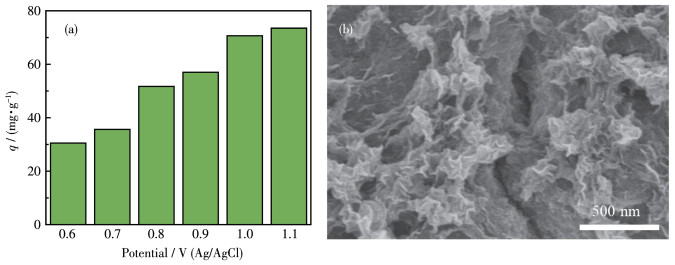
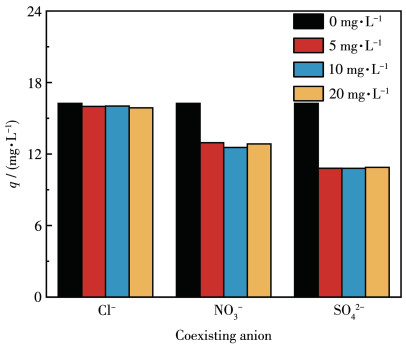
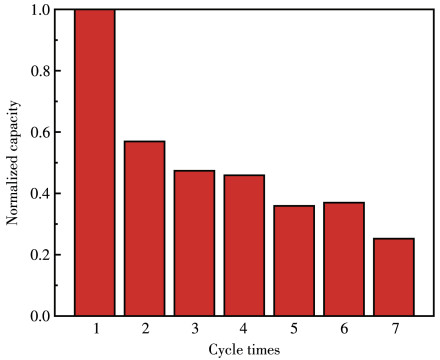
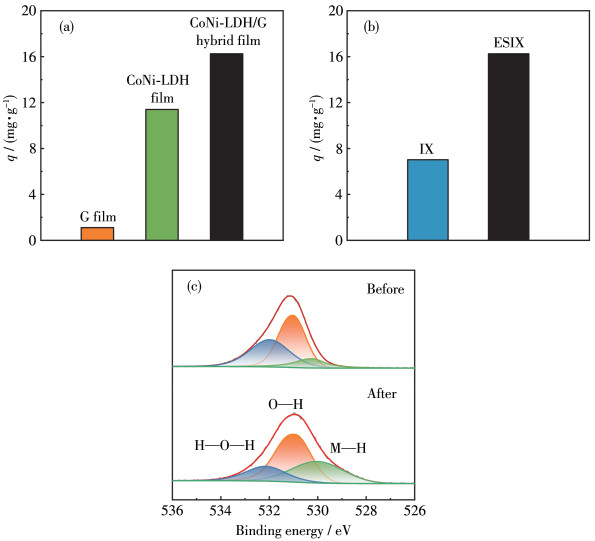
-
[1]
Zahed M A, Salehi S, Tabari Y, Farraji H, Ataei‑Kachooei S, Zinatizadeh A A, Kamali N, Mahjouri M. Phosphorus removal and recovery: State of the science and challenges[J]. Environ. Sci. Pollut. Res.,
2022,29(39):58561-58589.
doi: 10.1007/s11356-022-21637-5
-
[2]
Belarbi Z, Daramola D A, Trembly J P. Bench-scale demonstration and thermodynamic simulations of electrochemical nutrient reduction in wastewater via recovery as struvite[J]. J. Electrochem. Soc.,
2020,167(15)155524.
doi: 10.1149/1945-7111/abc58f
-
[3]
Childers D L, Corman J, Edwards M, Elser J J. Sustainability challenges of phosphorus and food: Solutions from closing the human phosphorus cycle[J]. Bioscience,
2011,61(2):117-124.
doi: 10.1525/bio.2011.61.2.6
-
[4]
Wang Y C, Kuntke P, Saakes M, van der Weijden R D, Buisman C J N, Lei Y. Electrochemically mediated precipitation of phosphate minerals for phosphorus removal and recovery: Progress and perspective[J]. Water Res.,
2022,209117891.
doi: 10.1016/j.watres.2021.117891
-
[5]
Bhambri A, Karn S K. Biotechnique for nitrogen and phosphorus removal: A possible insight[J]. Chem. Ecol.,
2020,36(8):785-809.
doi: 10.1080/02757540.2020.1777991
-
[6]
Borgstrom A, Hansson L A, Sjostedt J. Wetlands as a local scale management tool to reduce algal growth potential[J]. Wetlands,
2022,42(8)123.
doi: 10.1007/s13157-022-01640-9
-
[7]
Cornel P, Schaum C. Phosphorus recovery from wastewater: Needs, technologies and costs[J]. Water Sci. Technol.,
2009,59(6):1069-1076.
doi: 10.2166/wst.2009.045
-
[8]
Sun Y, Feng X L, Zheng W S. Nanoscale lanthanum carbonate hybridized with polyacrylic resin for enhanced phosphate removal from secondary effluent[J]. J. Chem. Eng. Data,
2020,65(9):4512-4522.
doi: 10.1021/acs.jced.0c00352
-
[9]
Wikstrom J, Bonaglia S, Ramo R, Renman G, Walve J, Hedberg J, Gunnarsson J S. Sediment remediation with new composite sorbent amendments to sequester phosphorus, organic contaminants, and metals[J]. Environ. Sci. Technol.,
2021,55(17):11937-11947.
doi: 10.1021/acs.est.1c02308
-
[10]
Wu B L, Lo I M C. Surface functional group engineering of CeO2 particles for enhanced phosphate adsorption[J]. Environ. Sci. Technol.,
2020,54(7):4601-4608.
doi: 10.1021/acs.est.9b06812
-
[11]
Lu J L, Jia P, Feng S W, Wang Y T, Zheng J, Ou S N, Wu Z H, Liao B, Shu W S, Liang J L, Li J T. Remarkable effects of microbial factors on soil phosphorus bioavailability: A country-scale study[J]. Global Change Biol.,
2022,28(14):4459-4471.
doi: 10.1111/gcb.16213
-
[12]
Kumar P S, Korving L, Keesman K J, van Loosdrecht M C M, Witkamp G J. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics[J]. Chem. Eng. J.,
2019,358:160-169.
doi: 10.1016/j.cej.2018.09.202
-
[13]
GAO F F, YANG Y Y, DU X, HAO X G, GUAN G Q, TANG B. Electrically switched ion membrane for ion selective separation and recovery: From ESIX to ESIPM[J]. Prog. Chem.,
2020,32(9):1344-1351.
-
[14]
Zhang X F, Wang J, Zhang Z L, Du X, Gao F F, Hao X G, Abudula A, Guan G Q, Liu Z, Li J. Modelling of pseudocapacitive ion adsorption of electrochemically switched ion exchange based on electroactive site concentration[J]. Sep. Purif. Technol.,
2022,286120451.
doi: 10.1016/j.seppur.2022.120451
-
[15]
Liu C, Zhang M Y, Pan G, Lundehoj L, Nielsen U G, Shi Y, Hansen H C B. Phosphate capture by ultrathin MgAl layered double hydroxide nanoparticles[J]. Appl. Clay Sci.,
2019,177:82-90.
doi: 10.1016/j.clay.2019.04.019
-
[16]
Gao F F, Du X, Hao X G, Li S S, Zheng J L, Yang Y Y, Han N C, Guan G Q. A potential‑controlled ion pump based on a three‑ dimensional PPy@GO membrane for separating dilute lead ions from wastewater[J]. Electrochim. Acta,
2017,236:434-442.
doi: 10.1016/j.electacta.2017.03.187
-
[17]
Hong S P, Yoon H, Lee J, Kim C, Kim S, Lee J, Lee C, Yoon J. Selective phosphate removal using layered double hydroxide/reduced graphene oxide (LDH/rGO) composite electrode in capacitive deionization[J]. J. Colloid Interface Sci.,
2020,564:1-7.
doi: 10.1016/j.jcis.2019.12.068
-
[18]
Liu G G, Wang G R, Jin Z L. Graphdiyne-modified NiV-layered double hydroxide nanostructures for supercapacitor applications[J]. ACS Appl. Nano Mater.,
2023,6(23):21803-21817.
doi: 10.1021/acsanm.3c03993
-
[19]
Gu Y L, Yang Z Z, Zhou J W, Fang Q Z, Tan X F, Long Q B. Graphene/LDHs hybrid composites synthesis and application in environmental protection[J]. Sep. Purif. Technol.,
2024,328125042.
doi: 10.1016/j.seppur.2023.125042
-
[20]
LIU J X, LIU C X, CHEN L J, ZHANG X G. Preparation and property of zwitterionic surfactants intercalation into graphene oxide-layered double hydroxide hybrid[J]. Chinese J. Inorg. Chem.,
2019,35(5):844-854.
-
[21]
Ding C X, Long X Y, Zeng G Y, Ouyang Y, Lei B W, Zeng R Y, Wang J, Zhou Z. Efficiency recycling and utilization of phosphate from wastewater using LDHs-modified biochar[J]. Int. J. Environ. Res. Public Health,
2023,20(4)3051.
doi: 10.3390/ijerph20043051
-
[22]
Zhao W H, Liu T T, Wu N D, Zhou B Y, Yan Y X, Ye Y C, Gong J F, Yang S G. Bimetallic electron-induced phase transformation of CoNi LDH-GO for high oxygen evolution and supercapacitor performance[J]. Sci. China Mater.,
2023,66(2):577-586.
doi: 10.1007/s40843-022-2170-6
-
[23]
Zhu Y, An S, Sun X, Lan D, Cui J, Zhang Y, He W. Core-branched NiCo2S4@CoNi-LDH heterostructure as advanced electrode with superior energy storage performance[J]. Chem. Eng. J.,
2020,383123206.
doi: 10.1016/j.cej.2019.123206
-
[24]
Liang H Y, Lin J H, Jia H N, Chen S L, Qi J L, Cao J, Lin T S, Fei W D, Feng J C. Hierarchical NiCo-LDH/NiCoP@NiMn-LDH hybrid electrodes on carbon cloth for excellent supercapacitors[J]. J. Mater. Chem. A,
2018,6(31):15040-15046.
doi: 10.1039/C8TA05065A
-
[25]
Wang J, Gao F F, Du X, Ma X L, Hao X G, Ma W B, Wang K Z, Guan G Q, Abudula A. A high-performance electroactive PPy/rGO/NiCo-LDH hybrid film for removal of dilute dodecyl sulfonate ions[J]. Electrochim. Acta,
2020,331135288.
doi: 10.1016/j.electacta.2019.135288
-
[26]
Song F, Zhang R, Zhang X Y, Qin J Q, Liu R P. Ni-Co double hydroxide grown on graphene oxide for enhancing lithium ion storage[J]. Energy Fuels,
2020,34(10):13032-13037.
doi: 10.1021/acs.energyfuels.0c01637
-
[27]
Ma P F, Zhu J W, Du X, Yang Y Y, Hao X Q, An X W, Hao X G, Prestigiacomo C. Specific separation and recovery of phosphate anions by a novel NiFe-LDH/rGO hybrid film based on electroactivity‑ variable valence[J]. J. Colloid Interface Sci.,
2022,626:47-58.
doi: 10.1016/j.jcis.2022.06.024
-
[28]
WANG X L, ZHANG D, SHI X M, QIAO X Y, CHENG Y, ZHAO H N, CHANG L M, YU Z Q, HUANG C H, YANG S B. Preparation by Co metal-organic framework template and capacitive properties of NiCo-layered double hydroxide/nickel foam composites[J]. Chinese J. Inorg. Chem.,
2023,39(4):607-616.
-
[29]
Youmbi B S, Pelisson C H, Denicourt-Nowicki A, Roucoux A, Greneche J M. Impact of the charge transfer process on the Fe2+/Fe3+ distribution at Fe3O4 magnetic surface induced by deposited Pd clusters[J]. Surf. Sci.,
2021,712121879.
doi: 10.1016/j.susc.2021.121879
-
[30]
YANG Y Y, LI Y G, ZHU X W, DU X, MA X L, HAO X G. Potential induced reversible removal/recovery of phosphate anions with high selectivity using an electroactive NiCo-layered double oxide film[J]. J. Inorg. Mater.,
2021,36(3):292-298.
-
[31]
Sun B, Hao X G, Wang Z D, Guan G Q, Zhang Z L, Li Y B, Liu S B. Separation of low concentration of cesium ion from wastewater by electrochemically switched ion exchange method: Experimental adsorption kinetics analysis[J]. J. Hazard. Mater.,
2012,233:177-183.
-
[32]
Cai J G, Zhang Y Y, Pan B C, Zhang W M, Lv L, Zhang Q X. Efficient defluoridation of water using reusable nanocrystalline layered double hydroxides impregnated polystyrene anion exchanger[J]. Water Res.,
2016,102:109-116.
doi: 10.1016/j.watres.2016.06.030
-
[33]
Yang Y Y, Du X, Abudula A, Zhang Z L, Ma X L, Tang K Y, Hao X G, Guan G Q. Highly efficient defluoridation using a porous MWCNT@NiMn-LDH composites based on ion transport of EDL coupled with ligand exchange mechanism[J]. Sep. Purif. Technol.,
2019,223:154-161.
doi: 10.1016/j.seppur.2019.04.052

 Login In
Login In






 DownLoad:
DownLoad: