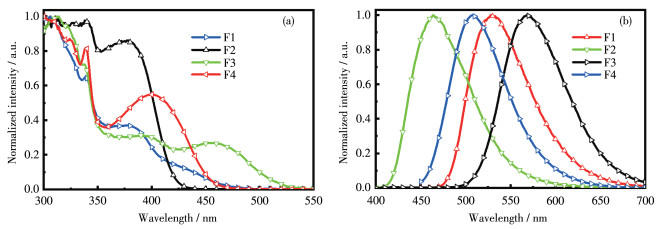
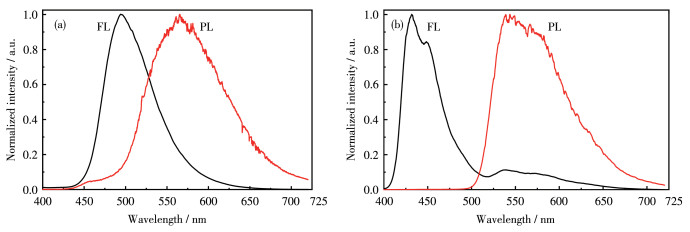
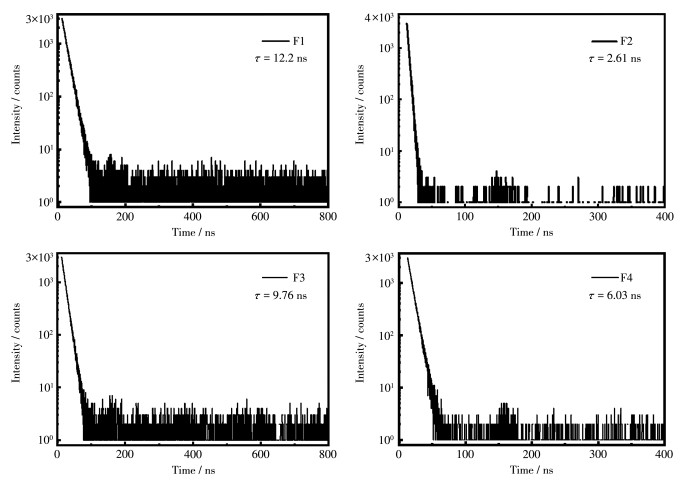
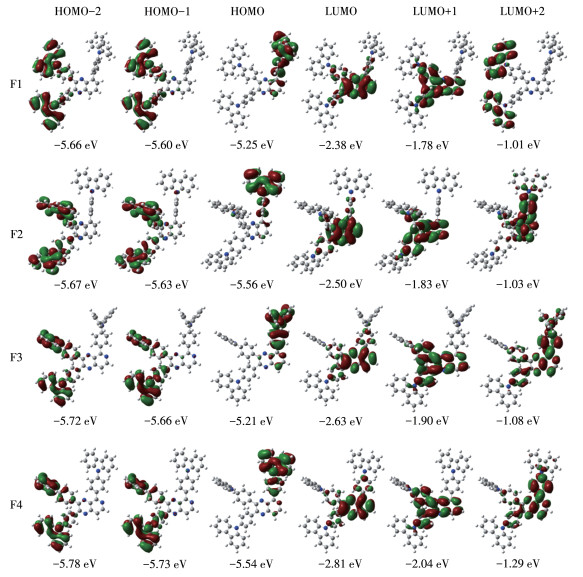
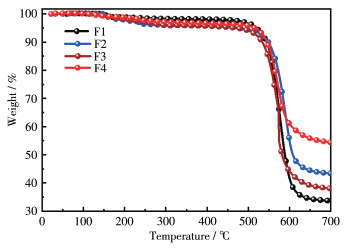
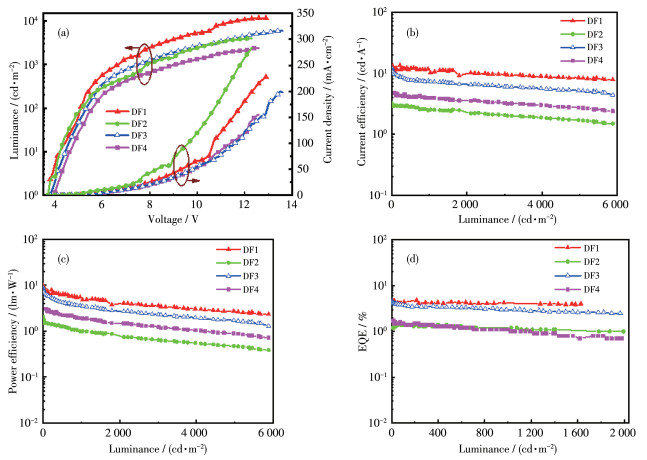
-
[1]
Tang C W, VanSlyke S A. Organic electroluminescent diodes[J]. Appl. Phys. Lett.,
1987,51:913-915.
doi: 10.1063/1.98799
-
[2]
Burroughes J H, Bradley D D C, Brown A R, Marks R N, Mackay K, Friend R H, Burns P L, Holmes A B. Light-emitting diodes based on conjugated polymers[J]. Nature,
1990,347:539-541.
doi: 10.1038/347539a0
-
[3]
Ma Y G, Zhang H Y, Shen J C, Che C M. Electroluminescence from triplet metal-ligand charge-transfer excited state of transition metal complexes[J]. Synth. Met.,
1998,94:245-248.
doi: 10.1016/S0379-6779(97)04166-0
-
[4]
Baldo M A, O'Brien D F, You Y, Shoustikov A, Sibley S, Thompson M E, Forrest S R. Highly efficient phosphorescent emission from organic electroluminescent devices[J]. Nature,
1998,395:151-154.
doi: 10.1038/25954
-
[5]
Wohlgenannt M, Tandon K, Mazumdar S, Ramasesha S, Vardeny Z V. Formation cross-sections of singlet and triplet excitons in π-conjugated polymers[J]. Nature,
2001,409:494-497.
doi: 10.1038/35054025
-
[6]
D'Andrade B W, Forrest S R. White organic light-emitting devices for solid-state lighting[J]. Adv. Mater.,
2004,16:1585-1595.
doi: 10.1002/adma.200400684
-
[7]
Xiao L X, Chen Z J, Qu B, Luo J X, Kong S, Gong Q H, Kido J J. Recent progresses on materials for electrophosphorescent organic light-emitting devices[J]. Adv. Mater.,
2011,23:926-952.
doi: 10.1002/adma.201003128
-
[8]
Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C. Highly efficient organic light-emitting diodes from delayed fluorescence[J]. Nature,
2012,492:234-238.
doi: 10.1038/nature11687
-
[9]
Reineke S, Thomschke M, Lüssem B, Leo K. White organic light-emitting diodes: Status and perspective[J]. Rev. Mod. Phys.,
2013,85:1245-1293.
doi: 10.1103/RevModPhys.85.1245
-
[10]
Tao Y, Yuan K, Ch en, T , Xu P, Li H H, Chen R F, Zheng C, Zhang L, Huang W. Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics[J]. Adv. Mater.,
2014,26:7931-7958.
doi: 10.1002/adma.201402532
-
[11]
Pan Y Y, Li W J, Zhang S T, Yao L, Gu C, Xu H, Yang B, Ma Y. High yields of singlet excitons in organic electroluminescence through two paths of cold and hot excitons[J]. Adv. Opt. Mater.,
2014,2:510-515.
doi: 10.1002/adom.201300467
-
[12]
Yao L, Zhang S T, Wang R, Li W J, Shen F Z, Yang B, Ma Y G. Highly efficient near-infrared organic light-emitting diode based on a butterfly-shaped donor-acceptor chromophore with strong solid-state fluorescence and a large proportion of radiative excitons[J]. Angew. Chem. Int. Ed.,
2014,53:2119-2123.
doi: 10.1002/anie.201308486
-
[13]
Mei J, Leung N L C, Kwok R T K, Lam J W Y, Tang B Z. Aggregation-induced emission: Together we shine, united we soar![J]. Chem. Rev.,
2015,115:11718-11721.
doi: 10.1021/acs.chemrev.5b00263
-
[14]
Hatakeyama T, Shiren K, Nakajima K, Nomura S, Nakatsuka S, Kinoshita K, Ni J, Ono Y, Ikuta T. Ultrapure blue thermally activated delayed fluorescence molecules: Efficient HOMO-LUMO separation by the multiple resonance effect[J]. Adv. Mater.,
2016,28:2777-2781.
doi: 10.1002/adma.201505491
-
[15]
Yang Z Y, Mao Z, Xie Z L, Zhang Y, Liu S W, Zhao J, Xu J R, Chi Z G, Aldred M P. Recent advances in organic thermally activated delayed fluorescence materials[J]. Chem. Soc. Rev.,
2017,46:915-1016.
doi: 10.1039/C6CS00368K
-
[16]
Liang X, Yan Z P, Han H B, Wu Z G, Zheng Y X, Meng H, Zuo J L, Huang W. Peripheral amplification of multi-resonance induced thermally activated delayed fluorescence for highly efficient OLEDs[J]. Angew. Chem. Int. Ed.,
2018,57:11316-11320.
doi: 10.1002/anie.201806323
-
[17]
Jeon S K, Lee H L, Yook K, Lee J Y. Recent progress of the lifetime of organic light-emitting diodes based on thermally activated delayed fluorescent material[J]. Adv. Mater.,
2019,311803524.
doi: 10.1002/adma.201803524
-
[18]
ZHOU Y H, KONG Q G, WANG Z M, WANG C C, ZHENG Y X. Synthesis and properties of two phosphorescence iridium complexes[J]. Chinese J. Inorg. Chem.,
2014,30(10):2288-2294.
-
[19]
Xu H, Chen R F, Sun Q, Lai W Y, Su Q Q, Huang W, Liu X G. Recent progress in metal-organic complexes for optoelectronic applications[J]. Chem. Soc. Rev.,
2014,43:3259-3302.
doi: 10.1039/C3CS60449G
-
[20]
Fan C, Yang C L. Yellow/orange emissive heavy-metal complexes as phosphors in monochromatic and white organic light-emitting devices[J]. Chem. Soc. Rev.,
2014,43:6439-6469.
doi: 10.1039/C4CS00110A
-
[21]
Yang X, Zhou G, Wong W Y. Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices[J]. Chem. Soc. Rev.,
2015,44:8484-8575.
doi: 10.1039/C5CS00424A
-
[22]
Li T Y, Wu J, Wu Z G, Zheng Y X, Zuo J L, Pan Y. Rational design of phosphorescent iridium complexes for emission color tunability and their applications in OLEDs[J]. Coord. Chem. Rev.,
2018,374:55-92.
doi: 10.1016/j.ccr.2018.06.014
-
[23]
Kumsampao J, Chaiwai C, Sukpattanacharoen C, Chawanpunyawat T, Nalaoh P, Chasing P, Kungwan N, Sudyoadsuka T, Promarak V. Self-absorption-free excited-state intramolecular proton transfer (ESIPT) emitters for high brightness and luminous efficiency organic fluorescent electroluminescent devices[J]. Mater. Chem. Front.,
2021,5:6212-6225.
doi: 10.1039/D1QM00455G
-
[24]
Pramanik S, Deol H, Bhalla V, Kumar V. AIEE active donor-acceptor-donor-based hexaphenylbenzene probe for recognition of aliphatic and aromatic amines[J]. ACS Appl. Mater. Interfaces,
2018,10:12112-12123.
doi: 10.1021/acsami.7b09791
-
[25]
Poddar M, Sivakumar G, Misra R. Donor-acceptor substituted 1, 8-naphthalimides: Design, synthesis, and structure-property relationship[J]. J. Mater. Chem. C,
2019,7:14798-14815.
doi: 10.1039/C9TC02634G
-
[26]
Justin T K R, Venkateswararao A, Joseph V, Kumar S, Jou J H. Polarity tuning of fluorene derivatives by chromophores to achieve efficient blue electroluminescent materials[J]. Org. Electron.,
2019,64:266-273.
doi: 10.1016/j.orgel.2018.10.029
-
[27]
Ye S F, Wang Y X, Guo R D, Zhang Q, Lv X L, Duan Y L, Leng P P, Sun S Q, Wang L. Asymmetric anthracene derivatives as multifunctional electronic materials for constructing simplified and efficient non-doped homogeneous deep blue fluorescent OLEDs[J]. Chem. Eng. J.,
2020,393124694.
doi: 10.1016/j.cej.2020.124694
-
[28]
Zhang T, Ye J Y, Luo A S, Liu D. Efficient deep blue emitter based on carbazole-pyrene hybrid for non-doped electroluminescent device[J]. Opt. Mater.,
2020,100109632.
doi: 10.1016/j.optmat.2019.109632
-
[29]
Yu Y, Cang M, Cui W, Xu L, Wang R Z, Sun M Z, Zhou H Y, Yang W J, Xue S F. Efficient red fluorescent OLEDs based on aggregation-induced emission combined with hybridized local and charge transfer state[J]. Dyes Pigment.,
2021,184108770.
doi: 10.1016/j.dyepig.2020.108770
-
[30]
Xu J W, Liu H, Li J S, Zhao Z J, Tang B Z. Multifunctional bipolar materials serving as emitters for efficient deep-blue fluorescent OLEDs and as hosts for phosphorescent and white OLEDs[J]. Adv. Opt. Mater.,
2021,92001840.
doi: 10.1002/adom.202001840
-
[31]
Tang X Y, Liu H, Liu F T, He X, Xu X H, Chen J W, Peng Q M, Lu P. Efficient red electroluminescence from phenanthro[9,10-d]imidazole-naphtho[2,3-c][1,2,5]thiadiazole donor-acceptor derivatives[J]. Chem.-Asian J.,
2021,16:1942-1948.
doi: 10.1002/asia.202100391
-
[32]
Wang Z Q, Yang T T, Dong S F, Wen Z J, Xu H X, Miao Y Q, Wang H, Yu SJ. Anthracene and carbazole based asymmetric fluorescent materials for high-efficiency deep-blue non-doped organic light emitting devices with CIEy=0.06[J]. Dyes Pigment.,
2022,199110047.
doi: 10.1016/j.dyepig.2021.110047
-
[33]
Ning S Y, Zhang Y F, Li Y X, Wu Y, Qin K, Wang D D, Wang X Y, Wu C M, Ma H L. Benzene[g]furan[2,3-B]quinoxaline-based red fluorescent material for non-doped organic light-emitting devices with low efficiency roll-off[J]. Chem. Phys. Lett.,
2022,787139199.
doi: 10.1016/j.cplett.2021.139199

 Login In
Login In

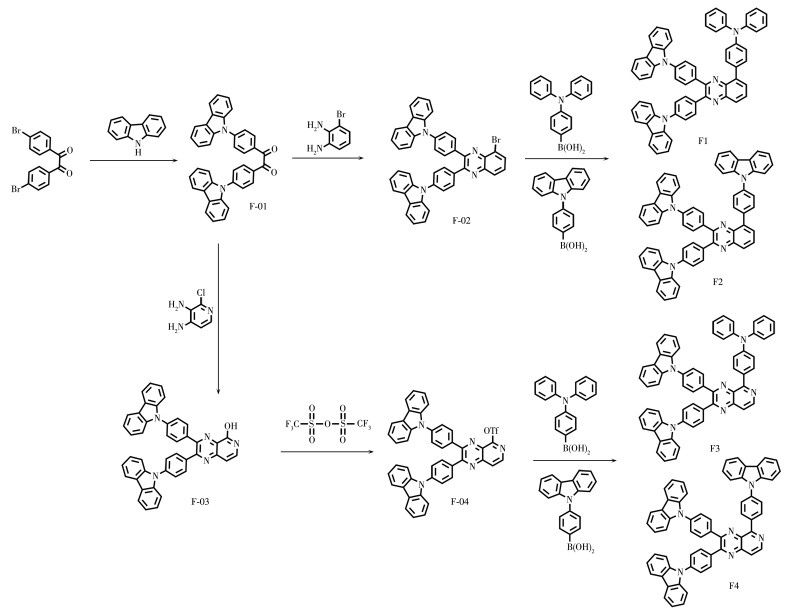





 DownLoad:
DownLoad: