Citation:
Zhou Mingdong, Du Haiwu, Gong Xinyu, Sun Jing. Progress in the Syntheses of Coumarin Derivatives through Tandem Radical Addition/Cyclization[J]. Chemistry,
;2019, 82(9): 789-795.

-
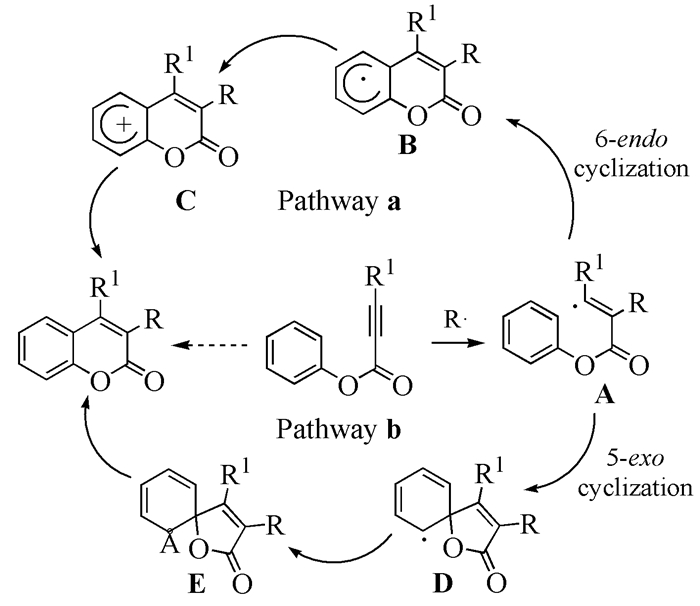
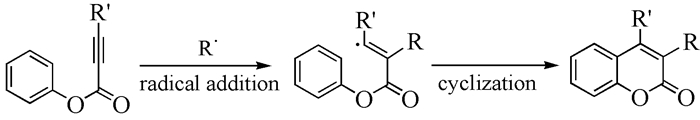
Coumarin and its derivatives are a key class of heterocyclic lactone compounds, which are widely applied in the fields of organic synthesis, pharmaceutics, fluorescent materials, and so on. The development of a novel simple and efficient synthetic strategy of coumarins has been paid much attention. Recently, the tandem radical addition/cyclization of alkynoate with radical precursor afforded a simple, green and efficient method to synthesize coumarins. In this review, the recent progress in the synthesis of coumarins through tandem radical addition/cyclization is summarized.
-
Keywords:
- Coumarin,
- Alkynoate,
- Radical cyclization,
- Functionalization
-

-
-
[1]
D Srikrishna, C Godugu, P Dubey. Mini-Rev. Med. Chem., 2018, 18:113~141.
- [2]
-
[3]
Y Hu, Z Xu, S Zhang et al. Eur. J. Med. Chem., 2017, 136:122~130.
- [4]
-
[5]
F Medina, J Marrero, M Macias-Alonso et al. Nat. Prod. Rep., 2015, 32:1472~1507.
-
[6]
T Silvia, M Miquel, Capo Xavier er al. Curr. Top. Med. Chem., 2017, 17:391~398.
-
[7]
P Anand, B Singh, N Singh. Bioorg. Med. Chem., 2012, 20:1175~1180.
-
[8]
X Peng, G Damu, C Zhou et al. Curr. Pharm. Des., 2013, 19:3884~3930.
-
[9]
X Mi, M Huang, J Zhang et al. Org. Lett., 2013, 15:6266~6269.
-
[10]
L Y Dian, H Zhao, D Zhang-Negrerie et al. Adv. Synth. Catal., 2016, 358:2422~2426.
-
[11]
F Jafarpour, M Abbasnia. J. Org. Chem., 2016, 81:11982~11986.
- [12]
-
[13]
B Niu, W Zhao, Y Ding et al. J. Org. Chem., 2015, 80:7251~7257.
-
[14]
C Mhiri, F Ladhar, R El Gharbi. Synth. Commun., 1999, 29:1451~1561.
-
[15]
S K De, R A Gibbs. Synthesis, 2005, 1231~1234.
-
[16]
M M Garazd, Y L Garazd, V P Khilya. Chem. Nat. Compd., 2005, 41:245~271.
-
[17]
T de A Fernandes, B G Vaz, M N Eberlin et al. J. Org. Chem., 2010, 75:7085~7091.
-
[18]
- [19]
-
[20]
T Liu, Q Ding, Q Zong et al. Org. Chem. Front., 2015, 2:670~673.
-
[21]
K Yan, D Yang, W Wei et al. J. Org. Chem., 2015, 80:1550~1556.
-
[22]
S Yang, H Tan, W Ji et al. Adv. Synth. Catal., 2017, 359:443~453.
-
[23]
H Yi, G Zhang, H Wang et al. Chem. Rev., 2017, 117:9016~9085.
-
[24]
X Mi, C Wang, M Huang et al. J. Org. Chem., 2015, 80:148~155.
-
[25]
K Kawaai, T Yamaguchi, E Yamaguchi et al. J. Org. Chem., 2018, 83:1988~1996.
-
[26]
Q Wang, C Yang, C Jiang. Org. Biomol. Chem., 2018, 16:8196~8204.
-
[27]
Y Liu, Q Wang, C Zhou et al. Tetrahed. Lett., 2018, 59:2038~2041.
-
[28]
W Wei, J Wen, D Yang et al. Chem. Commun., 2015, 51:768~771.
-
[29]
W Yang, S Yang, P Li et al. Chem. Commun., 2015, 51:7520~7523.
-
[30]
D Zheng, Y An, Z Li et al. Angew. Chem. Int. Ed., 2014, 53:2451~2454.
-
[31]
D Zheng, J Yu, J Wu. Angew. Chem. Int. Ed., 2016, 55:11925~11929.
-
[32]
X Wang, T Liu, D Zheng et al. Org. Chem. Front., 2017, 4:2455~2458.
-
[33]
Z Chen, N W Liu, M Bolte et al. Green Chem., 2018, 20:3059~3070.
- [34]
-
[35]
X Mi, C Wang, M Huang et al. Org. Lett., 2014, 16:3356~3359.
-
[36]
D Liu, J Chen, X Wang et al. Adv. Synth. Catal., 2017, 359:2773~2777.
- [37]
-
[38]
S Feng, J Li, Z Liu et al. Org. Biomol. Chem., 2017, 15:8820~8826.
- [39]
- [40]
-
[41]
L Chen, L Wu, W Duan et al. J. Org. Chem., 2018, 83:8607~8614.
-
[42]
W Fu, M Zhu, G Zou et al. J. Org. Chem., 2015, 80:4766~4770.
-
[43]
M Zhu, W Fu, Z Wang et al. Org. Biomol. Chem., 2017, 15:9057~9060.
-
[44]
J Fang, W Fan, B Feng. Chin. J. Org. Chem., 2018, 38:2666~2672.
- [45]
-
[46]
L Debien, B Quiclet-Sire, S Zard. Acc. Chem. Res., 2015, 48:1237~1253.
- [47]
-
[48]
B Quiclet-Sire, S Zard. Top. Curr. Chem., 2006, 264:201~236.
-
[49]
C Pan, R Chen, W Shao et al. Org. Biomol. Chem., 2016, 14:9033~9039.
-
[50]
S Feng, X Xie, W Zhang et al. Org Lett., 2016, 18:3846~3849.
-
[51]
D Kong, L Cheng, H Wu et al. Org. Biomol. Chem., 2016, 14:2210~2217.
-
[52]
Y Yu, S Zhuang, P Liu et al. J. Org. Chem., 2016, 81:11489~11495.
-
[53]
W Zhang, C Yang, Y L Pan et al. Org. Biomol. Chem., 2018, 16:5788~5792.
-
[54]
Y Zeng, D Tan, Y Chen et al. Org. Chem. Front., 2015, 2:1511~1515.
- [55]
- [56]
-
[57]
A Peng, F Hao, B Li et al. J. Org. Chem., 2008, 73:9012~9015.
-
[58]
Y Park, I Jeon, S Shin et al. J. Org. Chem., 2013, 78:10209~10220.
-
[59]
J Seo, Y Park, I Jeon et al. Org. Lett., 2013, 15:3358~3361.
-
[60]
Y Park, J Seo, S Park et al. Chem. Eur. J., 2013, 19, 16461~16468.
-
[61]
Y Unoh, Y Hashinoto, D Takeda et al Org. Lett., 2013, 15:3258~3261.
-
[62]
M Qiao, Y Liu, S Yao et al. J. Org. Chem., 2019, 84, 6798~6806.
-
[1]
-

-
-
[1]
Shengwen XU , Longlong YANG , Houji CAO , Deshuang TU , Xing WEI , Changsheng LU , Hong YAN . Research progress on light-induced functionalization of polyhedral carborane clusters. Chinese Journal of Inorganic Chemistry, 2025, 41(11): 2187-2200. doi: 10.11862/CJIC.20250192
-
[2]
Danqing Wu , Jiajun Liu , Tianyu Li , Dazhen Xu , Zhiwei Miao . Research Progress on the Simultaneous Construction of C—O and C—X Bonds via 1,2-Difunctionalization of Olefins through Radical Pathways. University Chemistry, 2024, 39(11): 146-157. doi: 10.12461/PKU.DXHX202403087
-
[3]
Qi Zhang , Ziyu Liu , Hongxia Tan , Jun Tong , Dazhen Xu . Research Progress on Direct Synthesis of β-Hydroxy Sulfones via Difunctionalization of Olefins. University Chemistry, 2025, 40(11): 199-209. doi: 10.12461/PKU.DXHX202412064
-
[4]
Hanxue LIU , Shijie LI , Meng REN , Xuling XUE , Hongke LIU . Design and antitumor properties of dehydroabietic acid functionalized cyclometalated iridium(Ⅲ) complex. Chinese Journal of Inorganic Chemistry, 2025, 41(8): 1483-1494. doi: 10.11862/CJIC.20250031
-
[5]
Zijian Zhao , Yanxin Shi , Shicheng Li , Wenhong Ruan , Fang Zhu , Jijun Jiang . A New Exploration of the Preparation of Polyacrylic Acid by Free Radical Polymerization Based on the Concept of Green Chemistry. University Chemistry, 2024, 39(5): 315-324. doi: 10.3866/PKU.DXHX202311094
-
[6]
Yujie WANG , Laobang WANG , Zheng ZHANG , Qi LIU , Jianping LANG . Construction of W/Cu/S cluster-based supramolecular compounds via alkynyl/sulfur cycloaddition and their third-order nonlinear optical properties. Chinese Journal of Inorganic Chemistry, 2025, 41(10): 2069-2077. doi: 10.11862/CJIC.20250129
-
[7]
Jiajia Li , Xiangyu Zhang , Zhihan Yuan , Zhengyang Qian , Jian Zhu . 3D Printing Based on Photo-Induced Reversible Addition-Fragmentation Chain Transfer Polymerization. University Chemistry, 2024, 39(5): 11-19. doi: 10.3866/PKU.DXHX202309073
-
[8]
Xinyu Zhu , Meili Pang . Application of Functional Group Addition Strategy in Organic Synthesis. University Chemistry, 2024, 39(3): 218-230. doi: 10.3866/PKU.DXHX202308106
-
[9]
Wen Jiang , Jieli Lin , Zhongshu Li . 低配位含磷官能团的研究进展. University Chemistry, 2025, 40(8): 138-151. doi: 10.12461/PKU.DXHX202409144
-
[10]
Benhua Wang , Chaoyi Yao , Yiming Li , Qing Liu , Minhuan Lan , Guipeng Yu , Yiming Luo , Xiangzhi Song . 一种基于香豆素氟离子荧光探针的合成、表征及性能测试——“科研反哺教学”在有机化学综合实验教学中的探索与实践. University Chemistry, 2025, 40(6): 201-209. doi: 10.12461/PKU.DXHX202408070
-
[11]
Yuanyuan Ping , Wangqing Kong . 光催化碳氢键官能团化合成1-苯基-1,2-乙二醇. University Chemistry, 2025, 40(6): 238-247. doi: 10.12461/PKU.DXHX202408092
-
[12]
Lei Shi . Nucleophilicity and Electrophilicity of Radicals. University Chemistry, 2024, 39(11): 131-135. doi: 10.3866/PKU.DXHX202402018
-
[13]
Min LIU , Huapeng RUAN , Zhongtao FENG , Xue DONG , Haiyan CUI , Xinping WANG . Neutral boron-containing radical dimers. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 123-130. doi: 10.11862/CJIC.20240362
-
[14]
Fugui XI , Du LI , Zhourui YAN , Hui WANG , Junyu XIANG , Zhiyun DONG . Functionalized zirconium metal-organic frameworks for the removal of tetracycline from water. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 683-694. doi: 10.11862/CJIC.20240291
-
[15]
Jie ZHAO , Huili ZHANG , Xiaoqing LU , Zhaojie WANG . Theoretical calculations of CO2 capture and separation by functional groups modified 2D covalent organic framework. Chinese Journal of Inorganic Chemistry, 2025, 41(2): 275-283. doi: 10.11862/CJIC.20240213
-
[16]
Tongyan Yu , Pan Xu . Visible-Light Photocatalyzed Radical Rearrangement Reaction. University Chemistry, 2025, 40(7): 169-176. doi: 10.12461/PKU.DXHX202409070
-
[17]
Yufan Pan , Xue Ding , Jiayu Lin , Haiting Wu , Hairong Huang , Cuixue Chen , Meiling Ye . Oil Cosmetics, Charming Chemistry: A Gradient Science Popularization Scheme for Cream Cosmetic Preparation. University Chemistry, 2025, 40(4): 382-389. doi: 10.12461/PKU.DXHX202406078
-
[18]
Hong RAO , Yang HU , Yicong MA , Chunxin LÜ , Wei ZHONG , Lihua DU . Synthesis and in vitro anticancer activity of phenanthroline-functionalized nitrogen heterocyclic carbene homo- and heterobimetallic silver/gold complexes. Chinese Journal of Inorganic Chemistry, 2024, 40(12): 2429-2437. doi: 10.11862/CJIC.20240275
-
[19]
.
CCS Chemistry | 超分子活化底物为自由基促进高效选择性光催化氧化
. CCS Chemistry, 2025, 7(10.31635/ccschem.025.202405229): -. -
[20]
Yinjie Xu , Suiqin Li , Lihao Liu , Jiahui He , Kai Li , Mengxin Wang , Shuying Zhao , Chun Li , Zhengbin Zhang , Xing Zhong , Jianguo Wang . Enhanced Electrocatalytic Oxidation of Sterols using the Synergistic Effect of NiFe-MOF and Aminoxyl Radicals. Acta Physico-Chimica Sinica, 2024, 40(3): 2305012-0. doi: 10.3866/PKU.WHXB202305012
-
[1]
Metrics
- PDF Downloads(6)
- Abstract views(845)
- HTML views(166)

 Login In
Login In




 DownLoad:
DownLoad: