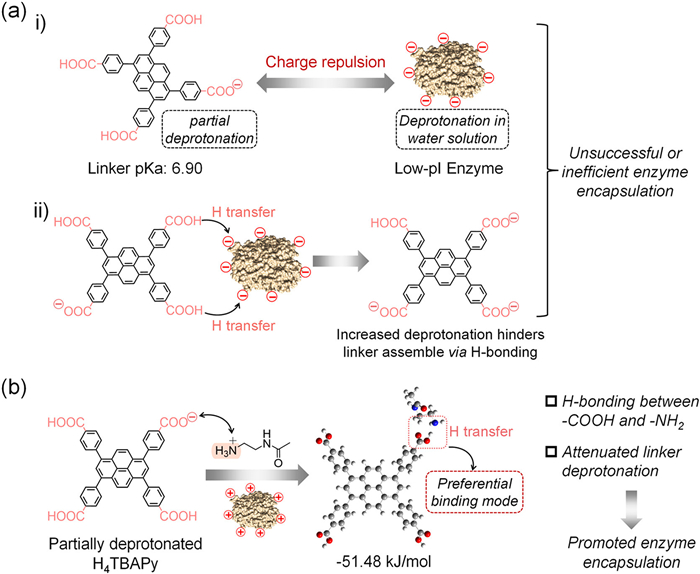
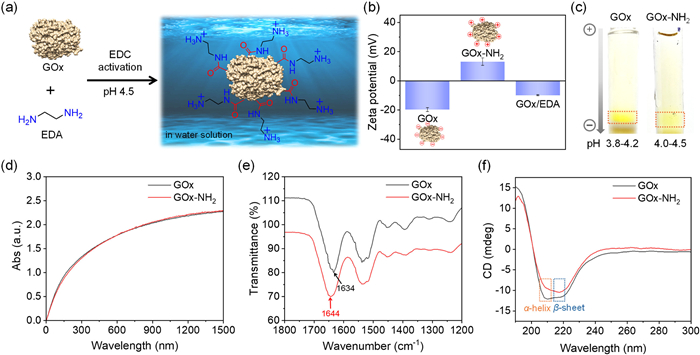
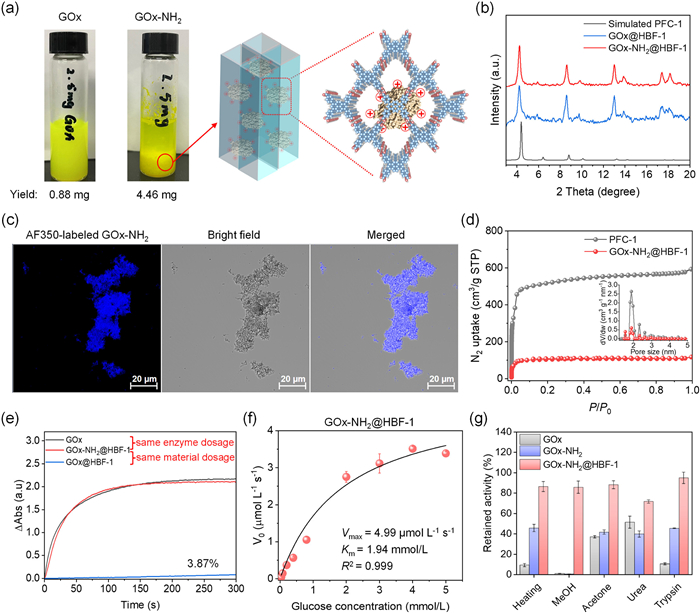
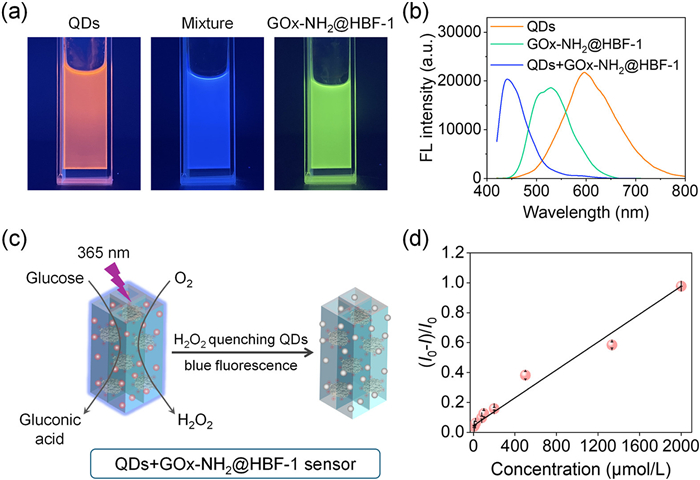
-
[1]
H.E. Schoemaker, D. Mink, M.G. Wubbolts, Science 299 (2003) 1694–1697.
doi: 10.1126/science.1079237
-
[2]
U.T. Bornscheuer, G.W. Huisman, R.J. Kazlauskas, et al., Nature 485 (2012) 185–194.
doi: 10.1038/nature11117
-
[3]
M. Pfefferkorn, S. Böhm, T. Schott, et al., Gut 67 (2018) 2045–2053.
doi: 10.1136/gutjnl-2017-313811
-
[4]
R. Jiang, G. Luo, G. Chen, et al., Sci. Adv. 10 (2024) eadp1796.
doi: 10.1126/sciadv.adp1796
-
[5]
S. Singh, P.K. Sharma, S. Chaturvedi, et al., Food Chem. 435 (2024) 137217.
doi: 10.1016/j.foodchem.2023.137217
-
[6]
M.M. Kristjánsson, J.E. Kinsella, Adv. Food Nutri. Res. 35 (1991) 237–316.
-
[7]
C. Selvaraj, O. Rudhra, A.S. Alothaim, M. Alkhanani, S.K. Singh, Adv. Protein Chem. Str. 130 (2022) 59–83.
-
[8]
M. Bilal, S.A. Qamar, D. Carballares, Á. Berenguer-Murcia, R. Fernandez-Lafuente, Biotechnol. Adv. 70 (2024) 108304.
doi: 10.1016/j.biotechadv.2023.108304
-
[9]
W. Liang, P. Wied, F. Carraro, et al., Chem. Rev. 121 (2021) 1077–1129.
doi: 10.1021/acs.chemrev.0c01029
-
[10]
Q. Zhu, Y. Zheng, Z. Zhang, Y. Chen, Nat. Protoc. 18 (2023) 3080–3125.
doi: 10.1038/s41596-023-00868-x
-
[11]
R.B. Lin, B. Chen, Chem 8 (2022) 2114–2135.
doi: 10.1016/j.chempr.2022.06.015
-
[12]
S. Huang, G. Chen, G. Ouyang, Chem. Soc. Rev. 51 (2022) 6824–6863.
doi: 10.1039/D1CS01011E
-
[13]
Z. Chen, K.O. Kirlikovali, P. Li, O.K. Farha, Acc. Chem. Res. 55 (2022) 579–591.
doi: 10.1021/acs.accounts.1c00707
-
[14]
K. Liang, R. Ricco, C.M. Doherty, et al., Nat. Commun. 6 (2015) 7240.
doi: 10.1038/ncomms8240
-
[15]
W. Liang, H. Xu, F. Carraro, et al., J. Am. Chem. Soc. 141 (2019) 2348–2355.
doi: 10.1021/jacs.8b10302
-
[16]
W. Liang, K. Flint, Y. Yao, et al., J. Am. Chem. Soc. 145 (2023) 20365–20374.
doi: 10.1021/jacs.3c05488
-
[17]
J.C. Díaz, B. Lozano-Torres, M. Giménez-Marqués, Chem. Mater. 34 (2022) 7817–7827.
doi: 10.1021/acs.chemmater.2c01338
-
[18]
I. Akpinar, X. Wang, K. Fahy, et al., J. Am. Chem. Soc. 146 (2024) 5108–5117.
doi: 10.1021/jacs.3c07785
-
[19]
T.H. Wei, S.H. Wu, Y.D. Huang, et al., Nat. Commun. 10 (2019) 5002.
-
[20]
H.Y. Guan, R.J. LeBlanc, S.Y. Xie, Y. Yue, Coordin. Chem. Rev. 369 (2018) 76–90.
-
[21]
Q. Liu, Y. Song, Y. Ma, et al., J. Am. Chem. Soc. 141 (2019) 488–496.
doi: 10.1021/jacs.8b11230
-
[22]
Z. Zhang, Y. Ye, S. Xiang, B. Chen, Acc. Chem. Res. 55 (2022) 3752–3766.
doi: 10.1021/acs.accounts.2c00686
-
[23]
Z.J. Lin, S.A.R. Mahammed, T.F. Liu, R. Cao, ACS Cent. Sci. 8 (2022) 1589–1608.
-
[24]
I. Hisaki, C. Xin, K. Takahashi, T. Nakamura, Angew. Chem. Int. Ed. 58 (2019) 11160–11170.
-
[25]
C. Huang, C. Zhao, Q. Deng, et al., Nat. Catal. 6 (2023) 729–739.
-
[26]
S. Huang, J. Li, Y. Lin, et al., J. Am. Chem. Soc. 146 (2024) 1967–1976.
-
[27]
G. Chen, S. Huang, X. Ma, R. He, G. Ouyang, Nat. Protoc. 18 (2023) 2032–2050.
-
[28]
A. Huang, H. Yang, S. Huang, G. Chen, G. Ouyang, Matter 6 (2023) 2635–2646.
-
[29]
W. Huang, H. Yuan, H. Yang, et al., Nat. Commun. 14 (2023) 3644.
-
[30]
G. Chen, L. Tong, S. Huang, et al., Nat. Commun. 13 (2023) 4816.
-
[31]
W. Huang, H. Yuan, H. Yang, et al., JACS Au 2 (2022) 2048–2058.
-
[32]
G. Chen, S. Huang, Y. Shen, et al., Chem 7 (2021) 2722–2742.
-
[33]
Z. Tang, X. Li, L. Tong, et al., Angew. Chem. Int. Ed. 60 (2021) 23608– 23613.
-
[34]
H. Yang, J. Fu, W. Huang, et al., Small Struct. 4 (2023) 2200346.
-
[35]
S. Hayashi, S. Nakamura, BBA–Enzymology 657 (1981) 40–51.
-
[36]
N.K. Maddigan, A. Tarzia, D.M. Huang, et al., Chem. Sci. 9 (2018) 4217–4223.
-
[37]
F.C. Church, D.H. Porter, G.L. Catignani, H.E. Swaisgood, Anal. Biochem. 146 (1985) 343–348.
-
[38]
B.B. Pinheiro, N.S. Rios, G. Zanatta, B.C. Pessela, L.R.B. Gonçalves, Process Biochem. 133 (2023) 292–302.
-
[39]
W.H. Chen, M. Vázquez-González, A. Zoabi, R. Abu-Reziq, I. Willner, Nat. Catal. 1 (2018) 689–695.
-
[40]
Z. Xu, Y.K. Cen, S.P. Zou, Y.P. Xue, Y.G. Zheng, Crit. Rev. Biotechnol. 40 (2019) 83–98.
-
[41]
N.G. Nezhad, R.N.Z.R.A. Rahman, Y.M. Normi, et al., Int. J. Biol. Macromol. 232 (2023) 123440.
-
[42]
N. Özer, M. Müftüoglu, D. Ataman, A. Ercan, I.H. Ögüs, J. Biochem. Bioph. Meth. 39 (1999) 153–159.
-
[43]
S. O'Connell, G. Walsh, Appl. Microbiol. Biotechnol. 86 (2010) 517–524.
-
[44]
Z. Li, J.R. Askim, K.S. Suslick, Chem. Rev. 119 (2019) 231–292.
-
[45]
H. Chen, H. Huang, H. Xu, et al., Small 20 (2024) 2308716.
-
[46]
L.A. Pérez-Márquez, M.D. Perretti, R. García-Rodríguez, F. Lahoz, R. Carrillo, Angew. Chem. Int. Ed. 61 (2022) e202205403.
-
[47]
Y. Wang, B. Li, T. Tian, et al., TrAC-Trends Anal. Chem. 149 (2022) 116565.
-
[48]
N.E. Azmi, N.I. Ramli, J. Abdullah, et al., Biosens. Bioelectron. 67 (2015) 129–133.
-
[49]
L. Lin, Y. Wen, Y. Liang, N. Zhang, D. Xiao, Anal. Methods 5 (2013) 457–464.
-
[50]
A. Singh, A.S.K. Sinha, Appl. Surface Sci. 430 (2018) 184–197.
-
[51]
X. Li, C. Li, L. Chen, New J. Chem. 39 (2015) 9976–9982.

 Login In
Login In






 DownLoad:
DownLoad: