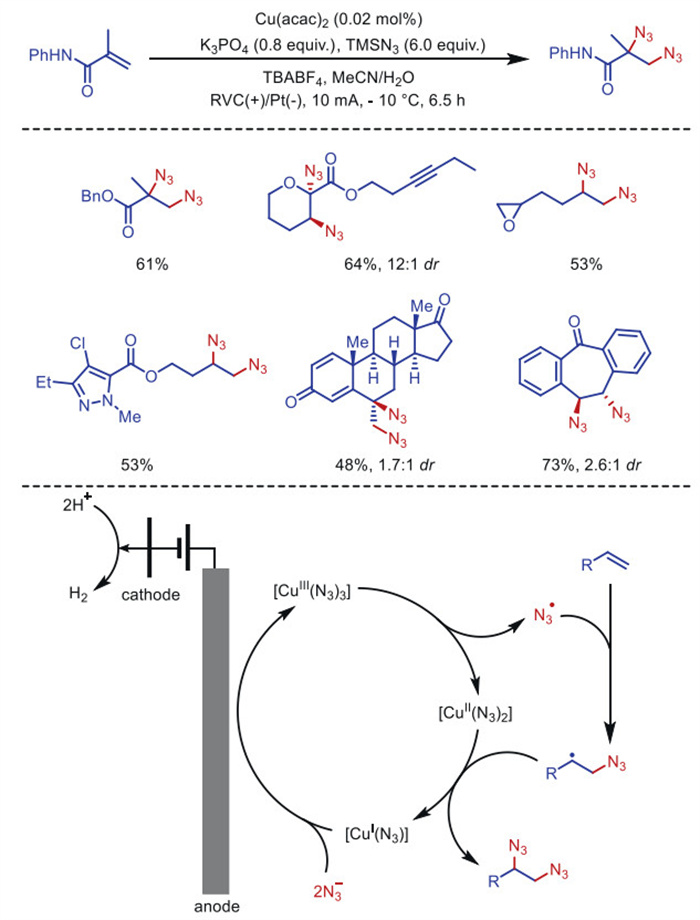
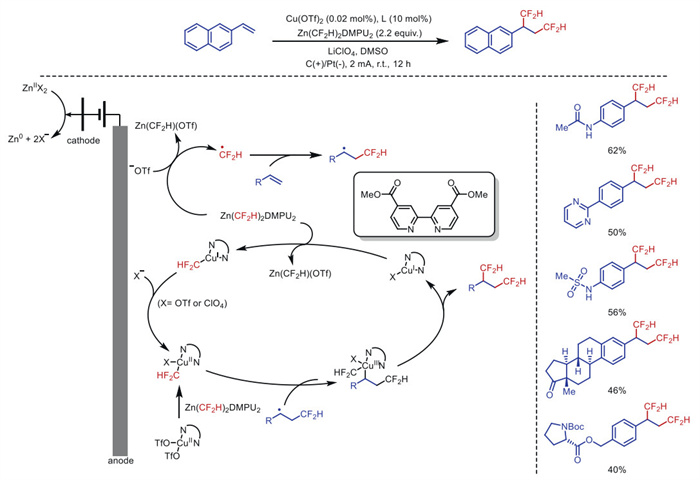
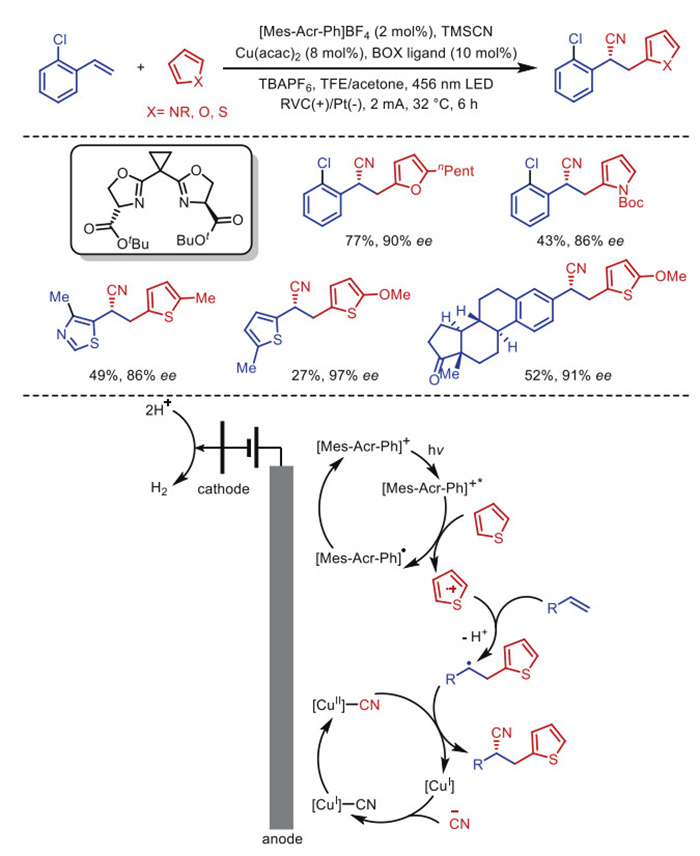
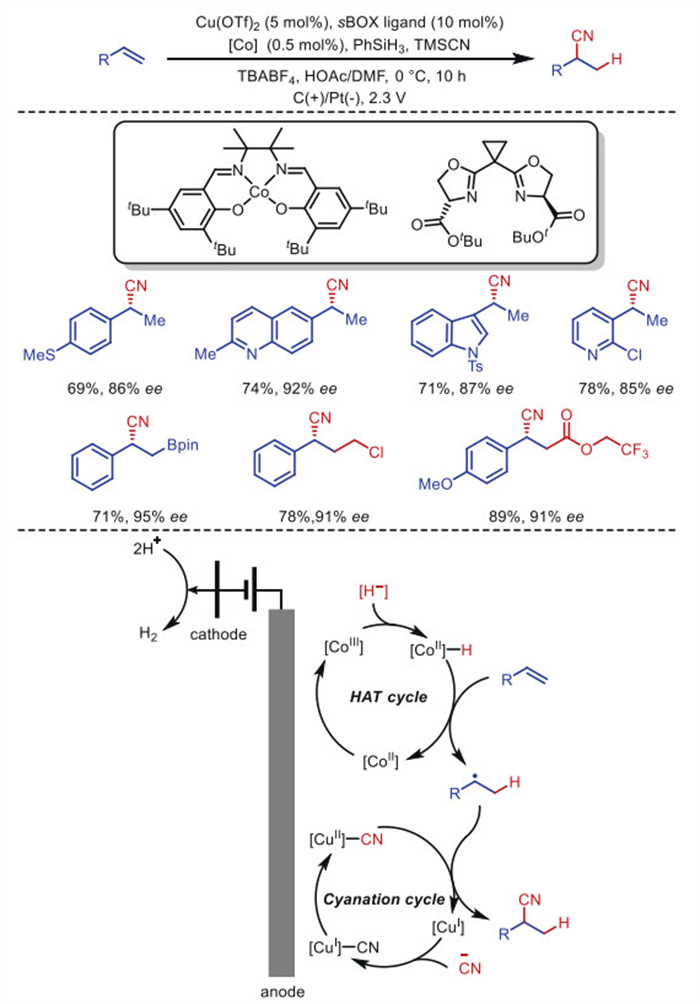
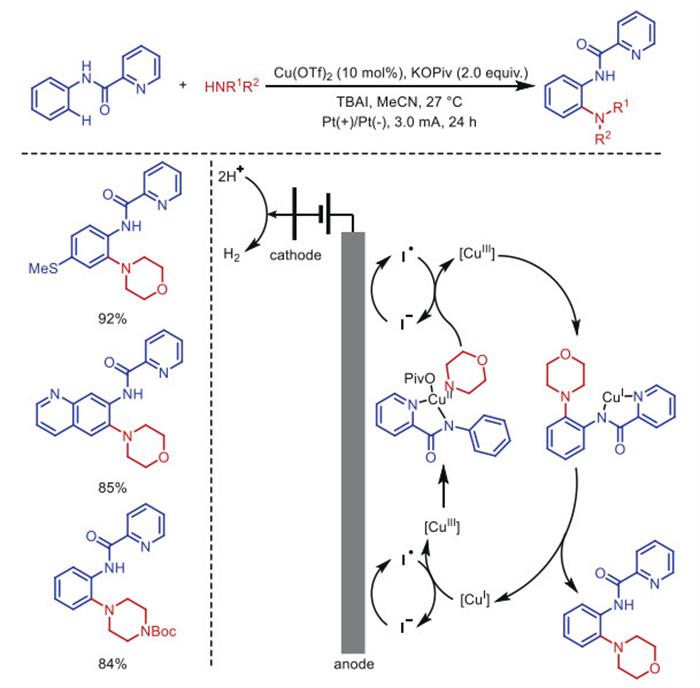
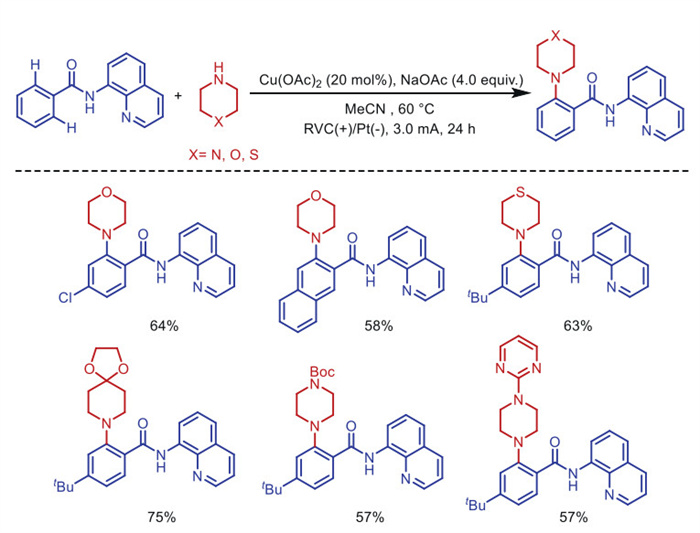
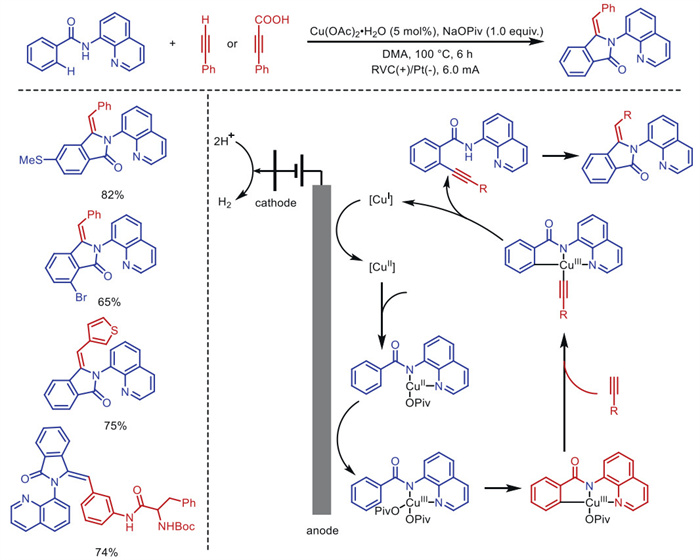
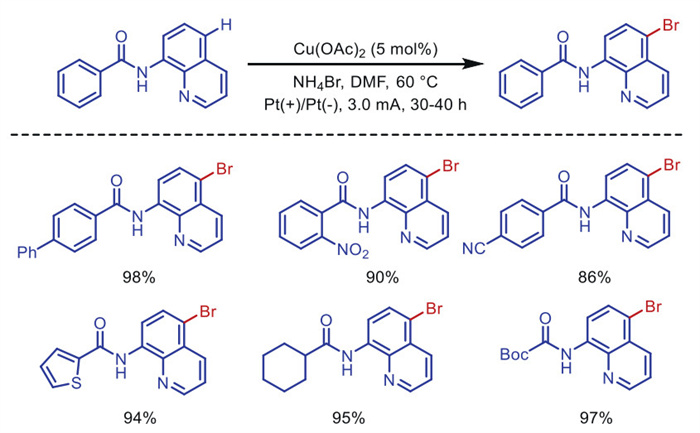
Citation:
Pan Zhou, Ting Zou, Hong-Jian Song, Yu-Xiu Liu, Qing-Min Wang. Advances in organoelectrochemical copper-catalyzed reactions[J]. Chinese Chemical Letters,
;2026, 37(1): 111673.
doi:
10.1016/j.cclet.2025.111673

-
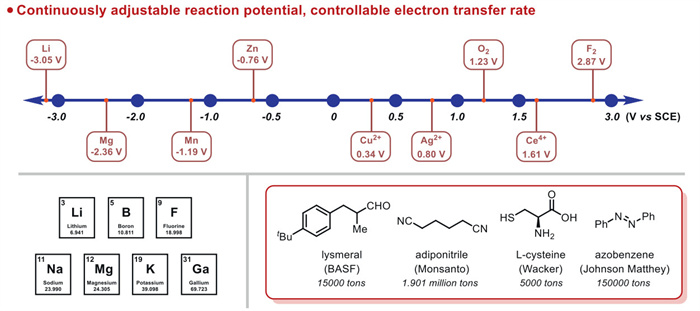
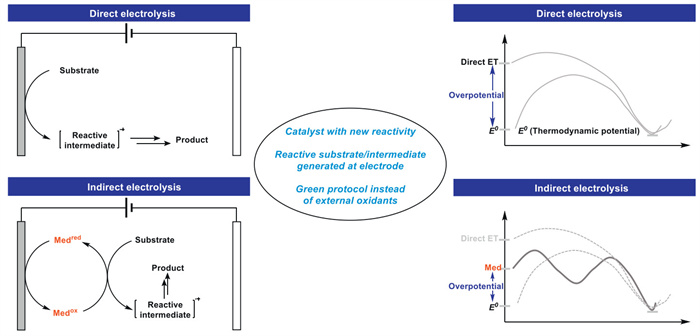
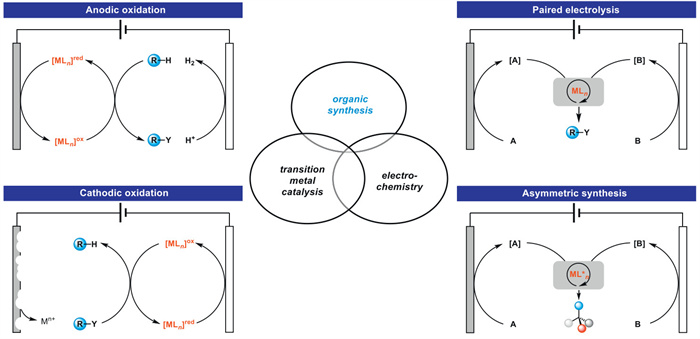
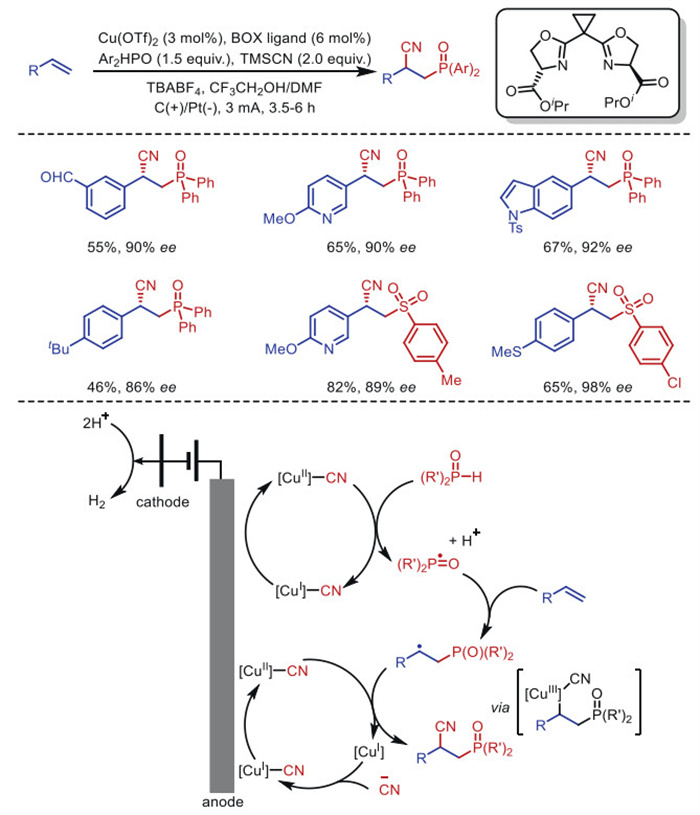
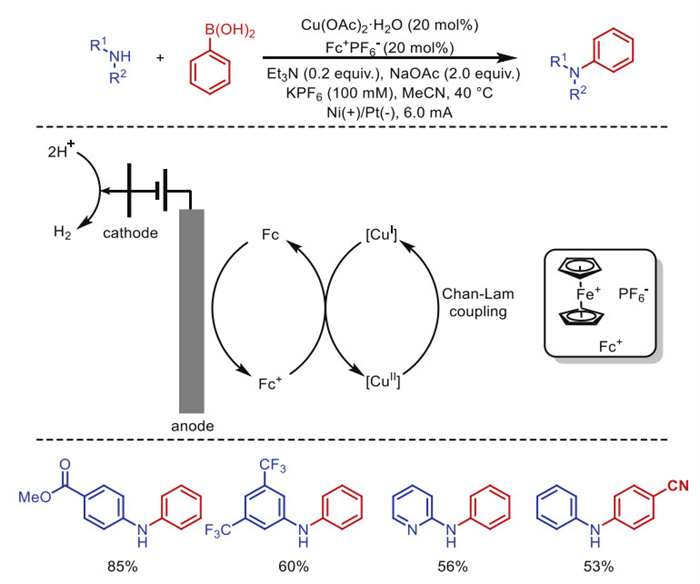
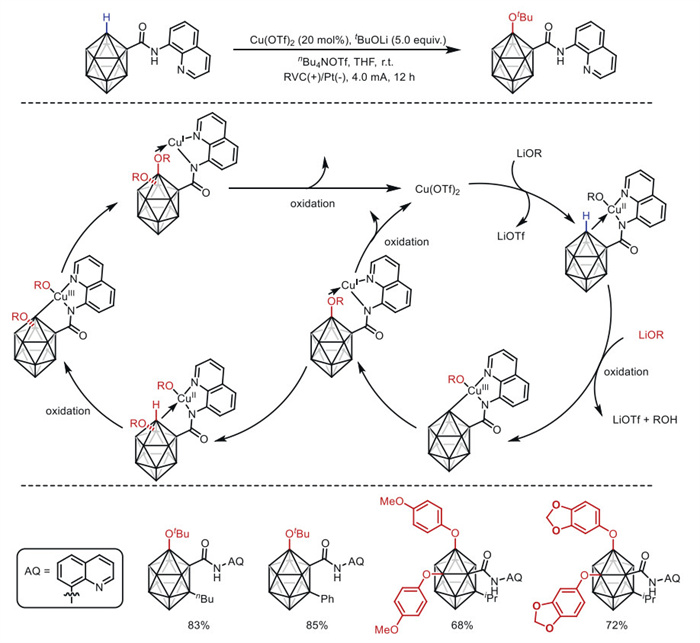
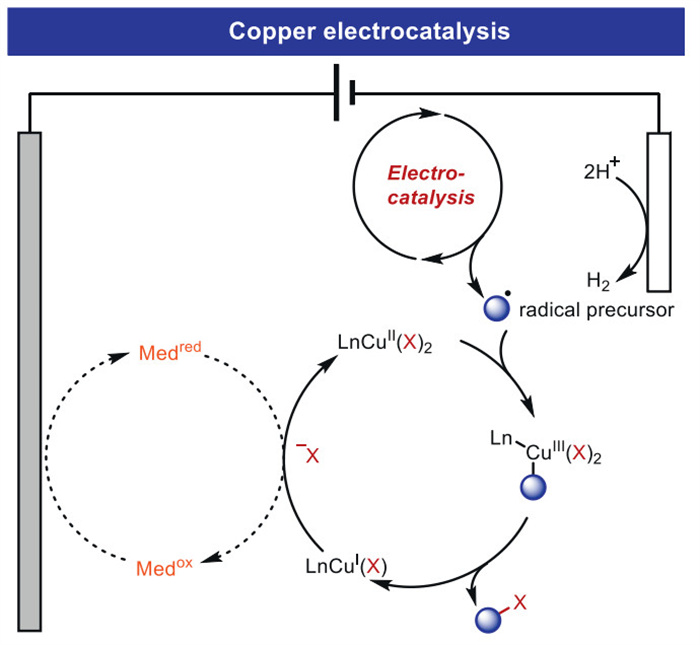
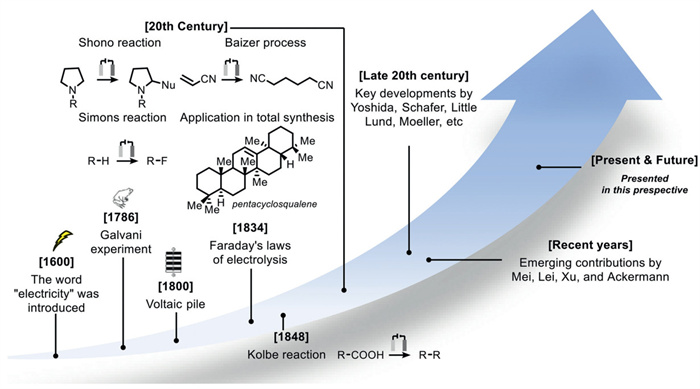
The combination of electrochemistry and metal catalysts has been a popular research topic in the field of organic synthesis due to the abundance and controllable valence states of transition metals, where electron transfer at the electrode produces catalysts with more valence states. Among these transition metal catalysts, electrochemical conversions catalyzed by inexpensive copper metals have received considerable attention. This article systematically investigated this field and reviewed the electrochemical copper catalytic methods applied in organic synthesis from the different activation modes of substrates, which can be broadly classified into the functionalization of C = C bonds, C−H bond activation, C−C and C−X bond activation, and so on.
-

-
-
[1]
A. Volta, Phil. Trans. R. Soc. Lond. 90 (1800) 403–431.
-
[2]
M. Faraday, Ann. Phys. 109 (1834) 433–451. doi: 10.1002/andp.18341092307
-
[3]
H. Kolbe, Ann. Chem. Pharm. 64 (1848) 339–341. doi: 10.1002/jlac.18480640346
-
[4]
H. Moissan, Comptesrendushebdomadaires des sé Ances de l'Académiedes Sciences 102 (1886) 1543–1544.
-
[5]
H.I. Davy, Phil. Trans. R. Soc. Lond. 98 (1808) 1–44.
-
[6]
M.M. Baizer, J. Electrochem. Soc. 111 (1964) 215. doi: 10.1149/1.2426086
-
[7]
T. Shono, Tetrahedron 40 (1984) 811–850. doi: 10.1016/S0040-4020(01)91472-3
-
[8]
J.H. Simons, J. Electrochem. Soc. 95 (1949) 47. doi: 10.1149/1.2776733
-
[9]
E.J. Corey, R.R. Sauers, J. Am. Chem. Soc. 81 (1959) 1739–1743. doi: 10.1021/ja01516a054
-
[10]
B.A. Frontana-Uribe, R.D. Little, J.G. Ibanez, et al., Green Chem. 12 (2010) 2099–2119. doi: 10.1039/c0gc00382d
-
[11]
C. Ma, P. Fang, Z.R. Liu, et al., Sci. Bull. 66 (2021) 2412–2429. doi: 10.1016/j.scib.2021.07.011
-
[12]
L.F.T. Novaes, J. Liu, Y. Shen, et al., Chem. Soc. Rev. 50 (2021) 7941–8002. doi: 10.1039/d1cs00223f
-
[13]
E.J. Horn, B.R. Rosen, P.S. Baran, ACS Cent. Sci. 2 (2016) 302–308. doi: 10.1021/acscentsci.6b00091
-
[14]
J. Yoshida, K. Kataoka, R. Horcajada, et al., Chem. Rev. 108 (2008) 2265–2299. doi: 10.1021/cr0680843
-
[15]
J.C. Siu, N. Fu, S. Lin, Acc. Chem. Res. 53 (2020) 547–560. doi: 10.1021/acs.accounts.9b00529
-
[16]
K. Yamamoto, M. Kuriyama, O. Onomura, Acc. Chem. Res. 53 (2019) 105–120. doi: 10.15261/serdj.26.105
-
[17]
N.W.J. Ang, J.C.A. Oliveira, L. Ackermann, et al., Angew. Chem. Int. Ed. 59 (2020) 12842–12847. doi: 10.1002/anie.202003218
-
[18]
B.R. Rosen, E.W. Werner, A.G. O'Brien, P.S. Baran, et al., J. Am. Chem. Soc. 136 (2014) 5571–5574. doi: 10.1021/ja5013323
-
[19]
K.J. Jiao, D. Liu, H.X. Ma, et al., Angew. Chem. Int. Ed. 59 (2020) 6520–6524. doi: 10.1002/anie.201912753
-
[20]
Z. Duan, L. Zhang, W. Zhang, et al., ACS Catal. 10 (2020) 3828–3831. doi: 10.1021/acscatal.0c00103
-
[21]
L. Niu, C. Jiang, Y. Liang, et al., J. Am. Chem. Soc. 142 (2020) 17693–17702. doi: 10.1021/jacs.0c08437
-
[22]
H. Yan, Z.W. Hou, H.C. Xu, et al., Angew. Chem. Int. Ed. 58 (2019) 4592–4595. doi: 10.1002/anie.201814488
-
[23]
M. Elsherbini, T. Wirth, Acc. Chem. Res. 52 (2019) 3287–3296. doi: 10.1021/acs.accounts.9b00497
-
[24]
C. Huang, X.X. Qian, H.C. Xu, Angew. Chem. Int. Ed. 58 (2019) 6650–6653. doi: 10.1002/anie.201901610
-
[25]
J. Hartwig (Ed.), Organotransition Metal Chemistry: from Bonding to Catalysis, University Science Books, New York, 2010.
-
[26]
K.L. Skubi, T.R. Blum, T.R. Yoon, Chem. Rev. 116 (2016) 10035–10074. doi: 10.1021/acs.chemrev.6b00018
-
[27]
J. Twilton, C. Le, P. Zhang, et al., Nat. Rev. Chem. 1 (2017) 0052.
-
[28]
J. Lu, Y. Wang, T. McCallum, N. Fu, et al., iScience 23 (2020) 101796. doi: 10.1016/j.isci.2020.101796
-
[29]
A.Y. Chan, I.B. Perry, N.B. Bissonnette, et al., Chem. Rev. 122 (2021) 1485–1542.
-
[30]
R.C. Samanta, T.H. Meyer, I. Siewert, et al., Chem. Sci. 11 (2020) 8657–8670. doi: 10.1039/d0sc03578e
-
[31]
X. Cheng, A. Lei, T. -S. Mei, et al., CCS Chem. 4 (2022) 1120–1152. doi: 10.31635/ccschem.021.202101451
-
[32]
C.A. Malapit, M.B. Prater, J.R. Cabrera-Pardo, et al., Chem. Rev. 122 (2022) 3180–3218. doi: 10.1021/acs.chemrev.1c00614
-
[33]
A. Jutand, Chem. Rev. 108 (2008) 2300–2347. doi: 10.1021/cr068072h
-
[34]
Y. Kim, W.J. Jang, Beilstein J. Org. Chem. 21 (2025) 155–178. doi: 10.3762/bjoc.21.9
-
[35]
F. Wang, P. Chen, G. Liu, Nat. Synth. 1 (2022) 107–116. doi: 10.1038/s44160-021-00016-x
-
[36]
L. Li, Y. Yao, N. Fu, Chem. Catal. 4 (2024) 100898.
-
[37]
S.E. Allen, R.R. Walvoord, R. Padilla-Salinas, et al., Chem. Rev. 113 (2013) 6234–6458. doi: 10.1021/cr300527g
-
[38]
N. Fu, L. Song, J. Liu, et al., J. Am. Chem. Soc. 141 (2019) 14480–14485. doi: 10.1021/jacs.9b03296
-
[39]
W. Liu, J.T. Groves, Acc. Chem. Res. 48 (2015) 1727–1735. doi: 10.1021/acs.accounts.5b00062
-
[40]
C.Y. Cai, Y.T. Zheng, J.F. Li, et al., J. Am. Chem. Soc. 144 (2022) 11980–11985. doi: 10.1021/jacs.2c05126
-
[41]
H. Kim, S. Kim, J. Am. Chem. Soc. 146 (2024) 22498–22508. doi: 10.1021/jacs.4c06207
-
[42]
S. Wu, J. Kaur, T.A. Karl, et al., Angew. Chem. Int. Ed. 61 (2022) e202107811. doi: 10.1002/anie.202107811
-
[43]
H. Huang, K.A. Steiniger, T.H. Lambert, J. Am. Chem. Soc. 144 (2022) 12567–12583. doi: 10.1021/jacs.2c01914
-
[44]
L. Qian, M. Shi, Chem. Commun. 59 (2023) 3487–3506. doi: 10.1039/d3cc00437f
-
[45]
X.L. Lai, H.C. Xu, J. Am. Chem. Soc. 145 (2023) 18753–18759. doi: 10.1021/jacs.3c07146
-
[46]
T.V. RajanBabu, A.L. Casalnuovo, J. Am. Chem. Soc. 114 (1992) 6265–6266. doi: 10.1021/ja00041a066
-
[47]
J. Wilting, M. Janssen, C. Müller, et al., J. Am. Chem. Soc. 128 (2006) 11374–11375. doi: 10.1021/ja064378u
-
[48]
A. Falk, A.L. Göderz, H.G. Schmalz, Angew. Chem. Int. Ed. 52 (2013) 1576–1580. doi: 10.1002/anie.201208082
-
[49]
X. Li, C. You, J. Yang, et al., Angew. Chem. Int. Ed. 58 (2019) 10928–10931. doi: 10.1002/anie.201906111
-
[50]
H. Zhang, X. Su, K. Dong, Org. Biomol. Chem. 18 (2020) 391–399. doi: 10.1039/c9ob02374g
-
[51]
L. Song, N. Fu, B.G. Ernst, et al., Nat. Chem. 12 (2020) 747–754. doi: 10.1038/s41557-020-0469-5
-
[52]
Q.L. Yang, X.Y. Wang, J.Y. Lu, et al., J. Am. Chem. Soc. 140 (2018) 11487–11494. doi: 10.1021/jacs.8b07380
-
[53]
S. Kathiravan, S. Suriyanarayanan, I.A. Nicholls, Org. Lett. 21 (2019) 1968–1972. doi: 10.1021/acs.orglett.9b00003
-
[54]
C. Tian, U. Dhawa, A. Scheremetjew, L. Ackermann, et al., ACS Catal. 9 (2019) 7690–7696. doi: 10.1021/acscatal.9b02348
-
[55]
X. Yang, Q.L. Yang, X.Y. Wang, et al., J. Org. Chem. 85 (2020) 3497–3507. doi: 10.1021/acs.joc.9b03223
-
[56]
X. Yi, X. Hu, Angew. Chem. Int. Ed. 58 (2019) 4700–4704. doi: 10.1002/anie.201814509
-
[57]
J. Poater, M. Solà, C. Viñas, F. Teixidor, Angew. Chem. Int. Ed. 53 (2014) 12191–12195. doi: 10.1002/anie.201407359
-
[58]
R. Núñez, M. Tarrés, A. Ferrer-Ugalde, et al., Chem. Rev. 116 (2016) 14307–14378. doi: 10.1021/acs.chemrev.6b00198
-
[59]
L. Yang, B.B. Jei, A. Scheremetjew, et al., Chem. Sci. 12 (2021) 12971–12976. doi: 10.1039/d1sc02905c
-
[60]
T. Cernak, K.D. Dykstra, S. Tyagarajan, et al., Chem. Soc. Rev. 45 (2016) 546–576. doi: 10.1039/C5CS00628G
-
[61]
Ł. Woźniak, J.F. Tan, Q.H. Nguyen, et al., Chem. Rev. 120 (2020) 10516–10543. doi: 10.1021/acs.chemrev.0c00559
-
[62]
H.M.L. Davies, R.E.J. Beckwith, Chem. Rev. 103 (2003) 2861–2904. doi: 10.1021/cr0200217
-
[63]
C.Y. Cai, X.L. Lai, Y. Wang, et al., Nat. Catal. 5 (2022) 943–951. doi: 10.1038/s41929-022-00855-7
-
[64]
W. Fan, X. Zhao, Y. Deng, et al., J. Am. Chem. Soc. 144 (2022) 21674–21682. doi: 10.1021/jacs.2c09366
-
[65]
P.S. Gao, X.J. Weng, Z.H. Wang, et al., Angew. Chem. Int. Ed. 59 (2020) 15254–15259. doi: 10.1002/anie.202005099
-
[66]
Z. He, H.L. Liu, Z.H. Wang, et al., J. Org. Chem. 88 (2023) 6203–6208. doi: 10.1021/acs.joc.3c00223
-
[67]
R.R. Naredla, D.A. Klumpp, Chem. Rev. 113 (2013) 6905–6948. doi: 10.1021/cr4001385
-
[68]
H. Wang, K. Liang, W. Xiong, et al., Sci. Adv. 6 (2020) eaaz0590. doi: 10.1126/sciadv.aaz0590
-
[69]
Q. Lin, Y. Duan, Y. Li, et al., Nat. Commun. 15 (2024) 6900. doi: 10.1038/s41467-024-50945-2
-
[70]
R. Francke, R.D. Little, Chem. Soc. Rev. 43 (2014) 2492–2521. doi: 10.1039/c3cs60464k
-
[71]
M. Yan, Y. Kawamata, P.S. Baran, Chem. Rev. 117 (2017) 13230–13319. doi: 10.1021/acs.chemrev.7b00397
-
[72]
A. Wiebe, T. Gieshoff, S. Möhle, et al., Angew. Chem. Int. Ed. 57 (2018) 5594–5619. doi: 10.1002/anie.201711060
-
[73]
L. Zeng, J. Wang, D. Wang, et al., Angew. Chem. Int. Ed. 62 (2023) e202309620. doi: 10.1002/anie.202309620
-
[74]
L. Zeng, Q. Yang, J. Wang, et al., Science 385 (2024) 216–223. doi: 10.1126/science.ado0875
-
[75]
Y. Abderrazak, A. Bhattacharyya, O. Reiser, Angew. Chem. Int. Ed. 60 (2021) 21100–21115. doi: 10.1002/anie.202100270
-
[76]
F. Juliá, ChemCatChem 14 (2022) e202200916. doi: 10.1002/cctc.202200916
-
[77]
X.L. Lai, M. Chen, Y. Wang, et al., J. Am. Chem. Soc. 144 (2022) 20201–20206. doi: 10.1021/jacs.2c09050
-
[78]
Y. Yuan, J. Yang, J. Zhang, Chem. Sci. 14 (2023) 705–710. doi: 10.1039/d2sc05428k
-
[79]
K. Yang, Y. Wang, S. Luo, N. Fu, Chem. Eur. J. 29 (2023) e202203962. doi: 10.1002/chem.202203962
-
[80]
O. Onomura, H. Arimoto, Y. Matsumura, Y. Demizu, Tetrahedron Lett. 48 (2007) 8668–8672. doi: 10.1016/j.tetlet.2007.10.014
-
[81]
D. Minato, H. Arimoto, Y. Nagasue, et al., Tetrahedron 64 (2008) 6675–6683. doi: 10.1016/j.tet.2008.05.015
-
[82]
X. Fang, X. Hu, Q.X. Li, et al., Angew. Chem. Int. Ed. 63 (2024) e202418277.
-
[83]
B.R. Walker, S. Manabe, A.T. Brusoe, C.S. Sevov, J. Am. Chem. Soc. 143 (2021) 6257–6265. doi: 10.1021/jacs.1c02103
-
[84]
Y.K. Au, H. Lyu, Y. Quan, Z. Xie, J. Am. Chem. Soc. 142 (2020) 6940–6945. doi: 10.1021/jacs.0c02490
-
[85]
D.E. Blanco, B. Lee, M.A. Modestino, Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 17683–17689. doi: 10.1073/pnas.1909985116
-
[86]
M. Santi, J. Seitz, R. Cicala, et al., Chem. Eur. J. 25 (2019) 16230–16235. doi: 10.1002/chem.201904711
-
[1]
-

-
-
[1]
Jing-Qi Tao , Shuai Liu , Tian-Yu Zhang , Hong Xin , Xu Yang , Xin-Hua Duan , Li-Na Guo . Photoinduced copper-catalyzed alkoxyl radical-triggered ring-expansion/aminocarbonylation cascade. Chinese Chemical Letters, 2024, 35(6): 109263-. doi: 10.1016/j.cclet.2023.109263
-
[2]
Guang Xu , Cuiju Zhu , Xiang Li , Kexin Zhu , Hao Xu . Copper-catalyzed asymmetric [4+1] annulation of yne–allylic esters with pyrazolones. Chinese Chemical Letters, 2025, 36(4): 110114-. doi: 10.1016/j.cclet.2024.110114
-
[3]
Yan-Bo Li , Yi Li , Liang Yin . Copper(Ⅰ)-catalyzed diastereodivergent construction of vicinal P-chiral and C-chiral centers facilitated by dual "soft-soft" interaction. Chinese Chemical Letters, 2024, 35(7): 109294-. doi: 10.1016/j.cclet.2023.109294
-
[4]
Wei-Bin Li , Xiao-Chao Huang , Pei Liu , Jie Kong , Guo-Ping Yang . Recent advances in directing group assisted transition metal catalyzed para-selective C-H functionalization. Chinese Chemical Letters, 2025, 36(6): 110543-. doi: 10.1016/j.cclet.2024.110543
-
[5]
Wujun Jian , Mong-Feng Chiou , Yajun Li , Hongli Bao , Song Yang . Cu-catalyzed regioselective diborylation of 1,3-enynes for the efficient synthesis of 1,4-diborylated allenes. Chinese Chemical Letters, 2024, 35(5): 108980-. doi: 10.1016/j.cclet.2023.108980
-
[6]
Kongchuan Wu , Dandan Lu , Jianbin Lin , Ting-Bin Wen , Wei Hao , Kai Tan , Hui-Jun Zhang . Elucidating ligand effects in rhodium(Ⅲ)-catalyzed arene–alkene coupling reactions. Chinese Chemical Letters, 2024, 35(5): 108906-. doi: 10.1016/j.cclet.2023.108906
-
[7]
Daheng Wen , Weiwei Fang , Yongmei Liu , Tao Tu . Valorization of carbon dioxide with alcohols. Chinese Chemical Letters, 2024, 35(7): 109394-. doi: 10.1016/j.cclet.2023.109394
-
[8]
Saima Perveen , Lulu Qin , Min Zhao , Zhengwei Ding , Yingying Wang , Zaicheng Nie , Pengfei Li . Recent development in radical cycloaddition reactions for the synthesis of carbo- and heterocycles. Chinese Chemical Letters, 2026, 37(1): 111886-. doi: 10.1016/j.cclet.2025.111886
-
[9]
Xue Xin , Qiming Qu , Islam E. Khalil , Yuting Huang , Mo Wei , Jie Chen , Weina Zhang , Fengwei Huo , Wenjing Liu . Hetero-phase zirconia encapsulated with Au nanoparticles for boosting electrocatalytic nitrogen reduction. Chinese Chemical Letters, 2024, 35(5): 108654-. doi: 10.1016/j.cclet.2023.108654
-
[10]
Zixuan Guo , Xiaoshuai Han , Chunmei Zhang , Shuijian He , Kunming Liu , Jiapeng Hu , Weisen Yang , Shaoju Jian , Shaohua Jiang , Gaigai Duan . Activation of biomass-derived porous carbon for supercapacitors: A review. Chinese Chemical Letters, 2024, 35(7): 109007-. doi: 10.1016/j.cclet.2023.109007
-
[11]
Qiang Cao , Xue-Feng Cheng , Jia Wang , Chang Zhou , Liu-Jun Yang , Guan Wang , Dong-Yun Chen , Jing-Hui He , Jian-Mei Lu . Graphene from microwave-initiated upcycling of waste polyethylene for electrocatalytic reduction of chloramphenicol. Chinese Chemical Letters, 2024, 35(4): 108759-. doi: 10.1016/j.cclet.2023.108759
-
[12]
Yuemin Chen , Yunqi Wu , Guoao Wang , Feihu Cui , Haitao Tang , Yingming Pan . Electricity-driven enantioselective cross-dehydrogenative coupling of two C(sp3)-H bonds enabled by organocatalysis. Chinese Chemical Letters, 2024, 35(9): 109445-. doi: 10.1016/j.cclet.2023.109445
-
[13]
Li Li , Zhi-Xin Yan , Chuan-Kun Ran , Yi Liu , Shuo Zhang , Tian-Yu Gao , Long-Fei Dai , Li-Li Liao , Jian-Heng Ye , Da-Gang Yu . Electro-reductive carboxylation of CCl bonds in unactivated alkyl chlorides and polyvinyl chloride with CO2. Chinese Chemical Letters, 2024, 35(12): 110104-. doi: 10.1016/j.cclet.2024.110104
-
[14]
Yan-Jiang Li , Shu-Lei Chou , Yao Xiao . Detecting dynamic structural evolution based on in-situ high-energy X-ray diffraction technology for sodium layered oxide cathodes. Chinese Chemical Letters, 2025, 36(2): 110389-. doi: 10.1016/j.cclet.2024.110389
-
[15]
Shuai Chen , Anzai Shi , Guoqing Yang , Pengfei Xie , Feng Liu , Youai Qiu . Electrochemical demethoxyl-cyanation of methoxyarenes via SNAr. Chinese Chemical Letters, 2025, 36(9): 110810-. doi: 10.1016/j.cclet.2024.110810
-
[16]
Ruilong Geng , Lingzi Peng , Chang Guo . Dynamic kinetic stereodivergent transformations of propargylic ammonium salts via dual nickel and copper catalysis. Chinese Chemical Letters, 2024, 35(8): 109433-. doi: 10.1016/j.cclet.2023.109433
-
[17]
Rong-Nan Yi , Wei-Min He . Visible light/copper catalysis enabled radial type ring-opening of sulfonium salts. Chinese Chemical Letters, 2025, 36(4): 110787-. doi: 10.1016/j.cclet.2024.110787
-
[18]
Hongping Zhao , Weiming Yuan . Merging catalytic electron donor-acceptor complex and copper catalysis: Enantioselective radical carbocyanation of alkenes. Chinese Chemical Letters, 2025, 36(10): 110894-. doi: 10.1016/j.cclet.2025.110894
-
[19]
Jialin Huang , Liying Fu , Zhanyong Tang , Xiaoqiang Ma , Xingda Zhao , Depeng Zhao . Cross-coupling of trifluoromethylarenes with alkynes C(sp)-H bonds and azoles C(sp2)-H bonds via photoredox/copper dual catalysis. Chinese Chemical Letters, 2025, 36(7): 110505-. doi: 10.1016/j.cclet.2024.110505
-
[20]
Pengzi Wang , Wenjing Xiao , Jiarong Chen . Copper-Catalyzed C―O Bond Formation by Kharasch-Sosnovsky-Type Reaction. University Chemistry, 2025, 40(4): 239-244. doi: 10.12461/PKU.DXHX202406090
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(8)
- HTML views(1)

 Login In
Login In




 DownLoad:
DownLoad: