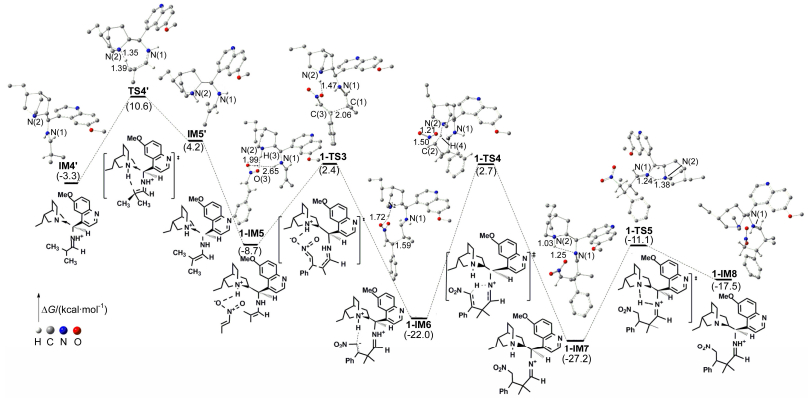
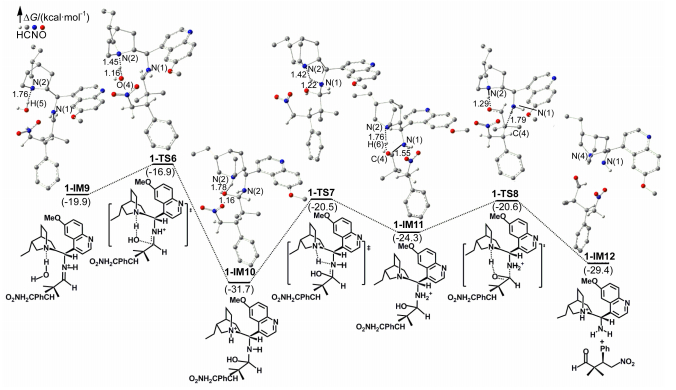
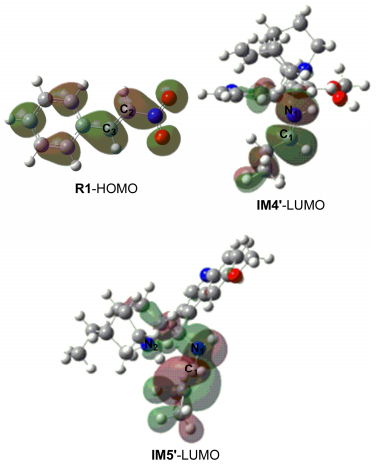
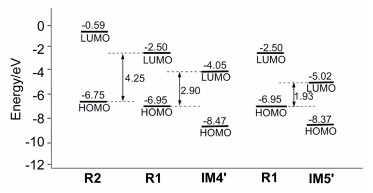
-
[1]
Dalko, P. I.; Moisan, L. Angew. Chem., Int. Ed. 2004, 43, 5138.
doi: 10.1002/(ISSN)1521-3773
-
[2]
Seayad, J.; List, B. Org. Biomol. Chem. 2005, 3, 719.
doi: 10.1039/b415217b
-
[3]
Berkessel, A. ; Gröger, H. Asymmetric Organocatalysis, Wiley-VCH, Weinheim, 2005.
-
[4]
Dalko, P. I. Enantioselective Organocatalysis, Wiley-VCH, Weinheim, 2007.
-
[5]
Wang, T. L.; Yu, Z. Y.; Hoon, D. L.; Lan, Y.; Lu, Y. X. J. Am. Chem. Soc. 2016, 138, 265.
doi: 10.1021/jacs.5b10524
-
[6]
Wang, T. L.; Yu, Z. Y.; Hoon, D. L.; Huang, K. W.; Lan, Y.; Lu, Y. X. Chem. Sci. 2015, 6, 4912.
doi: 10.1039/C5SC01614B
-
[7]
Li, J. H.; Du, D. M. Chin. J. Chem. 2015, 33, 418.
doi: 10.1002/cjoc.v33.4
-
[8]
Zhou, H. Y.; Li, N. N.; Yang, J. Y.; Li, T. Y.; Li, Z. Chin. J. Org. Chem. 2016, 36, 502(in Chinese).
-
[9]
Berner, O. M.; Tedeschi, L.; Enders, D. Eur. J. Org. Chem. 2002, 12, 1877.
-
[10]
List, B.; Pojarliev, P.; Martin, H. J. Org. Lett. 2001, 3, 2423.
doi: 10.1021/ol015799d
-
[11]
Zu, L.; Wang, J.; Li, H.; Wang, W. Org. Lett. 2006, 8, 3077.
doi: 10.1021/ol061053+
-
[12]
Mossé, S.; Alexakis, A. Org. Lett. 2006, 8, 3577.
doi: 10.1021/ol0614727
-
[13]
Luo, S.; Mi, X.; Zhang, L.; Liu, S.; Xu, H.; Cheng, J. P. Angew. Chem., Int. Ed. 2006, 45, 3093.
doi: 10.1002/(ISSN)1521-3773
-
[14]
Luo, S.; Mi, X.; Zhang, L.; Cheng, J. P. Chem. Commun. 2006, 3687.
-
[15]
Li, Y.; Liu, X. Y.; Zhao, G. Tetrahedron:Asymmetry 2006, 17, 2034.
doi: 10.1016/j.tetasy.2006.07.004
-
[16]
Zhu, M. K.; Cun, L. F.; Mi, A. Q.; Jiang, Y. Z.; Gong, L. Z. Tetrahedron:Asymmetry 2006, 17, 491.
doi: 10.1016/j.tetasy.2006.01.034
-
[17]
Cao, C. L.; Ye, M. C.; Sun, X. L.; Tang, Y. Org. Lett. 2006, 8, 2901.
doi: 10.1021/ol060481c
-
[18]
Palomo, C.; Vera, S.; Mielgo, A.; Gómez-Bengoa, E. Angew. Chem., Int. Ed. 2006, 45, 5984.
doi: 10.1002/(ISSN)1521-3773
-
[19]
Pansare, S. V.; Pandya, K. J. Am. Chem. Soc. 2006, 128, 9624.
doi: 10.1021/ja062701n
-
[20]
Mossé, S.; Laars, M.; Kriis, K.; Kanger, T.; Alexakis, A. Org. Lett. 2006, 8, 2559.
doi: 10.1021/ol0607490
-
[21]
Wang, J.; Li, H.; Lou, B.; Zu, L.; Guo, H.; Wang, W. Chem.-Eur. J. 2006, 12, 4321.
doi: 10.1002/(ISSN)1521-3765
-
[22]
Tsogoeva, S. B.; Wei, S. Chem. Commun. 2006, 24, 1451.
-
[23]
Wang, B.; Xu, T.; Zhu, L.; Lan, Y.; Wang, J. D. Org. Chem. Front. 2017, 4, 1266.
doi: 10.1039/C7QO00124J
-
[24]
Huang, H.; Jacobsen, E. N. J. Am. Chem. Soc. 2006, 128, 7170.
doi: 10.1021/ja0620890
-
[25]
Yalonde, M. P.; Chen, Y.; Jacobsen, E. N. Angew. Chem., Int. Ed. 2006, 45, 6366.
doi: 10.1002/(ISSN)1521-3773
-
[26]
Xu, Y.; Zou, W.; Sundén, H.; Ibrahem, I.; Córdova, A. Adv. Synth. Catal. 2006, 348, 418.
doi: 10.1002/(ISSN)1615-4169
-
[27]
Jiang, L.; Chen, Y. C. Catal. Sci. Technol. 2011, 1, 354.
doi: 10.1039/c0cy00096e
-
[28]
Hansen, H. M.; Longbottom, D. A.; Ley, S. V. Chem. Commun. 2006, 4838.
-
[29]
Wascholowski, V.; Hansen, H. M.; Longbottom, D. A.; Ley, S. V.; Synthesis 2008, 1269.
-
[30]
Xie, J. W.; Chen, W.; Li, R.; Zeng, M.; Du, W.; Yue, L.; Chen, Y. C.; Wu, Y.; Zhu, J.; Deng, J. G. Angew. Chem., Int. Ed. 2007, 46, 389.
doi: 10.1002/(ISSN)1521-3773
-
[31]
Li, X. F.; Cun, L. F.; Lian, C. X.; Zhong, L.; Chen, Y. C.; Liao, J.; Zhu, J.; Deng, J. G. Org. Biomol. Chem. 2008, 6, 349.
doi: 10.1039/B713129A
-
[32]
Li, X. M.; Wang, B.; Zhang, J. M.; Yan, M. Org. Lett. 2011, 13, 374.
doi: 10.1021/ol102570b
-
[33]
Yue, L.; Du, W.; Liu, Y. K.; Chen, Y. C. Tetrahedron Lett. 2008, 49, 3881.
doi: 10.1016/j.tetlet.2008.04.069
-
[34]
Lu, X.; Deng, L. Angew. Chem. 2008, 120, 7824.
doi: 10.1002/ange.v120:40
-
[35]
McCooey, S. H.; Connon, S. J. Org. Lett. 2007, 9, 599.
doi: 10.1021/ol0628006
-
[36]
Gonzalez, C.; Schlegel, H. B. J. Chem. Phys. 1989, 90, 2154.
doi: 10.1063/1.456010
-
[37]
Gonzalez, C.; Schlegel, H. B. J. Phys. Chem. 1990, 94, 5523.
doi: 10.1021/j100377a021
-
[38]
Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
doi: 10.1021/cr00088a005
-
[39]
Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
doi: 10.1063/1.449486
-
[40]
Frisch, M. J. ; Trucks, G. W. ; Schlegel, H. B. ; Scuseria, G. E. ; Robb, M. A. ; Cheeseman, J. R. ; Kudin, K. N. ; Burant, J. C. ; Millam, J. M. ; Iyengar, S. S. ; Tomasi, J. ; Barone, V. ; Mennucci, B. ; Cossi, M. ; Scalmani, G. ; Rega, N. ; Petersson, G. A. ; Nakatsuji, H. ; Hada, M. ; Ehara, M. ; Toyota, K. ; Fukuda, R. ; Hasegawa, J. ; Ishida, M. ; Nakajima, T. ; Honda, Y. ; Kitao, O. ; Nakai, H. ; Klene, M. ; Li, X. ; Knox, J. E. ; Hratchian, H. P. ; Cross, J. B. ; Adamo, C. ; Jaramillo, J. ; Gomperts, R. ; Stratmann, R. E. ; Yazyev, O. ; Austin, A. J. ; Cammi, R. ; Pomelli, C. ; Zakrzewski, V. G. ; Dapprich, S. ; Daniels, A. D. ; Strain, M. C. ; Farkas, O. ; Malick, D. K. ; Rabuck, A. D. ; Raghavachari, K. ; Foresman, J. B. ; Ortiz, J. V. ; Cui, Q. ; Baboul, A. G. ; Clifford, S. ; Piskorz, P. ; Komaromi, I. ; Martin, R. L. ; Fox, D. J. ; Keith, T. ; Challacombe, M. ; Johnson, B. ; Chen, W. ; Wong, M. W. ; Gonzalez. C. ; Pople, J. A. Gaussian 09, revision A. 1, Gaussian Inc., Wallingford CT, 2009.
-
[41]
Jones, G. O.; Li, X.; Hayden, A. E.; Houk, K. N.; Danishefsky, S. J. Org. Lett. 2008, 10, 4093.
doi: 10.1021/ol8016287
-
[42]
Peles, D. N.; Thoburn, J. D. J. Org. Chem. 2008, 73, 3135.
doi: 10.1021/jo702668u
-
[43]
Rehbein, J.; Hiersemann, M. J. Org. Chem. 2009, 74, 4336.
doi: 10.1021/jo900635k
-
[44]
Su, Z. S.; Lee, H. W.; Kim, C. K. Eur. J. Org. Chem. 2013, 2013, 1706.
-
[45]
Jiang, H. Y.; Feng, W.; Sun, Y. W.; Liu, H. L.; Huang, X. R. Chem. J. Chin. Univ. 2014, 35, 1500(in Chinese).
doi: 10.7503/cjcu20140136
-
[46]
Fukui, K. ; Fujimoto, H. Frontier Orbitals and Reaction Paths: Selected Papers of Kenichi Fukui, World Scientific, Singapore, 1997
-
[47]
Hoffmann, R. Rev. Mod. Phys. 1988, 60, 601.
doi: 10.1103/RevModPhys.60.601
-
[48]
Zhang, L. L.; Zhou, Z. J.; Jiang, H. Y.; Liu, H. L.; Huang, X. R. Tetrahedron:Asymmetry 2013, 24, 1.
doi: 10.1016/j.tetasy.2012.11.014

 Login In
Login In






 DownLoad:
DownLoad: