Citation:
Na LIU. Mechanism of the effect of carbon coating on high temperature cycle performance of LiFePO4[J]. Chinese Journal of Inorganic Chemistry,
;2023, 39(12): 2287-2294.
doi:
10.11862/CJIC.2023.210

-
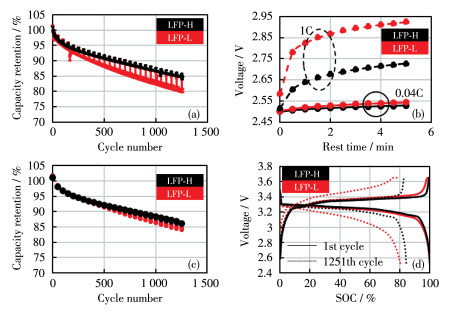
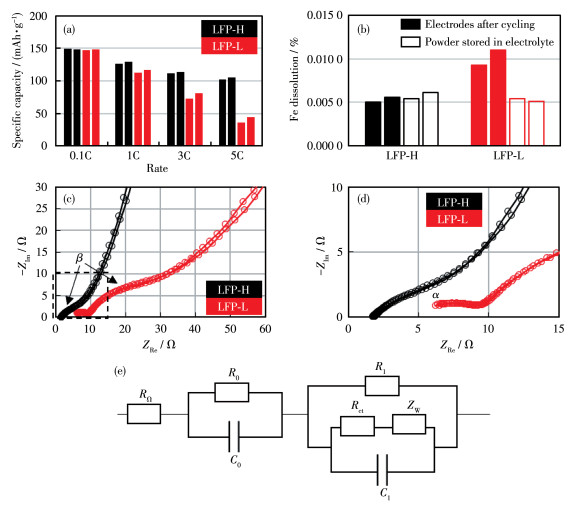
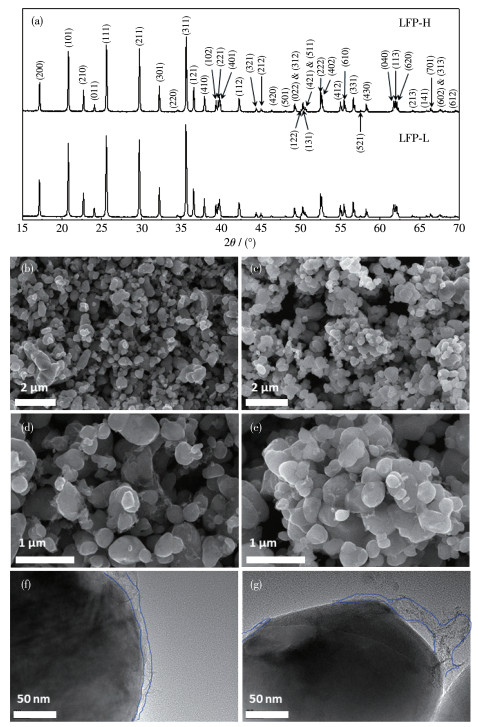
To investigate mechanism of carbon coating on the high-temperature cycle performance of widely used LiFePO4/graphite batteries, two types of LiFePO4 cathode material with different carbon coating degrees were prepared. According to characterization results from X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscope (TEM), powder resistance, and coin cell, two types of LiFePO4 were almost identical with respect to the crystal structure, particle size, and specific capacity. Then LiFePO4/graphite pouch cells were prepared and cycled at 1C under 60 ℃. It turns out that carbon coating can improve capacity retention from 80.4% to 84.9% after 1 251 cycles. The capacity improvement for polarization capacity and thermodynamic capacity account for 76% and 24%, respectively. This demonstrates that the mechanism of carbon coating is to reduce the polarization capacity loss by forming integrated conducting networks. In contrast, carbon coating can not inhibit Fe dissolution directly. Instead, it may be an indirect interaction through the reduction of moisture.
-

-
-
[1]
Gong Z L, Yang Y. Recent advances in the research of polyanion-type cathode materials for Li-ion batteries[J]. Energy Environ. Sci, 2011,4:3223-3242. doi: 10.1039/c0ee00713g
-
[2]
LIU L S. Application status and development trend of lithium iron phosphate battery[J]. Chinese Battery Industry, 2021,25(5):263-265. doi: 10.3969/j.issn.1008-7923.2021.05.007
-
[3]
Dubarry M, Liaw B Y. Identify capacity fading mechanism in a commercial LiFePO4 cell[J]. J. Power Sources, 2009,194(1):541-549. doi: 10.1016/j.jpowsour.2009.05.036
-
[4]
Zhang Y C, Wang C Y, Tang X D. Cycling degradation of an automotive LiFePO4 lithium-ion battery[J]. J. Power Sources, 2011,196(3):1513-1520. doi: 10.1016/j.jpowsour.2010.08.070
-
[5]
Safari M, Delacourt C. Aging of a commercial graphite/LiFePO4 cell[J]. J. Electrochem. Soc., 2011,158(10):A1123-A1135. doi: 10.1149/1.3614529
-
[6]
Dubarry M, Truchot C, Liaw B Y. Cell degradation in commercial LiFePO4 cells with high-power and high-energy designs[J]. J. Power Sources, 2014,258:408-419. doi: 10.1016/j.jpowsour.2014.02.052
-
[7]
Cao W P, Li J, Wu Z B. Cycle-life and degradation mechanism of LiFePO4-based lithium-ion batteries at room and elevated temperatures[J]. Ionics, 2016,22:1791-1799. doi: 10.1007/s11581-016-1703-4
-
[8]
Liang J L, Gan Y H, Yao M L, Li Y. Numerical analysis of capacity fading for a LiFePO4 battery under different current rates and ambient temperatures[J]. Int. J. Heat Mass Transf., 2021,165120615. doi: 10.1016/j.ijheatmasstransfer.2020.120615
-
[9]
ZHENG Y, LI J L, WANG X D. Capacity fading mechanism of LiFePO4/graphite power battery at high temperature[J]. Mater. Rep., 2016,30(10):15-18.
-
[10]
Amine K, Liu J, Belharouak I. High-temperature storage and cycling of C-LiFePO4/graphite Li-ion cells[J]. Electrochem. Commun., 2005,7(7):669-673. doi: 10.1016/j.elecom.2005.04.018
-
[11]
Chang H H, Wu H C, Wu N L. Enhanced high-temperature cycle performance of LiFePO4/carbon batteries by an ion-sieving metal coating on negative electrode[J]. Electrochem. Commun., 2008,10(12):1823-1826. doi: 10.1016/j.elecom.2008.09.022
-
[12]
Doeff M M, Hu Y Q, McLarnon F, Kostecki R. Effect of surface carbon structure on the electrochemical performance of LiFePO4[J]. Electrochem. Solid-State Lett., 2003,6(10):A207-A209. doi: 10.1149/1.1601372
- [13]
-
[14]
Meng Y S, Xia J, Wang L, Wang G R, Zhu F L, Zhang Y. A comparative Study on LiFePO4/C by in-situ coating with different carbon sources for high-performance lithium batteries[J]. Electrochim. Acta, 2018,261:96-103. doi: 10.1016/j.electacta.2017.12.127
-
[15]
Cho Y D, Fey G T K, Kao H M. The effect of carbon coating thickness on the capacity of LiFePO4/C composite cathodes[J]. J. Power Sources, 2009,189:256-262. doi: 10.1016/j.jpowsour.2008.09.053
-
[16]
Ong C W, Lin Y K, Chen J S. Effect of various organic precursors on the performance of LiFePO4/C composite cathode by coprecipitation method[J]. J. Electrochem. Soc., 2007,154(6):A527-A533. doi: 10.1149/1.2720714
-
[17]
Choi D, Kumta P N. Surfactant based sol-gel approach to nanostructured LiFePO4 for high rate Li-ion batteries[J]. J. Power Sources, 2007,163:1064-1069. doi: 10.1016/j.jpowsour.2006.09.082
-
[18]
Kim K, Jeong J H, Kim I J, Kim H S. Carbon coatings with olive oil, soybean oil and butter on nano-LiFePO4[J]. J. Power Sources, 2007,167(2):524-528. doi: 10.1016/j.jpowsour.2007.01.097
-
[19]
Hsu K F, Tsay S Y, Hwang B J. Synthesis and characterization of nano-sized LiFePO4 cathode materials prepared by a citric acid-based sol-gel route[J]. J. Mater. Chem., 2004,14:2690-2695. doi: 10.1039/B406774F
-
[20]
Zhi X K, Liang G C, Wang L, Ou Q X, Gao L M, Jie X F. Optimization of carbon coatings on LiFePO4: Carbonization temperature and carbon content[J]. J. Alloy. Compd., 2010,503:370-374. doi: 10.1016/j.jallcom.2010.02.173
-
[21]
ZHANG N, LIU Y C, CHEN C C, ZHU Z Q, TAO Z L, CHEN J. Research progress in carbon coating on LiFePO4 cathode materials for lithium ion batteries[J]. J. Electrochem., 2015,21(3):201-210.
-
[22]
HU G R, PENG Q Y, PENG Z D, CAO Y B, DU K. Comparison on properties of lithium iron phosphate/graphene composite prepared by two methods[J]. Chinese J. Inorg. Chem., 2015,31(6):1153-1158. doi: 10.11862/CJIC.2015.167
-
[23]
TONG H, HU G H, HU G R, PENG Z D, ZHANG X L. Synthesis of LiFePO4/C cathode material for lithium-ion battery[J]. Chinese J. Inorg. Chem., 2006,22(12):2159-2164.
-
[24]
Huang Y G, Zheng F H, Zhang X H, Li Q Y, Wang H Q. Effect of carbon coating on cycle performance of LiFePO4/C composite cathodes using Tween80 as carbon source[J]. Electrochim. Acta, 2014,130:740-747. doi: 10.1016/j.electacta.2014.03.091
-
[25]
Koltypin M, Aurbach D, Nazar L, Ellis B. On the stability of LiFePO4 olivine cathodes under various conditions (electrolyte solutions, temperatures)[J]. Electrochem. Solid-State Lett., 2007,10(2):A40-A44. doi: 10.1149/1.2403974
-
[26]
Koltypin M, Aurbach D, Nazar L, Ellis B. More on the performance of LiFePO4 electrodes—The effect of synthesis route, solution composition, aging, and temperature[J]. J. Power Sources, 2007,174(2):1241-1250. doi: 10.1016/j.jpowsour.2007.06.045
-
[27]
Heider U, Oesten R, Jungnitz M. Challenge in manufacturing electrolyte solutions for lithium and lithium ion batteries quality control and minimizing contamination level[J]. J. Power Sources, 1999,81-82:119-122. doi: 10.1016/S0378-7753(99)00142-1
-
[28]
Gaberscek M, Moskon J, Erjavec B, Dominko R, Jamnik J. The importance of interphase contacts in Li ion electrodes: The meaning of the high-frequency impedance Arc[J]. Electrochem. Solid-State Lett., 2008,11(10):A170-A174. doi: 10.1149/1.2964220
-
[1]
-

-
-
[1]
Xintong Zhu , Bin Cao , Chong Yan , Cheng Tang , Aibing Chen , Qiang Zhang . Advances in coating strategies for graphite anodes in lithium-ion batteries. Acta Physico-Chimica Sinica, 2025, 41(9): 100096-0. doi: 10.1016/j.actphy.2025.100096
-
[2]
Zhicheng JU , Wenxuan FU , Baoyan WANG , Ao LUO , Jiangmin JIANG , Yueli SHI , Yongli CUI . MOF-derived nickel-cobalt bimetallic sulfide microspheres coated by carbon: Preparation and long cycling performance for sodium storage. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 661-674. doi: 10.11862/CJIC.20240363
-
[3]
Jingshuo Zhang , Yue Zhai , Ziyun Zhao , Jiaxing He , Wei Wei , Jing Xiao , Shichao Wu , Quan-Hong Yang . Research Progress of Functional Binders in Silicon-Based Anodes for Lithium-Ion Batteries. Acta Physico-Chimica Sinica, 2024, 40(6): 2306006-0. doi: 10.3866/PKU.WHXB202306006
-
[4]
Ying Li , Yushen Zhao , Kai Chen , Xu Liu , Tingfeng Yi , Li-Feng Chen . Rational Design of Cross-Linked N-Doped C-Sn Nanofibers as Free-Standing Electrodes towards High-Performance Li-Ion Battery Anodes. Acta Physico-Chimica Sinica, 2024, 40(3): 2305007-0. doi: 10.3866/PKU.WHXB202305007
-
[5]
Qingtang ZHANG , Xiaoyu WU , Zheng WANG , Xiaomei WANG . Performance of nano Li2FeSiO4/C cathode material co-doped by potassium and chlorine ions. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1689-1696. doi: 10.11862/CJIC.20240115
-
[6]
Xinpeng LIU , Liuyang ZHAO , Hongyi LI , Yatu CHEN , Aimin WU , Aikui LI , Hao HUANG . Ga2O3 coated modification and electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material. Chinese Journal of Inorganic Chemistry, 2024, 40(6): 1105-1113. doi: 10.11862/CJIC.20230488
-
[7]
Zhihuan XU , Qing KANG , Yuzhen LONG , Qian YUAN , Cidong LIU , Xin LI , Genghuai TANG , Yuqing LIAO . Effect of graphene oxide concentration on the electrochemical properties of reduced graphene oxide/ZnS. Chinese Journal of Inorganic Chemistry, 2024, 40(7): 1329-1336. doi: 10.11862/CJIC.20230447
-
[8]
Xueyu Lin , Ruiqi Wang , Wujie Dong , Fuqiang Huang . Rational Design of Bimetallic Oxide Anodes for Superior Li+ Storage. Acta Physico-Chimica Sinica, 2025, 41(3): 100021-0. doi: 10.3866/PKU.WHXB202311005
-
[9]
Bowen Yang , Rui Wang , Benjian Xin , Lili Liu , Zhiqiang Niu . C-SnO2/MWCNTs Composite with Stable Conductive Network for Lithium-based Semi-Solid Flow Batteries. Acta Physico-Chimica Sinica, 2025, 41(2): 100015-0. doi: 10.3866/PKU.WHXB202310024
-
[10]
Yuting ZHANG , Zunyi LIU , Ning LI , Dongqiang ZHANG , Shiling ZHAO , Yu ZHAO . Nickel vanadate anode material with high specific surface area through improved co-precipitation method: Preparation and electrochemical properties. Chinese Journal of Inorganic Chemistry, 2024, 40(11): 2163-2174. doi: 10.11862/CJIC.20240204
-
[11]
Yuanchao LI , Weifeng HUANG , Pengchao LIANG , Zifang ZHAO , Baoyan XING , Dongliang YAN , Li YANG , Songlin WANG . Effect of heterogeneous dual carbon sources on electrochemical properties of LiMn0.8Fe0.2PO4/C composites. Chinese Journal of Inorganic Chemistry, 2024, 40(4): 751-760. doi: 10.11862/CJIC.20230252
-
[12]
Yifeng Xu , Jiquan Liu , Bin Cui , Yan Li , Gang Xie , Ying Yang . “Xiao Li’s School Adventures: The Working Principles and Safety Risks of Lithium-ion Batteries”. University Chemistry, 2024, 39(9): 259-265. doi: 10.12461/PKU.DXHX202404009
-
[13]
Wen Tang , Luyu Sui , Qian Chen , Jun Shao , Xinwen Peng , Jianwen Jiang , Shuiliang Chen . Project-based Teaching of “the Condensed State of Polymers”: Unveiling the Lithium-Ion Battery Separator. University Chemistry, 2025, 40(11): 115-126. doi: 10.12461/PKU.DXHX202412108
-
[14]
Siyu Zhang , Kunhong Gu , Bing'an Lu , Junwei Han , Jiang Zhou . Hydrometallurgical Processes on Recycling of Spent Lithium-lon Battery Cathode: Advances and Applications in Sustainable Technologies. Acta Physico-Chimica Sinica, 2024, 40(10): 2309028-0. doi: 10.3866/PKU.WHXB202309028
-
[15]
Qi Li , Pingan Li , Zetong Liu , Jiahui Zhang , Hao Zhang , Weilai Yu , Xianluo Hu . Fabricating Micro/Nanostructured Separators and Electrode Materials by Coaxial Electrospinning for Lithium-Ion Batteries: From Fundamentals to Applications. Acta Physico-Chimica Sinica, 2024, 40(10): 2311030-0. doi: 10.3866/PKU.WHXB202311030
-
[16]
Aoyu Huang , Jun Xu , Yu Huang , Gui Chu , Mao Wang , Lili Wang , Yongqi Sun , Zhen Jiang , Xiaobo Zhu . Tailoring Electrode-Electrolyte Interfaces via a Simple Slurry Additive for Stable High-Voltage Lithium-Ion Batteries. Acta Physico-Chimica Sinica, 2025, 41(4): 100037-0. doi: 10.3866/PKU.WHXB202408007
-
[17]
Junke LIU , Kungui ZHENG , Wenjing SUN , Gaoyang BAI , Guodong BAI , Zuwei YIN , Yao ZHOU , Juntao LI . Preparation of modified high-nickel layered cathode with LiAlO2/cyclopolyacrylonitrile dual-functional coating. Chinese Journal of Inorganic Chemistry, 2024, 40(8): 1461-1473. doi: 10.11862/CJIC.20240189
-
[18]
Liangliang Song , Haoyan Liang , Shunqing Li , Bao Qiu , Zhaoping Liu . Challenges and strategies on high-manganese Li-rich layered oxide cathodes for ultrahigh-energy-density batteries. Acta Physico-Chimica Sinica, 2025, 41(8): 100085-0. doi: 10.1016/j.actphy.2025.100085
-
[19]
Xuechen Hu , Qiuying Xia , Fan Yue , Xinyi He , Zhenghao Mei , Jinshi Wang , Hui Xia , Xiaodong Huang . Electrochemical Characteristics of LiNbO3 Anode Film and Its Applications in All-Solid-State Thin-Film Lithium-Ion Battery. Acta Physico-Chimica Sinica, 2024, 40(2): 2309046-0. doi: 10.3866/PKU.WHXB202309046
-
[20]
Zhenming Xu , Mingbo Zheng , Zhenhui Liu , Duo Chen , Qingsheng Liu . Experimental Design of Project-Driven Teaching in Computational Materials Science: First-Principles Calculations of the LiFePO4 Cathode Material for Lithium-Ion Batteries. University Chemistry, 2024, 39(4): 140-148. doi: 10.3866/PKU.DXHX202307022
-
[1]
Metrics
- PDF Downloads(97)
- Abstract views(3671)
- HTML views(1191)

 Login In
Login In




 DownLoad:
DownLoad: