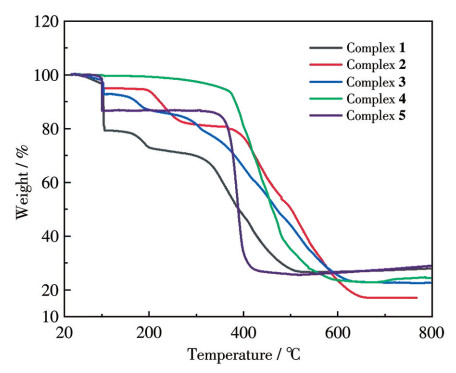
-
[1]
YAGHI O M, LI G, LI H. Selective binding and removal of guests in a microporous metal-organic framework[J]. Nature, 1995, 378: 703-706
doi: 10.1038/378703a0
-
[2]
FURUKAWA H, CORDOVA K E, O′KEEFFE M, YAGHI O M. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341: 1230444
doi: 10.1126/science.1230444
-
[3]
LI J D, FENG J Y, REN H M, LI G. Proton conductive properties of a Hf(Ⅳ)-based metal-organic framework built by 2,5-dibromophenyl-4, 6-dicarboxylic acid[J]. Chinese J. Inorg. Chem., 2025, 41(6): 1094-1110
-
[4]
QIU J H, ZHANG X G, FENG Y, ZHANG X F, WANG H, YAO J F. Modified metal-organic frameworks as photocatalysts[J]. Appl. Catal. B-Environ., 2018, 231: 317-342
doi: 10.1016/j.apcatb.2018.03.039
-
[5]
CUI P P, LI X, CHEN Y L, CHENG Z L, GAO F Y, GUO X, YAN W N, DENG Y C. Transition metal coordination polymers with flexible dicarboxylate ligand: Synthesis, characterization, and photoluminescence property[J]. Chinese J. Inorg. Chem., 2024, 40(11): 2221-2231
-
[6]
LUO X L, ZOU P T, WANG X Y, LIU Z, KONG X F, TANG Q, WANG S. Synthesis, crystal structures, and properties of lanthanide metal-organic frameworks based on 2,5-dibromoterephthalic acid ligand[J]. Chinese J. Inorg. Chem., 2024, 40(6): 1143-1150
-
[7]
WANG Z H, LI Z Z, NG M, MILNER P J. Rapid mechanochemical synthesis of metal-organic frameworks using exogenous organic base[J]. Dalton Trans., 2020, 49: 16238-16244
doi: 10.1039/D0DT01240H
-
[8]
WAUTERAERTS N, TU M, CHANUT N, RODRÍGUEZ-HERMIDA S, GANDARA-LOE J, AMELOOT R. Vapor-assisted synthesis of the MOF-74 metal-organic framework family from zinc, cobalt, and magnesium oxides[J]. Dalton Trans., 2023, 52: 17873-17880
doi: 10.1039/D3DT01785K
-
[9]
HEISKA J, KARPPINEN M. Gas-phase deposition of di- and tetra-lithium salts of 2,5-dihydroxyterephthalic acid[J]. Dalton Trans., 2022,51: 4246-4251
doi: 10.1039/D2DT00055E
-
[10]
EDDAOUDI M, KIM J, ROSI N, VODKA D, WACHTER J, KEEFFE M, YAGHI O M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science, 2002, 295: 469-472
doi: 10.1126/science.1067208
-
[11]
ROSI N L, KIM J, EDDAOUDI M, CHEN B, KEEFFE M, Y AGHI O M. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units[J]. J. Am. Chem. Soc., 2005, 127: 1504-1518
doi: 10.1021/ja045123o
-
[12]
ROWSELL J L C, MILLWARD A R, PARK K S, YAGHI O M. Hydrogen sorption in functionalized metal-organic frameworks[J]. J. Am. Chem. Soc., 2004, 126: 5666-5667
doi: 10.1021/ja049408c
-
[13]
DIETZEL P D C, BLOM R, FJELLVÅG H. A scandium coordination polymer constructed from trimeric octahedral building blocks and 2,5-dihydroxyterephthalate[J]. Dalton Trans., 2006, 35: 2055-2057
-
[14]
DIETZEL P D C, PANELLA B, HIRSCHER M, BLOM R, FJELLVÅG H. Hydrogen adsorption in a nickel based coordination polymer with open metal sites in the cylindrical cavities of the desolvated framework[J]. Chem. Commun., 2006, 42: 959-961
-
[15]
GUO X G, YANG W B, WU X Y, ZHANG Q K, LU C Z. Construction of coordination polymers based on methylenebis(3, 5-dimethylpyrazole) and varied aromatic carboxylic acids[J]. CrystEngComm, 2013, 15: 10107-10115
doi: 10.1039/c3ce41575a
-
[16]
HAN L, QIN L, YAN X Z, XU L P, SUN J L, YU L, CHEN H B, ZOU X D. Two isomeric magnesium metal-organic frameworks with [24-MC-6] metallacrown cluster[J]. Cryst. Growth Des., 2013, 13: 1807-1811
doi: 10.1021/cg4000318
-
[17]
DUNCAN M J, WHEATLEY P S, COGHILL E M, VORNHOLT S M, WARRENDER S J, MORRIS R E. Antibacterial efficacy from NO-releasing MOF-polymer films[J]. Mater. Adv., 2020, 1: 2509-2519
doi: 10.1039/D0MA00650E
-
[18]
NONG W Q, WU J, GHILADI R A, GUAN Y G. The structural appeal of metal-organic frameworks in antimicrobial applications[J]. Coord. Chem. Rev., 2021, 442: 214007
doi: 10.1016/j.ccr.2021.214007
-
[19]
BUKONJIĆ A M, TOMOVIĆ D L, NIKOLIĆ M V, MIJAJLOVIĆ M Ž, JEVTIĆ V V, RATKOVIĆ Z R, NOVAKOVIĆ S B, BOGDANOVIĆ G A, RADOJEVIĆ I D, MAKSIMOVIĆ J Z, VASIĆ S M, ČOMIĆ L R, TRIFUNOVIĆ S R, RADIĆ G P. Antibacterial, antibiofilm and antioxidant screening of copper(Ⅱ)-complexes with some S-alkyl derivatives of thiosalicylic acid. Crystal structure of the binuclear copper(Ⅱ)-complex with S-propyl derivative of thiosalicylic acid[J]. J. Mol. Struct., 2017, 1128: 330-337
doi: 10.1016/j.molstruc.2016.08.086
-
[20]
NAYAK M, SINGH A K, PRAKASH P, KANT R, BHATTACHARYA S. Structural studies on thiosalicylate complexes of Zn(Ⅱ) & Hg(Ⅱ). First insight into Zn(Ⅱ)-thiosalicylate complex as potential antibacterial, antibiofilm and anti-tumour agent[J]. Inorg. Chim. Acta, 2020, 501: 119263
doi: 10.1016/j.ica.2019.119263
-
[21]
WU C Y, SHAN Y N, LUO J M, FAN X D, ZHENG R, GUO S H, CAI X J. Silver(Ⅰ) complexes containing bioactive salicylic acid derivatives: Synthesis, characterization, antibacterial activity, and their underlying mechanism[J]. J. Inorg. Biochem., 2025, 266: 112845
doi: 10.1016/j.jinorgbio.2025.112845
-
[22]
XIONG X T, YI X G, XIAO P L, PENG D Y, XIONG Z Q, NIE X L. Hydrothermal preparation, crystal structure, Hirshfeld surface analysis, photophysical properties and antifungal activity of a series of complexes assembled by 5-bromo-2-(carboxymethoxy) benzoic acid ligands[J]. J. Mol. Struct., 2024, 1312: 138593
doi: 10.1016/j.molstruc.2024.138593
-
[23]
XIONG X T, XIONG Z Q, XIAO P L, NIE X L, SONG X Y, YI X G. Synthesis, crystal structures, Hirshfeld surface analysis and antifungal activity of two complexes Na(Ⅰ)/Cd(Ⅱ) assembled by 5-bromo-2-hydroxybenzoic acid ligands[J]. Chinese J. Inorg. Chem., 2024, 40(9): 1661-1670
-
[24]
HUANG C X, GU J, XIONG W M, CHEN J Z, NIE X L, SHANGGUAN X C. Synthesis, crystal structures and antibacterial activities of two complexes of Zn(Ⅱ)/Cd(Ⅱ) assembled by 4-carboxymethoxycinnamic acid ligand[J]. Chinese J. Inorg. Chem., 2021, 37(7): 1197-1203
-
[25]
XIAO P L, GU J, PENG D Y, XIONG W M, NIE X L. Synthesis and crystal structures of two Cd(Ⅱ) coordination polymers assembled by 4-carboxymethoxy-3-phenylacrylic acid ligands[J]. J. Chem. Crystallogr., 2023, 53: 16-24
doi: 10.1007/s10870-022-00938-0
-
[26]
WANG A, WALDEN M, ETTLINGER R, KIESSLING F, GASSENSMITH J J, LAMMERS T, WUTTKE S, PEÑA Q. Biomedical metal-organic framework materials: Perspectives and challenges[J]. Adv. Funct. Mater., 2024, 34: 2308589
doi: 10.1002/adfm.202308589
-
[27]
KALHORIZADEH T, DAHRAZMA B, ZARGHAMI R, MIRZABABAEI S, KIRILLOV A M, ABAZARI R. Quick removal of metronidazole from aqueous solutions using metal-organic frameworks[J]. New J. Chem., 2022, 46: 9440-9450
doi: 10.1039/D1NJ06107K
-
[28]
ABAZARI R, SANATI S, MORSALI A, KIRILLOV A M. Instantaneous sonophotocatalytic degradation of tetracycline over NU-1000@ZnIn2S4 core-shell nanorods as a robust and eco-friendly catalyst[J]. Inorg. Chem., 2021, 60: 9660-9672
doi: 10.1021/acs.inorgchem.1c00951
-
[29]
ALAVIJEH R K, BEHESHTI S, AKHBARI K, MORSALI A. Investigation of reasons for metal-organic framework′s antibacterial activities[J]. Polyhedron, 2018, 156: 257-278
doi: 10.1016/j.poly.2018.09.028
-
[30]
WYSZOGRODZKA G, MARSZALEK B, GIL B, DOROŻYŃSKI P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications[J]. Drug Discov. Today, 2016, 21: 1009-1018
doi: 10.1016/j.drudis.2016.04.009
-
[31]
BRUKER. APEX2, SAINT and SADABS[CP]. Bruker AXS Inc. : Madison, WI, USA, 2005.
-
[32]
SHELDRICK G M. A short history of SHELX[J]. Acta Crystallogr. Sect. A, 2008, A64: 112-122
-
[33]
SHELDRICK G M. Crystal structure refinement with SHELXL[J]. Acta Crystallogr. Sect. C, 2015, C71: 3-8
-
[34]
CHEN G Q, ZHU L N, HE J X, ZHANG S, LI Y H, GUO X L, SUN D, TIAN Y E, LIU S M, HUANG X B, CHE Z P. Combinatorial synthesis of novel 1-sulfonyloxy/acyloxyeugenol derivatives as fungicidal agents[J]. Comb. Chem. High Throughput Screen, 2022, 25: 1545-1551
doi: 10.2174/1386207324666210813114829
-
[35]
YAO C Y, LIU C, LI X J, BAI J K, XU M, QIU R, CHEN Y G, KANG Y B, LI S J. The screening of the root rot prevention of tobacco sickle bacteria[J]. Journal of Henan Agricultural Sciences, 2022,51(4): 87-94
-
[36]
XIAO P L, SONG X Y, XIONG X T, PENG D Y, NIE X L. Synthesis, crystal structure, spectral characterization and antifungal activity of novel phenolic acid triazole derivatives[J]. Molecules, 2023, 28: 6970
doi: 10.3390/molecules28196970
-
[37]
YANG K Z, ZOU R, LIAO W M, YI X G, ZHANG J B. Preparation, structure, characterization and properties of a novel [Y(HIA)3(H2O)2]n·nYCl3 (HIA=isonicotinic acid)[J]. Acta Chim. Slov., 2023, 70: 310-317
doi: 10.17344/acsi.2023.8005
-
[38]
LEE C T, YANG W T, PARR R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density[J]. Phys. Rev. B, 1988, 37: 785-789
doi: 10.1103/PhysRevB.37.785

 Login In
Login In






 DownLoad:
DownLoad: