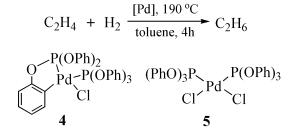Citation:
Hu Ziqiao, Liu Guichuan, Kong Yong, Lin Yongxue, Jin Junbin. Syntheses and Catalytic Applications of Palladacycles Complexes[J]. Chemistry,
;2019, 82(1): 32-36.

-
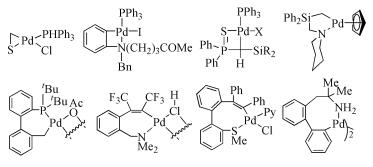
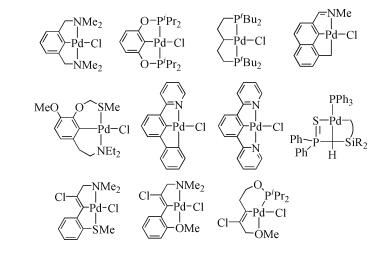
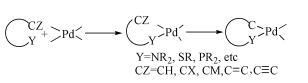
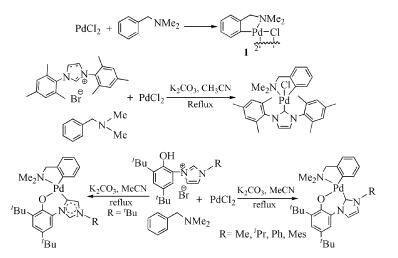
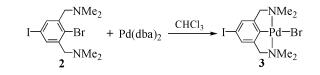
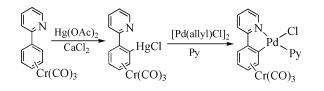
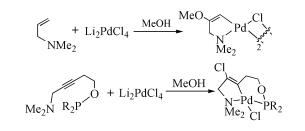
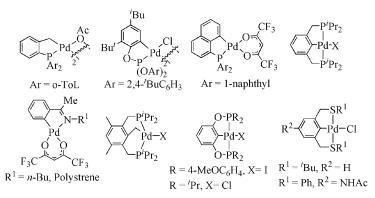
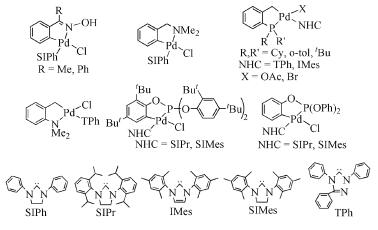
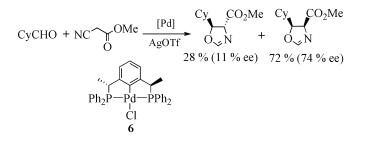
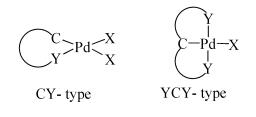
Due to their abundant structures, high stability and excellent catalytic properties, palladacycles complexes have become one of the hotspots in the research of palladium chemistry. A number of synthesis methods have been developed, such as C-H bond activation, oxidation addition, metal transfer, nucleophilic addition, and ligand exchange. The CY type palladacycles complexes from ternary ring to eleven-membered ring and YCY type palladacycles complexes have been afforded. Palladacycles complexes have been utilized in olefin hydrogenation, coupling reaction, asymmetric catalysis, and other reactions. In this article, the types of palladacycles complexes were briefly introduced. The synthesis methods and application for catalytic reactions of palladacycles complexes were focused on. The development suggestions of palladacycles complexes for synthesis and catalytic application were proposed.
-
Keywords:
- Palladacycles,
- Synthesis,
- Catalysis
-

-
- [1]
- [2]
- [3]
- [4]
-
[5]
I P Beletskaya, A V Cheprakov. J. Organomet. Chem., 2004, 689(24):4055~4082.
-
[6]
J Dupont, C S Consorti, J Spencer. Chem. Rev., 2005, 105(6):2527~2571.
-
[7]
A C Cope, E C Friedrich. J. Am. Chem. Soc., 1968, 90(4):909~911.
-
[8]
J Dupont, M Pfeffer, J Spencer. Eur. J. Inorg. Chem., 2001, (8):1917~1927.
-
[9]
D Sole, L Vallverdu, X Solans et al. J. Am. Chem. Soc., 2003, 125(6):1587~1594.
- [10]
-
[11]
D Morales-Morales, R Redon, C Yung et al. Chem. Commun., 2000, (17):1619~1634.
-
[12]
S G Koller, R Martín-Romo, J S Melero et al. Organometallics, 2014, 33(24):7329~7332.
-
[13]
M Oliva-Madrid, J García-López, I Saura-Llamas et al. Organometallics, 2014, 33(22):6420~6430.
-
[14]
G Ebeling, M R Meneghetti, F Rominger et al. Organometallics, 2002, 21(15):3221~3227.
-
[15]
S Molitor, C Schwarz, V H Gessner. Organometallics, 2016, 35(2):159~167.
-
[16]
C S Consorti, G Ebeling, F Rodembusch et al. Inorg. Chem., 2004, 43(2):530~536.
- [17]
-
[18]
E A B Kantchev, G Peh, C Zhang. Org. Lett., 2008, 10(8):3949~3952.
-
[19]
E A B Kantchev, J Y Ying. Organometallics, 2009, 28(1):289~299.
-
[20]
Y Kong, L Wen, H Song et al. Organometallics, 2011, 30(1):153~159.
-
[21]
-
[22]
M Li, H Song, B Wang. Organometallics, 2015, 34(10):1969~1977.
-
[23]
-
[24]
- [25]
-
[26]
L Dang, H Song, B Wang. Organometallics, 2014, 33(23):6812~6818.
-
[27]
S Sara, A M Jose, P Eduardo. Organometallics, 2013, 32(4):1112~1120.
- [28]
- [29]
- [30]
- [31]
- [32]
-
[33]
M Liu, P Yang, M K Karunananda et al. J. Am. Chem. Soc., 2018, 140(17):5805~5813.
-
[34]
M Nowotny, U Hanefeld, H Koningsveld et al. Chem. Commun., 2000, (19):1877~1878.
-
[35]
X Gai, R Grigg, M I Ramzan et al. Chem. Commun., 2000, (20):2053~2054.
-
[36]
-
[37]
M L O'Duill, R Matsuura, Y Wang et al. J. Am. Chem. Soc., 2017, 139(44):15576~15579.
- [38]
- [39]
-
[40]
D Morales-Morales, R Redon, C Yung et al. Chem. Commun., 2000, (17):1619~1670.
-
[41]
D A Lonso, C Nejera, M C Pacheco. Org. Lett., 2000, 2(13):1823~1826.
-
[42]
D E Bergbreiter, P L Osburn, A Wilson et al. J. Am. Chem. Soc., 2000, 122(38):9058~9064.
-
[43]
A S Gruber, D Zim, G Ebeling et al. Org. Lett., 2000, 2(9):1287~1290
-
[44]
D Zim, A S Gruber, G Ebeling et al. Org. Lett., 2000, 2(18):2881~2884.
-
[45]
X Gai, R Grigg, M I Ramzan et al. Chem. Commun., 2000, (20):2053~2054.
-
[46]
S Darses, G Michaud, J P Genet. Eur. J. Org. Chem., 1999, (8):1875~1883.
-
[47]
M S Viciu, R A Ⅲ Kelly, E D Stevens et al. Org. Lett., 2003, 5(9):1479~1482.
-
[48]
G D Frey, J Schütz, E Herdtweck et al. Organometallics, 2005, 24(18):4416~4426.
-
[49]
O Navarro, N Marion, Y Oonishi et al. J. Org. Chem., 2006, 71(2):685~692.
-
[50]
L Jin, W Wei, N Sun et al. Org. Chem. Front., 2018, 5(16):2484~2491.
-
[51]
J Broggi, H Clavier, S P Nolan. Organometallics, 2008, 27(21):5525~5531.
-
[52]
B Alexandre, R Maxime, M Alami et al. ACS Catal., 2015, 5(2):1386~1396.
-
[53]
N Marion, S P Nolan. Acc. Chem. Res., 2008, 41(11):1440~1449.
-
[54]
R Navarro, E P Urriolabeitia, C Cativiela et al. J. Mol. Catal. A, 1996, 105(3):111~116.
-
[55]
M A Stark, G Jones, C J Richards. Organometallics, 2000, 19(7):1282~1291.
-

-
-
[1]
Jiaming Xu , Yu Xiang , Weisheng Lin , Zhiwei Miao . Research Progress in the Synthesis of Cyclic Organic Compounds Using Bimetallic Relay Catalytic Strategies. University Chemistry, 2024, 39(3): 239-257. doi: 10.3866/PKU.DXHX202309093
-
[2]
Wenjuan SHI , Yuke LU , Xiuyuan LI , Lei HOU , Yaoyu WANG . Mg(Ⅱ) metal-organic frameworks based on biphenyltetracarboxylic acid: Synthesis and CO2 adsorption and catalytic conversion performance. Chinese Journal of Inorganic Chemistry, 2025, 41(12): 2455-2463. doi: 10.11862/CJIC.20250220
-
[3]
Ying Chen , Ronghua Yan , Weiyan Yin . Research Progress on the Synthesis of Metal Single-Atom Catalysts and Their Applications in Electrocatalytic Hydrogen Evolution Reactions. University Chemistry, 2025, 40(9): 344-353. doi: 10.12461/PKU.DXHX202503066
-
[4]
Jing WU , Puzhen HUI , Huilin ZHENG , Pingchuan YUAN , Chunfei WANG , Hui WANG , Xiaoxia GU . Synthesis, crystal structures, and antitumor activities of transition metal complexes incorporating a naphthol-aldehyde Schiff base ligand. Chinese Journal of Inorganic Chemistry, 2024, 40(12): 2422-2428. doi: 10.11862/CJIC.20240278
-
[5]
Xinting XIONG , Zhiqiang XIONG , Panlei XIAO , Xuliang NIE , Xiuying SONG , Xiuguang YI . Synthesis, crystal structures, Hirshfeld surface analysis, and antifungal activity of two complexes Na(Ⅰ)/Cd(Ⅱ) assembled by 5-bromo-2-hydroxybenzoic acid ligands. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1661-1670. doi: 10.11862/CJIC.20240145
-
[6]
Lifang HE , Wenjie TANG , Yaoze LUO , Mingsheng LIANG , Jianxin TANG , Yuxuan WU , Fuxing ZHANG , Xiaoming ZHU . Synthesis, structure, and anticancer activity of two dialkyltin complexes constructed based on 2, 2′-bipyridin-6, 6′-dicarboxylic acid. Chinese Journal of Inorganic Chemistry, 2025, 41(8): 1601-1609. doi: 10.11862/CJIC.20250012
-
[7]
Xinyi Zhang , Kai Ren , Yanning Liu , Zhenyi Gu , Zhixiong Huang , Shuohang Zheng , Xiaotong Wang , Jinzhi Guo , Igor V. Zatovsky , Junming Cao , Xinglong Wu . Progress on Entropy Production Engineering for Electrochemical Catalysis. Acta Physico-Chimica Sinica, 2024, 40(7): 2307057-0. doi: 10.3866/PKU.WHXB202307057
-
[8]
Ran Yu , Chen Hu , Ruili Guo , Ruonan Liu , Lixing Xia , Cenyu Yang , Jianglan Shui . Catalytic Effect of H3PW12O40 on Hydrogen Storage of MgH2. Acta Physico-Chimica Sinica, 2025, 41(1): 100001-0. doi: 10.3866/PKU.WHXB202308032
-
[9]
Xiaogang Liu , Mengyu Chen , Yanyan Li , Xiantao Ma . Experimental Reform in Applied Chemistry for Cultivating Innovative Competence: A Case Study of Catalytic Hydrogen Production from Liquid Formaldehyde Reforming at Room Temperature. University Chemistry, 2025, 40(7): 300-307. doi: 10.12461/PKU.DXHX202408007
-
[10]
Lixing ZHANG , Yaowen WANG , Xu HAN , Junhong ZHOU , Jinghui WANG , Liping LI , Guangshe LI . Research progress in the synthesis of fluorine-containing perovskites and their derivatives. Chinese Journal of Inorganic Chemistry, 2025, 41(9): 1689-1701. doi: 10.11862/CJIC.20250007
-
[11]
Bin SUN , Heyan JIANG . Glucose-modified bis-Schiff bases: Synthesis and bio-activities in Alzheimer′s disease therapy. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1338-1350. doi: 10.11862/CJIC.20240428
-
[12]
Yongxin LIU , Xingchen LI , Hongjia LIU , Danni LI , Tao ZHANG , Xi CHEN . Enhancement effect of Fe3O4 conversion to MIL-100(Fe) on activation of persulfate for degradation of antibiotic. Chinese Journal of Inorganic Chemistry, 2025, 41(12): 2503-2513. doi: 10.11862/CJIC.20250169
-
[13]
Shiyan Cheng , Yonghong Ruan , Lei Gong , Yumei Lin . Research Advances in Friedel-Crafts Alkylation Reaction. University Chemistry, 2024, 39(10): 408-415. doi: 10.12461/PKU.DXHX202403024
-
[14]
Chi Li , Jichao Wan , Qiyu Long , Hui Lv , Ying Xiong . N-Heterocyclic Carbene (NHC)-Catalyzed Amidation of Aldehydes with Nitroso Compounds. University Chemistry, 2024, 39(5): 388-395. doi: 10.3866/PKU.DXHX202312016
-
[15]
Lei Feng , Ze-Min Zhu , Ying Yang , Zongbin He , Jiafeng Zou , Man-Bo Li , Yan Zhao , Zhikun Wu . Long-Pursued Structure of Au23(S-Adm)16 and the Unexpected Doping Effects. Acta Physico-Chimica Sinica, 2024, 40(5): 2305029-0. doi: 10.3866/PKU.WHXB202305029
-
[16]
Chunling Qin , Shuang Chen , Hassanien Gomaa , Mohamed A. Shenashen , Sherif A. El-Safty , Qian Liu , Cuihua An , Xijun Liu , Qibo Deng , Ning Hu . Regulating HER and OER Performances of 2D Materials by the External Physical Fields. Acta Physico-Chimica Sinica, 2024, 40(9): 2307059-0. doi: 10.3866/PKU.WHXB202307059
-
[17]
Geyang Song , Dong Xue , Gang Li . Recent Advances in Transition Metal-Catalyzed Synthesis of Anilines from Aryl Halides. University Chemistry, 2024, 39(2): 321-329. doi: 10.3866/PKU.DXHX202308030
-
[18]
Lili Jiang , Shaoyu Zheng , Xuejiao Liu , Xiaomin Xie . Copper-Catalyzed Oxidative Coupling Reactions for the Synthesis of Aryl Sulfones: A Fundamental and Exploratory Experiment for Undergraduate Teaching. University Chemistry, 2025, 40(7): 267-276. doi: 10.12461/PKU.DXHX202408004
-
[19]
Yan Kong , Wei Wei , Lekai Xu , Chen Chen . Electrochemical Synthesis of Organonitrogen Compounds from N-integrated CO2 Reduction Reaction. Acta Physico-Chimica Sinica, 2024, 40(8): 2307049-0. doi: 10.3866/PKU.WHXB202307049
-
[20]
Zhiqiang XING , Jinling LIU , Mingmin SU , Lei ZHANG , Lijun YANG . CoNi dual-single-atom catalyst for electrocatalytic H2O2 production and in situ electro-Fenton degradation of pollutants. Chinese Journal of Inorganic Chemistry, 2025, 41(12): 2479-2490. doi: 10.11862/CJIC.20250181
-
[1]
Metrics
- PDF Downloads(6)
- Abstract views(533)
- HTML views(56)

 Login In
Login In




 DownLoad:
DownLoad: