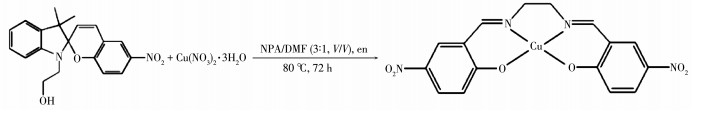
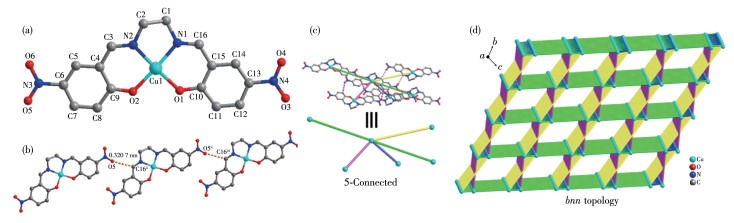
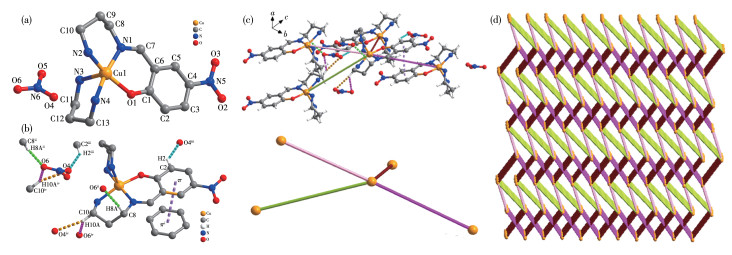
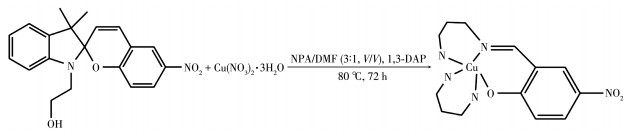-
[1]
Al-Sahlanee T Q M, Al-Amery M H. Synthesis, Characterization, Antioxident and Anticancer Human Studies of New Metal Ion Complexes of Poly Schiff Base Derived from 4-Aminocetophenone with Salicylaldehyde and 4-Bromoaniline[J]. Asian J. Pharm. Clin. Res.,
2018,11:489-493.
-
[2]
Zoubi W A, Al-Hamdani A A S, Kaseem M, Ahmed S D, Ko Y G. A New Azo-Schiff Base: Synthesis, Characterization, Biological Activity and Theoretical Studies of Its Complexes[J]. Appl. Organomet. Chem.,
2016,30:810-817.
doi: 10.1002/aoc.3506
-
[3]
Malik M A, Dar O A, Gull P, Wani M Y. Heterocyclic Schiff Base Transition Metal Complexes in Antimicrobial and Anticancer Chemotherapy[J]. MedChemComm,
2018,9:409-436.
doi: 10.1039/C7MD00526A
-
[4]
Gayen F R, Ali A A, Bora D, Roy S, Saha S, Saikia L, Goswamee R L, Saha B. Correction: A Ferrocene Functionalized Schiff Base Containing Cu(Ⅱ) Complex: Synthesis, Characterization and Parts-Per-Million Level Catalysis for Azide Alkyne Cycloaddition[J]. Dalton Trans.,
2020,49:6578-6586.
doi: 10.1039/D0DT00915F
-
[5]
Xu Y J, Kaneko K, Kanai M, Shibasaki M, Matsunaga S. Regiodivergent Kinetic Resolution of Terminal and Internal rac-Aziridines with Malonates under Dinuclear Schiff Base Catalysis[J]. J. Am. Chem. Soc.,
2014,136(25):9190-9194.
doi: 10.1021/ja5039165
-
[6]
Liu X, Hamon J R. Recent Developments in Penta-, Hexa- and Hepta-dentate Schiff Base Ligands and Their Metal Complexes[J]. Coord. Chem. Rev.,
2019,389(15):94-118.
-
[7]
Cozzi P G. Metal-Salen Schiff Base Complexes in Catalysis: Practical Aspects[J]. Chem. Soc. Rev.,
2004,33(7):410-421.
doi: 10.1039/B307853C
-
[8]
Shellaiah M, Wu Y H, Singh A, Raju M V R, Lin H C. Novel Pyrene- and Anthracene-Based Schiff Base Derivatives as Cu2+ and Fe3+ Fluorescence Turn-On Sensors and for Aggregation Induced Emissions[J]. J. Mater. Chem. A,
2013,1:1310-1318.
doi: 10.1039/C2TA00574C
-
[9]
Berbasova T, Nosrati M, Vasileiou C, Wang W J, Lee K S S, Yapici I, Geiger J H, Borhan B. Rational Design of a Colorimetric pH Sensor from a Soluble Retinoic Acid Chaperone[J]. J. Am. Chem. Soc.,
2013,135(43):16111-16119.
doi: 10.1021/ja404900k
-
[10]
El-Shishtawy R M, Al-Ghamdi H A, Alam M M, Al-Amshany Z M, Asiri A M, Rahman M M. Development of Cd2+ Sensor Based on BZNA/Nafion/Glassy Carbon Electrode by Electrochemical Approach[J]. Chem. Eng. J.,
2018,352(15):225-231.
-
[11]
Liu X, Fu C H, Ren X L, Liu H Y, Li L L, Meng X W. Fluorescence Switching Method for Cascade Detection of Salicylaldehyde and Zinc(Ⅱ) Ion Using Protein Protected Gold Nanoclusters[J]. Biosens. Bioelectron.,
2015,74(15):322-328.
-
[12]
Das M, Biswas A, Kundu B K, Charmier M A J, Mukherjee A, Mobin S M, Udayabhanu G, Mukhopadhyay S. Enhanced Pseudo-Halide Promoted Corrosion Inhibition by Biologically Active Zinc(Ⅱ) Schiff Base Complexes[J]. Chem. Eng. J.,
2019,357(1):447-457.
-
[13]
Bedair M A, El-Sabbah M M B, Fouda A S, Elaryian H M. Synthesis, Electrochemical and Quantum Chemical Studies of Some Prepared Surfactants Based on Azodye and Schiff Base as Corrosion Inhibitors for Steel in Acid Medium[J]. Corros. Sci.,
2017,128:54-72.
doi: 10.1016/j.corsci.2017.09.016
-
[14]
Saha S Kr, Banerjee P. Introduction of Newly Synthesized Schiff Base Molecules as Efficient Corrosion Inhibitors for Mild Steel in 1 M HCl Medium: An Experimental, Density Functional Theory and Molecular Dynamics Simulation Study[J]. Mater. Chem. Front.,
2018,2:1674-1691.
doi: 10.1039/C8QM00162F
-
[15]
Sun H, Sun S S, Han F F, Ni Z H, Zhang R, Li M D. A New Tetraphenylethene-Based Schiff Base: Two Crystalline Polymorphs Exhibiting Totally Different Photochromic and Fluorescence Properties[J]. J. Mater. Chem. C,
2019,7:7053-7060.
-
[16]
Guo S L, Liu G, Fan C B, Pu S Z. A New Diarylethene-Derived Probe for Colorimetric Sensing of Cu(Ⅱ) and Fluorometric Sensing of Cu(Ⅱ) and Zn(Ⅱ): Photochromism and High Selectivity[J]. Sens. Actuators B,
2018,266(1):603-613.
-
[17]
Pu S Z, Tong Z P, Liu G, Wang R J. Multi-Addressable Molecular Switches Based on a New Diarylethene Salicylal Schiff Base Derivative[J]. J. Mater. Chem. C,
2013,1:4726-4739.
doi: 10.1039/c3tc30804a
-
[18]
Mukhopadhyay A, Maka V K, Moorthy J N. Fluoride-Triggered Ring-Opening of Photochromic Diarylpyrans into Merocyanine Dyes: Naked-Eye Sensing in Subppm Levels[J]. J. Org. Chem.,
2016,81(17):7741-7750.
doi: 10.1021/acs.joc.6b01361
-
[19]
Zhang J J, Fu Y X, Han H H, Zang Y, Li J, He X P, Feringa B L, Tian H. Remote Light-Controlled Intracellular Target Recognition by Photochromic Fluorescent Glycoprobes[J]. Nat. Commun.,
2017,8987.
doi: 10.1038/s41467-017-01137-8
-
[20]
Lin S Y, Gutierrez-Cuevas K G, Zhang X F, Guo J B, Li Q. Fluorescent Photochromic Alpha-Cyanodiarylethene Molecular Switches: An Emerging and Promising Class of Functional Diarylethene[J]. Adv. Funct. Mater.,
20202007957.
-
[21]
Ali A A, Kharbash R, Kim Y. Chemo- and Biosensing Applications of Spiropyran and Its Derivatives: A Review[J]. Anal. Chim. Acta,
2020,1110(8):199-223.
-
[22]
Zacharias P, Gather M C, Kohnen A, Rehmann N, Meerholz K. Photoprogrammable Organic Light-Emitting Diodes[J]. Angew. Chem. Int. Ed.,
2009,48:4038-4041.
doi: 10.1002/anie.200805969
-
[23]
Feuerstein T J, Muller R, Barner-Kowollik C, Roesky P W. Investigating the Photochemistry of Spiropyran Metal Complexes with Online LED-NMR[J]. Inorg. Chem.,
2019,58(22):15479-15486.
doi: 10.1021/acs.inorgchem.9b02547
-
[24]
Wales D J, Cao Q, Kastner K, Karjalainen E, Newton G N, Sans V. 3D-Printable Photochromic Molecular Materials for Reversible Information Storage[J]. Adv. Mater.,
2018,30(26)1870193.
doi: 10.1002/adma.201870193
-
[25]
Tian H. Data Processing on a Unimolecular Platform[J]. Angew. Chem. Int. Ed.,
2010,49(28):4710-4712.
doi: 10.1002/anie.200906834
-
[26]
Raymo F M, Alvarado R J, Giordani S, Cejas M A. Memory Effects Based on Intermolecular Photoinduced Proton Transfer[J]. J. Am. Chem. Soc.,
2003,125(8):2361-2364.
doi: 10.1021/ja027977j
-
[27]
Miguez F B, Menzonatto T G, Netto J F Z, Silva I M S, Verano-Braga T, Lopes J F, DeSousa F B. Photo-Dynamic and Fluorescent Zinc Complex Based on Spiropyran Ligand[J]. J. Mol. Struct.,
2020,1211(5)128105.
-
[28]
Funasako Y, Miyazaki H, Sasaki T, Goshima K, Inokuchi M. Synthesis, Photochromic Properties, and Crystal Structures of Salts Containing a Pyridinium-Fused Spiropyran: Positive and Negative Photochromism in the Solution and Solid State[J]. J. Phys. Chem. B,
2020,124(33):7251-7257.
doi: 10.1021/acs.jpcb.0c04994
-
[29]
Qu L, Xu X M, Song J T, Wu D H, Wang L, Zhou W L, Zhou X G, Xiang H F. Solid-State Photochromic Molecular Switches Based on Axially Chiral and Helical Spiropyrans[J]. Dyes Pigm.,
2020,181108597.
doi: 10.1016/j.dyepig.2020.108597
-
[30]
Cui H Q, Liu H, Chen S, Wang R M. Synthesis of Amphiphilic Spiropyran-Based Random Copolymer by Atom Transfer Radical Polymerization for Co2+ Recognition[J]. Dyes Pigm.,
2015,115:50-57.
doi: 10.1016/j.dyepig.2014.12.008
-
[31]
Hu S Z, Lv L H, Chen S H, You M L, Fu Z Y. Zn-MOF-Based Photoswitchable Dyad that Exhibits Photocontrolled Luminescence[J]. Cryst. Growth Des.,
2016,16(12):6705-6708.
doi: 10.1021/acs.cgd.6b01129
-
[32]
Zhang H, Kou X X, Zhang Q, Qu D H, Tian H. Altering Intercomponent Interactions in a Photochromic Multi-State Rotaxane[J]. Org. Biomol. Chem.,
2011,9:4051-4056.
doi: 10.1039/c1ob05307h
-
[33]
Poelma S O, Oh S S, Helmy S, Knight A S, Burnett G L, Soh H T, Hawker C, Dealaniz J R. Controlled Drug Release to Cancer Cells from Modular One-Photon Visible Light-Responsive Micellar System[J]. Chem. Commun.,
2016,52:10525-10528.
doi: 10.1039/C6CC04127B
-
[34]
Guo X, Shao B H, Zhou S B, Aprahamian I, Chen Z. Visualizing Intracellular Particles and Precise Control of Drug Release Using an Emissive Hydrazone Photochrome[J]. Chem. Sci.,
2020,11:3016-3021.
doi: 10.1039/C9SC05321B
-
[35]
Cardano F, Delcanto E, Giordani S. Spiropyrans for Light-Controlled Drug Delivery[J]. Dalton Trans.,
2019,48:15537-15544.
doi: 10.1039/C9DT02092F
-
[36]
Tong R, Hemmati H D, Langer R, Kohane D S. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery[J]. J. Am. Chem. Soc.,
2012,134(21):8848-8855.
doi: 10.1021/ja211888a
-
[37]
Achilleos D S, Hatton T A, Vamvakaki M. Light-Regulated Supramolecular Engineering of Polymeric Nanocapsules[J]. J. Am. Chem. Soc.,
2012,134(13):5726-5729.
doi: 10.1021/ja212177q
-
[38]
Ye Z W, Yu H B, Yang W, Zheng Y, Li N, Bian H, Wang Z C, Liu Q, Song Y T, Zhang M Y, Xiao Y. Strategy to Lengthen the On-Time of Photochromic Rhodamine Spirolactam for Super-resolution Photoactivated Localization Microscopy[J]. J. Am. Chem. Soc.,
2019,141(16):6527-6536.
doi: 10.1021/jacs.8b11369
-
[39]
Deniz E, Tomasulo M, Cusido J, Yildiz I, Petriella M, Bossi M L, Sortino S, Raymo F M. Photoactivatable Fluorophores for Super-Resolution Imaging Based on Oxazine Auxochromes[J]. J. Phys. Chem. C,
2012,116(10):6058-6068.
-
[40]
Xie J L, Batten S R, Zou Y, Ren X M. Observation of In Situ Ligand Reactions during the Assembly of Crystalline Zn-S Clusters[J]. Cryst. Growth Des.,
2011,11:16-20.
doi: 10.1021/cg100926g
-
[41]
Liu W L, Yu J H, Jiang J H, Yuan L M, Xu B, Liu Q A, Qu B T, Zhang G Q, Yan C G. Hydrothermal Syntheses, Structures and Luminescent Properties of Zn Coordination Polymers Assembled with Benzene-1, 2, 3-tricarboxylic Acid Involving In Situ Ligand Reactions[J]. CrystEngComm,
2011,13:2764-2773.
-
[42]
Hou Y L, PengY L, Diao Y X, Liu J, Chen L Z, Li D. Side Chain Induced Self-Assembly and Selective Catalytic Oxidation Activity of Copper-Copper-N-4 Complexes[J]. Cryst. Growth Des.,
2020,20:1237-1241.
-
[43]
Wei R P, Dong Y T, Zhang Y Y, Zhang R, Al-Tahan M A, Zhang J M. In-Situ Self-Assembled Hollow Urchins F-Co-MOF on rGO as Advanced Anodes for Lithium-Ion and Sodium-Ion Batteries[J]. J. Colloid Interface Sci.,
2021,582:236-245.
-
[44]
Zhang W Q, Kang Y F, Guo L L, Yang J J. Synthesis, Structure and Fluorescent Property of a Novel 3D Rod-Packing Microporous Zn(Ⅱ) MOF Based on a Temperature-Induced In Situ Ligand Reaction[J]. ChemistrySelect,
2020,5:1439-1442.
-
[45]
Dunning S G, Reynolds J E, Walsh K M, Kristek D J, Lynch V M, Kunal P, Humphrey S M. Direct, One-Pot Syntheses of MOFs Decorated with Low-Valent Metal-Phosphine Complexes[J]. Organometallics,
2019,38:3406-3411.
-
[46]
Baldrighi M, Locatelli G, Desper J, Aakeroy C B, Giordani S. Probing Metal Ion Complexation of Ligands with Multiple Metal Binding Sites: The Case of Spiropyrans[J]. Chem. Eur. J.,
2016,22(39):13976-13984.

 Login In
Login In






 DownLoad:
DownLoad: