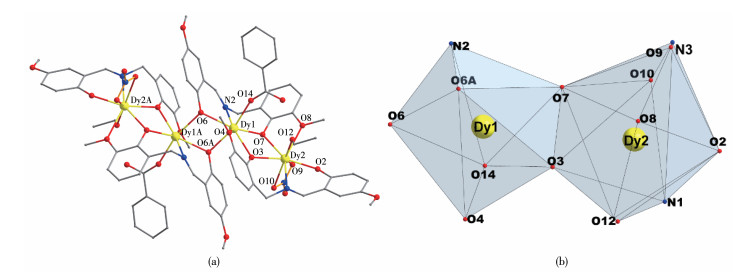
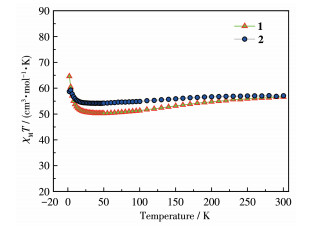
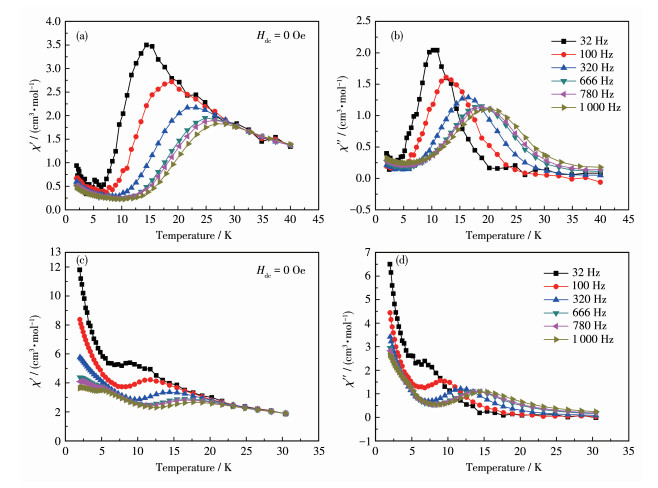
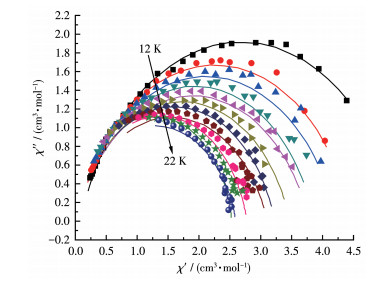
-
[1]
Ferrando-Soria J, Vallejo J, Castellano M, MartínezLillo J, Pardo E, Cano J, Castro I, Lloret F, Ruiz-García R, Julve M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint[J]. Coord. Chem. Rev.,
2017,339:17-103.
doi: 10.1016/j.ccr.2017.03.004
-
[2]
Affronte M. Molecular nanomagnets for information technologies[J]. J. Mater. Chem.,
2009,19:1731-1737.
doi: 10.1039/B809251F
-
[3]
ZHANG L, ZENG S Y, LIU T, SUN J S, DOU J M, JIANG J Z. Mixed tetrapyrrole terbium triple-decker single molecule magnets with bulky inorganic polyhedral oligomeric silsesquioxanes moieties at outer porphyrin peripheries[J]. Chinese J. Inorg. Chem.,
2015,31(9):1761-1773.
-
[4]
ZHANG K, WANG H S, YU L Y, CHEN Y, PAN Z Q. Research progress and prospect on the first series transition metal-Dy single molecule magnets[J]. Chinese J. Inorg. Chem.,
2020,36(12):2205-2226.
-
[5]
Torres F, Herńandez J M, Bohigas X, Tejada J. Giant and time-dependent magnetocaloric effect in high-spin molecular magnets[J]. Appl. Phys. Lett.,
2000,77:3248-3250.
doi: 10.1063/1.1325393
-
[6]
REN M, ZHENG L M. Lanthanide-based single molecule magnets[J]. Acta Chim. Sinica,
2015,73(11):1091-1113.
-
[7]
Ishikawa N, Sugita M, Ishikawa T, Koshihara S Y, Kaizu Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level[J]. J. Am. Chem. Soc.,
2003,125:8694-8695.
doi: 10.1021/ja029629n
-
[8]
Liu K, Zhang X J, Meng X X, Shi W, Cheng P, Powell A K. Constraining the coordination geometries of lanthanide centers and magnetic building blocks in frameworks: A new strategy for molecular nanomagnets[J]. Chem. Soc. Rev.,
2016,45:2423-2439.
doi: 10.1039/C5CS00770D
-
[9]
Liddle S T, Van Slageren J. Improving f-element single molecule magnets[J]. Chem. Soc. Rev.,
2015,44:6655-6669.
doi: 10.1039/C5CS00222B
-
[10]
Dolai M, Ali Molla H, Rogez G, Ali M. Two[Mn3(μ3-O)]7+ based single chain magnets with different solvent ligation[J]. Polyhedron,
2017,127:248-256.
doi: 10.1016/j.poly.2017.01.043
-
[11]
Nguyen T N, Abboud K A, Christou G. MOF-like supramolecular network of Mn3 single-molecule magnets formed by extensive π-π stacking[J]. Polyhedron,
2016,103:150-156.
doi: 10.1016/j.poly.2015.09.039
-
[12]
Thomas-Hargreaves L R, Hunger D, Kern M, Wooles A J, Slageren J V, Chilton N F, Liddle S T. Insights into D4h@ metal-symmetry single-molecule magnetism: The case of a dysprosium-bis(boryloxide) complex[J]. Chem. Commun.,
2021,57:733-736.
doi: 10.1039/D0CC07446B
-
[13]
Blagg R J, Muryn C A, McInnes E J L, Tuna F, Winpenny R E P. Single pyramid magnets: Dy5 pyramids with slow magnetic relaxation to 40 K[J]. Angew. Chem. Int. Ed.,
2011,50:6530-6533.
doi: 10.1002/anie.201101932
-
[14]
Guo Y N, Xu G F, Gamez P, Zhao L, Lin S Y, Deng R P, Tang J K, Zhang H J. Two-step relaxation in a linear tetranuclear dysprosium(Ⅲ) aggregate showing single-molecule magnet behavior[J]. J. Am. Chem. Soc.,
2010,132:8538-8539.
doi: 10.1021/ja103018m
-
[15]
Zhang Y C, Wang Q L, Chen G, Shi P F, Wang W M. Two linear-shaped Gd4 clusters based on a multidentate ligand: Synthesis, structures, and magnetic refrigeration[J]. Polyhedron,
2019,169:247-252.
doi: 10.1016/j.poly.2019.05.002
-
[16]
Anwar M U, Thompson L K, Dawe L N, Habibb F, Murugesu M. Predictable self-assembled[2 × 2] Ln(Ⅲ)4 square grids (Ln=Dy, Tb)-SMM behaviour in a new lanthanide cluster motif[J]. Chem. Commun.,
2012,48:4576-4578.
doi: 10.1039/c2cc17546k
-
[17]
Xue S F, Zhao L, Guo Y N, Chen X H, Tang J K. Field enhanced thermally activated mechanism in a square Dy4 aggregate[J]. Chem. Commun.,
2012,48:7031-7033.
doi: 10.1039/c2cc31864d
-
[18]
Wang W M, Li X Z, Zhang L, Chen J L, Wang J H, Wu Z L, Cui J Z. A series of[2×2] square grid Ln4Ⅲ clusters: A large magnetocaloric effect and single-molecule-magnet behavior[J]. New J. Chem.,
2019,43:7419-7426.
doi: 10.1039/C8NJ04454F
-
[19]
Zangana K H, Pineda E M, Winpenny R E P. Tetrametallic lanthanide(Ⅲ) phosphonate cages: Synthetic, structural and magnetic studies[J]. Dalton Trans.,
2014,43:17101-17107.
doi: 10.1039/C4DT02630F
-
[20]
Wang X X, Han X R, Si X, Fang M, Wang W M, Peng H K, Fang M. A rhombus-shaped tetranuclear dysprosium cluster showing single-molecule magnet behavior[J]. Polyhedron,
2017,137:306-310.
doi: 10.1016/j.poly.2017.08.033
-
[21]
Wang W M, Zhang L, Li X Z, He L Y, Wang X X, Shi Y, Wang J, Dong J, Wu Z L. Structures, fluorescence properties and magnetic properties of a series of rhombus-shaped Ln4Ⅲ clusters: Magnetocaloric effect and single-molecule-magnet behavior[J]. New J. Chem.,
2019,43:12941-12949.
doi: 10.1039/C9NJ02872B
-
[22]
Wu Z L, Ran Y G, Wu X Y, Xia Y P, Fang M, Wang W M. Butterfly shaped tetranuclear dysprosium compound displaying slow magnetic relaxation features[J]. Polyhedron,
2017,126:282-286.
doi: 10.1016/j.poly.2017.01.024
-
[23]
Xue S F, Guo Y N, Zhao L, Zhang P, Tang J K. Unique Y-shaped lanthanide aggregates and single-molecule magnet behaviour for the Dy4 analogue[J]. Dalton Trans.,
2014,43:1564-1570.
doi: 10.1039/C3DT52444B
-
[24]
Lin P H, Burchell T J, Ungur L, Chibotaru L F, Wernsdorfer W, Murugesu M. A polynuclear lanthanide single-molecule magnet with a record anisotropic barrier[J]. Angew. Chem. Int. Ed.,
2009,48:9489-9492.
doi: 10.1002/anie.200903199
-
[25]
Zagol-Ikapitte I, Vmarnath A, Bala M, Roberts L J, Oates J A, Boutaud O. Characterization of scavengers of γ-ketoaldehydes that do not inhibit prostaglandin biosynthesis[J]. Chem. Res. Toxicol.,
2010,23:240-250.
doi: 10.1021/tx900407a
-
[26]
Li D W, Ding M M, Huang Y, Tello Yepes D F, Li H Y, Li Y H, Zhang Y Q, Yao J L. Evolution from a single relaxation process to two-step relaxation processes of Dy2 single-molecule magnets via the modulations of the terminal solvent ligands[J]. Dalton Trans.,
2021,50:217-228.
doi: 10.1039/D0DT03093G
-
[27]
Sheldrick G M. A short history of SHELX[J]. Acta Crystallogr. Sect. A,
2008,A64:112-122.
-
[28]
Sheldrick G M. Crystal structure refinement with SHELXL[J]. Acta Crystallogr. Sect. C,
2015,C71:3-8.
-
[29]
Fang M, Shao L J, Shi T X, Chen Y Y, Yu H, Li P F, Wang W M, Zhao B. Four tetra-nuclear lanthanide complexes based on 8-hydroxyquinolin derivatives: Magnetic refrigeration and single-molecule magnet behaviour[J]. New J. Chem.,
2018,42:11847-11853.
doi: 10.1039/C8NJ02161A
-
[30]
Chen J H, Peng S, Pan H D, Wu C E, Teng Q H, Li Y, Zhang X Q, Liang F P, Wang K. Dysprosium clusters from 3-ethoxysalicylidene terminal-decorated acylhydrazone ligands: In situ ligand transformation-assisted assembly and zero-field single-molecule magnet behaviors[J]. Cryst. Growth Des.,
2023,23:4927-4938.
doi: 10.1021/acs.cgd.3c00169
-
[31]
Ke H S, Xu G F, Guo Y N, Gamez P, Beavers C M, Teat S J, Tang J K. A linear tetranuclear dysprosium(Ⅲ) compound showing single-molecule magnet behaviour[J]. Chem. Commun.,
2010,46:6057-6059.
doi: 10.1039/c0cc01067g
-
[32]
Lin S Y, Zhao L, Ke H S, Guo Y N, Tang J K, Guo Y, Dou J M. Steric hindrances create a discrete linear Dy4 complex exhibiting SMM behaviour[J]. Dalton Trans.,
2012,41:3248-3252.
doi: 10.1039/c2dt11539e
-
[33]
Lu J J, Li X L, Jin C Y, Yu Y, Tang J K. Dysprosium-based linear helicate clusters: Syntheses, structures, and magnetism[J]. New J. Chem.,
2020,44:994-1000.
doi: 10.1039/C9NJ05192A
-
[34]
Wang W M, Hu X Y, Yang Y, Zhao J Q, Zhang Y X, Kang X M, Wu Z L. Modulation of magnetic relaxation behaviors via replacing coordinated solvents in a series of linear tetranuclear Dy4 complexes[J]. New J. Chem.,
2020,44:8494-8502.
doi: 10.1039/D0NJ01830A
-
[35]
Li R P, Liu Q Y, Wang Y L, Liu C M, Liu S J. Evolution from linear tetranuclear clusters into one-dimensional chains of Dy(Ⅲ) single-molecule magnets with an enhanced energy barrier[J]. Inorg. Chem. Front.,
2017,4:1149-1156.
-
[36]
Corredoira-Vázquez J, González-Barreira C, Fondo M, García-Deibe A M, Sanmartín-Matalobos J, Hernández-Rodríguez M A, Carlos L D. Luminescence thermometry in a Dy4 single molecule magnet[J]. Dalton Trans.,
2022,51:15593-15600.
doi: 10.1039/D2DT02250H
-
[37]
Bala S, Bishwas M S, Pramanik B, Khanra S, Fromm K M, Poddar P, Mondal R. Construction of polynuclear lanthanide (Ln=DyⅢ, TbⅢ, and NdⅢ) cage complexes using pyridine-pyrazole-based ligands: Versatile molecular topologies and SMM behavior[J]. Inorg. Chem.,
2015,54:8197-8206.
doi: 10.1021/acs.inorgchem.5b00334
-
[38]
Xu Y, Yu Y S, Huang X D, Bao S S, Ding H M, Ma Y Q, Zheng L M. Counteranion modulated crystal growth and function of one-dimensional homochiral coordination polymers: Morphology, structures, and magnetic properties[J]. Inorg. Chem.,
2018,57:12143-12154.
doi: 10.1021/acs.inorgchem.8b01762
-
[39]
Zhang L, Zhang Y Q, Zhang P, Zhao L, Guo M, Tang J K. Single-molecule magnet behavior enhanced by synergic effect of single-ion anisotropy and magnetic interactions[J]. Inorg. Chem.,
2017,56:7882-7889.
doi: 10.1021/acs.inorgchem.7b00625
-
[40]
Wu J F, Jung J L, Zhang P, Zhang H X, Tang J K, Guennic B L. Cis-trans isomerism modulates the magnetic relaxation of dysprosium single-molecule magnets[J]. Chem. Sci.,
2016,7:3632-3639.
doi: 10.1039/C5SC04510J
-
[41]
Lu J J, Zhang Y Q, Li X L, Guo M, Wu J F, Zhao L, Tang J K. Influence of magnetic interactions and single-ion anisotropy on magnetic relaxation within a family of tetranuclear dysprosium complexes[J]. Inorg. Chem.,
2019,58:5715-5724.
doi: 10.1021/acs.inorgchem.9b00067
-
[42]
Huang W, Shen F X, Wu S Q, Liu L, Wu D, Zheng Z, Xu J, Zhang M, Huang X C, Jiang J, Pan F, Li Y, Zhu K, Sato O. Metallogrid single-molecule magnet: Solvent-induced nuclearity transformation and magnetic hysteresis at 16 K[J]. Inorg. Chem.,
2016,55:5476-5484.
doi: 10.1021/acs.inorgchem.6b00500
-
[43]
Guo P H, Liu J L, Zhang Z M, Ungur L, Chibotaru L F, Leng J D, Guo F S, Tong M L. The first {Dy4} single-molecule magnet with a toroidal magnetic moment in the ground state[J]. Inorg. Chem.,
2012,51:1233-1235.
doi: 10.1021/ic202650f
-
[44]
Wang W M, Kang X M, Shen H Y, Wu Z L, Gao H L, Cui J Z. Modulating single-molecule magnet behavior towards multiple magnetic relaxation processes through structural variation in Dy4 clusters[J]. Inorg. Chem. Front.,
2018,5:1876-1885.
doi: 10.1039/C8QI00214B
-
[45]
Guo P H, Liu J L, Jia J H, Wang J, Guo F S, Chen Y C, Lin W Q, Leng J D, Bao D H, Zhang X D, Luo J H, Tong M L. Multifunctional Dy4Ⅲ cluster exhibiting white-emitting, ferroelectric and single-molecule magnet behavior[J]. Chem.-Eur. J.,
2013,19:8769-8773.
doi: 10.1002/chem.201300299
-
[46]
Aquilante F, Autschbach J, Carlson R K, Chibotaru L F, Delcey M G, Vico L D, Galván I F, Ferré N, Frutos L M, Gagliardi L, Garavelli M, Giussani A, Hoyer C E, Li Manni G, Lischka H, Ma D, Malmqvist P Å, Müller T, Nenov A, Olivucci M, Pedersen T B, Peng D, Plasser F, Pritchard B, Reiher M, Rivalta I, Schapiro I, Segarra-Martí J, Stenrup M, Truhlar D G, Ungur L, Valentini A, Vancoillie S, Veryazov V, Vysotskiy V P, Weingart O, Zapata F, Lindh R. MOLCAS 8: New capabilities for multiconfigurational quantum chemical calculations across the periodic table[J]. J. Comput. Chem.,
2016,37:506-541.
doi: 10.1002/jcc.24221
-
[47]
Chibotaru L F, Ungur L, Soncini A. The origin of nonmagnetic Kramers doublets in the ground state of dysprosium triangles: Evidence for a toroidal magnetic moment[J]. Angew. Chem. Int. Ed.,
2008,47:4126-4129.
doi: 10.1002/anie.200800283
-
[48]
Lu F, Ding M M, Li J X, Wang B L, Zhang Y Q. Why lanthanide ErⅢ SIMs cannot possess huge energy barriers: A theoretical investigation[J]. Dalton Trans.,
2020,49:14576-14583.
doi: 10.1039/D0DT02868A
-
[49]
Langley S K, Wielechowski D P, Vieru V, Chilton N F, Moubaraki B, Abrahams B F, Chibotaru L F, Murray K S. A {Cr2ⅢDy2Ⅲ} single-molecule magnet: Enhancing the blocking temperature through 3d magnetic exchange[J]. Angew. Chem. Int. Ed.,
2013,52:12014-12019.
doi: 10.1002/anie.201306329
-
[50]
Lines M E. Orbital angular momentum in the theory of paramagnetic clusters[J]. J. Chem. Phys.,
1971,55:2977-2984.
doi: 10.1063/1.1676524

 Login In
Login In






 DownLoad:
DownLoad: