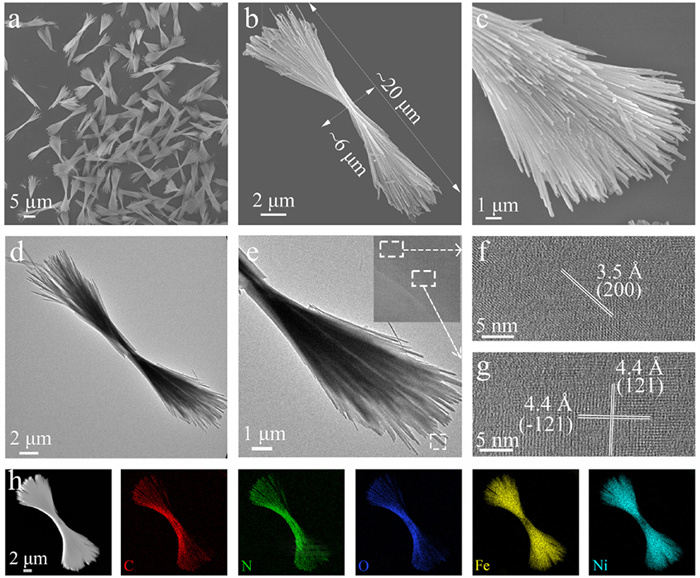
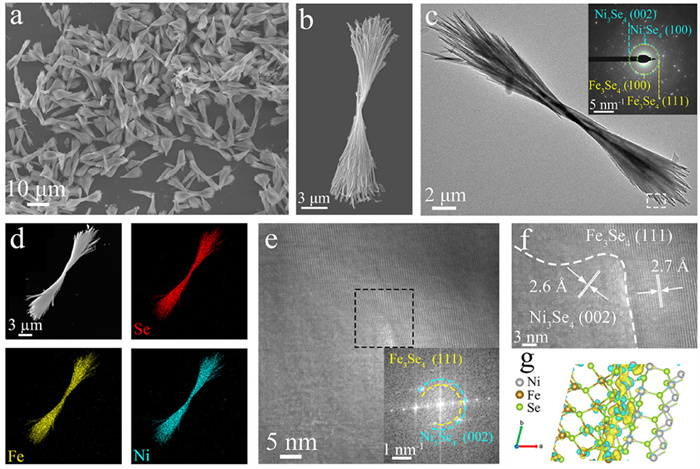
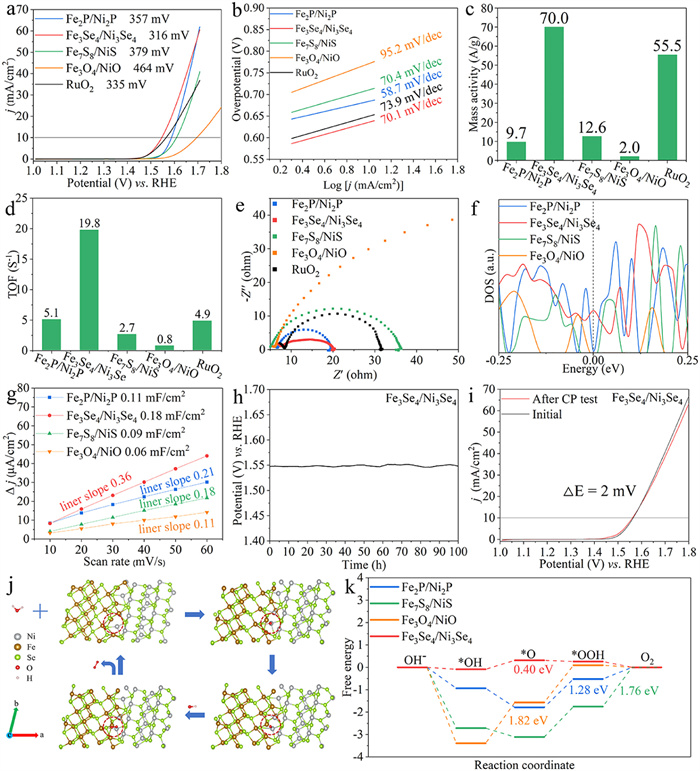
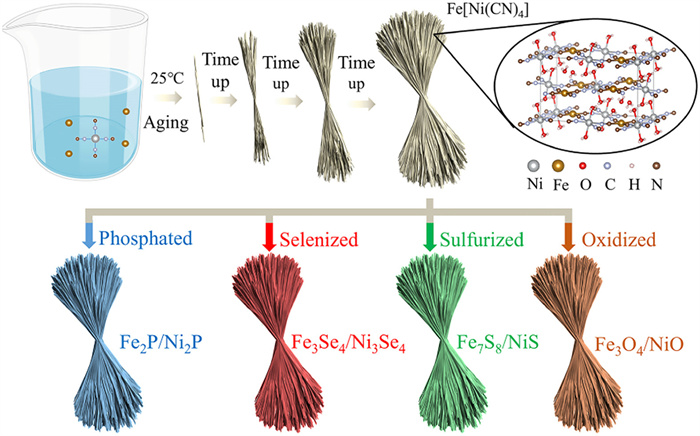
-
[1]
E. Kabir, P. Kumar, S. Kumar, et al., Renew. Sust. Energ. Rev. 82 (2018) 894–900.
-
[2]
P. Sadorsky, J. Clean Prod. 289 (2021) 125779.
-
[3]
B.Q. Lin, Z. Li, Appl. Energy 307 (2022) 118160.
-
[4]
J. Di, W. Jiang, Mater. Today. Catal. 1 (2023) 100001.
-
[5]
H. Ishaq, I. Dincer, C. Crawford, Int. J. Hydrog. Energy 47 (2022) 26238–26264.
-
[6]
M.D. Ji, J.L. Wang, Int. J. Hydrog. Energy 46 (2021) 38612–38635.
-
[7]
L.M. Cao, D. Lu, D.C. Zhong, et al., Coord. Chem. Rev. 407 (2020) 213156.
-
[8]
M. Chatenet, B.G. Pollet, D.R. Dekel, et al., Chem. Soc. Rev. 51 (2022) 4583–4762.
doi: 10.1039/d0cs01079k
-
[9]
W. Yang, S.W. Chen, Chem. Eng. J. 393 (2020) 124726.
-
[10]
E. Fabbri, A. Habereder, K. Waltar, et al., Catal. Sci. Technol. 4 (2014) 3800–3821.
-
[11]
X.H. Xie, L. Du, L.T. Yon, et al., Adv. Energy Mater. 32 (2022) 2110036.
-
[12]
Q. Wang, Y. Gong, X. Zi, et al., Angew. Chem. Int. Ed. 63 (2024) e202405438.
doi: 10.1002/anie.202405438
-
[13]
K. Xiao, Y. Wang, P. Wu, et al., Angew. Chem. Int. Ed. 62 (2023) e202301408.
-
[14]
J.H. Huang, Y.H. Xie, L. Yan, et al., Energy Environ. Sci. 14 (2021) 883–889.
doi: 10.1039/d0ee03639k
-
[15]
L.J. Zhang, H. Jang, H.H. Liu, et al., Angew. Chem. Int. Ed. 60 (2021) 18821–18829.
doi: 10.1002/anie.202106631
-
[16]
J.Y. Chen, P.X. Cui, G.Q. Zhao, et al., Angew. Chem. Int. Ed. 58 (2019) 12540–12544.
doi: 10.1002/anie.201907017
-
[17]
Y. Fu, H.Y. Yu, C. Jiang, et al., Adv. Funct. Mater. 28 (2018) 1705094.
-
[18]
H. Zhang, T. Gao, Q. Zhang, et al., Mater. Today. Catal. 3 (2023) 100013.
-
[19]
S. Guo, C. Chen, M. Qiu, et al., Mater. Today. Catal. 3 (2023) 100022.
-
[20]
Z.P. Wu, X.F. Lu, S.Q. Zang, et al., Adv. Energy Mater. 30 (2020) 1910274.
-
[21]
H. Xu, H.Y. Shang, C. Wang, et al., Coord. Chem. Rev. 418 (2020) 213374.
-
[22]
X. Long, J.K. Li, S. Xiao, et al., Angew. Chem. Int. Ed. 53 (2014) 7584–7588.
doi: 10.1002/anie.201402822
-
[23]
Q. Zhang, W. Xiao, H.C. Fu, et al., ACS Catal. 13 (2023) 14975–14986.
doi: 10.1021/acscatal.3c03804
-
[24]
J. Mohammed-Ibrahim, J. Power Sources 448 (2020) 227375.
-
[25]
W. Cao, X.H. Gao, J. Wu, et al., ACS Catal. 14 (2024) 3640–3646.
doi: 10.1021/acscatal.3c06180
-
[26]
M. Wang, Y.S. Chen, Z.B. Yu, et al., J. Colloid Interface Sci. 640 (2023) 1–14.
-
[27]
S. Kang, C. Im, I. Spanos, et al., Angew. Chem. Int. Ed. 61 (2022) e202214541.
-
[28]
H. Liao, X. Zhang, S. Niu, et al., Appl. Catal. B: Environ. 307 (2022) 121150.
-
[29]
H. Liao, T. Luo, P. Tan, et al., Adv. Energy Mater. 31 (2021) 2102772.
-
[30]
X.J. Li, H.K. Zhang, Q. Hu, et al., Angew. Chem. Int. Ed. 62 (2023) e202300478.
-
[31]
Y. Hao, X. Cao, C. Lei, et al., Mater. Today. Catal. 2 (2023) 100012.
-
[32]
H. Liao, G. Ni, P. Tan, et al., Adv. Mater. 35 (2023) 2300347.
-
[33]
L. Magnier, G. Cossard, V. Martin, et al., Nat. Mater. 23 (2024) 252–261.
doi: 10.1038/s41563-023-01744-5
-
[34]
J. Zhuang, D. Wang, Mater. Today. Catal. 2 (2023) 100009.
-
[35]
Z.K. Wang, Y.Y. Wang, N. Zhang, et al., J. Mater. Chem. A 10 (2022) 10342–10349.
doi: 10.1039/d2ta01931k
-
[36]
H. Yang, L.Q. Gong, H.M. Wang, et al., Nat. Commun. 11 (2020) 5075.
-
[37]
Y. Chen, B. Zhang, Y. Liu, et al., Mater. Today. Catal. 1 (2023) 100003.
-
[38]
S. Xie, H. Jin, C. Wang, et al., Chin. Chem. Lett. 34 (2023) 107681.
-
[39]
Q.C. Xu, H. Jiang, X.Z. Duan, et al., Nano Lett. 21 (2021) 492–499.
doi: 10.1021/acs.nanolett.0c03950
-
[40]
J.Y. Zou, X.F. Ren, New J. Chem. 47 (2023) 14408–14417.
doi: 10.1039/d3nj02351f
-
[41]
Y.K. Bai, Y. Wu, X.C. Zhou, et al., Nat. Commun. 13 (2022) 6094.
-
[42]
Y.C. Wu, Y.J. Wang, Z.W. Wang, et al., J. Mater. Chem. A 9 (2021) 23574–23581.
doi: 10.1039/d1ta06574b
-
[43]
Q. Jiang, S.H. Wang, C.R. Zhang, et al., Nat. Commun. 14 (2023) 6826.
-
[44]
J.W. Nai, X.Z. Xu, Q.F. Xie, et al., Adv. Mater. 34 (2022) 2104405.
-
[45]
P. Xiao, W. Chen, X. Wang, Adv. Energy Mater. 5 (2015) 1500985.
-
[46]
P.R. Chen, J.S. Ye, H. Wang, et al., J. Alloy. Compd. 883 (2021) 160833.
-
[47]
K. Chu, Y.P. Liu, Y.B. Li, et al., J. Mater. Chem. A 7 (2019) 4389–4394.
doi: 10.1039/c9ta00016j
-
[48]
X.X. Yu, Z.Y. Yu, X.L. Zhang, et al., J. Am. Chem. Soc. 141 (2019) 7537–7543.
doi: 10.1021/jacs.9b02527
-
[49]
R. Boppella, J. Tan, W. Yang, et al., Adv. Energy Mater. 29 (2019) 1807976.
-
[50]
G.Y. Wen, X.P. Zhang, Y.L. Sui, et al., Chem. Eng. J. 430 (2022) 133041.
-
[51]
G.Y. Wen, Y.L. Sui, X.P. Zhang, et al., J. Colloid Interface Sci. 589 (2021) 208–216.
-
[52]
P. Guo, L. Shi, D. Liu, et al., Mater. Today. Catal. 1 (2023) 100002.
-
[53]
G. Zhong, S. Xu, B. Fang, Mater. Today. Catal. 4 (2024) 100045.
-
[54]
J. Chen, Q. Long, K. Xiao, et al., Sci. Bull. 66 (2021) 1063–1072.
doi: 10.1109/tcpmt.2021.3084966
-
[55]
J. Wei, K. Xiao, Y. Chen, et al., Energy Environ. Sci. 15 (2022) 4592–4600.
doi: 10.1039/d2ee02151j
-
[56]
H. Song, M. Wu, Z. Tang, et al., Angew. Chem. Int. Ed. 133 (2021) 7310–7320.
doi: 10.1002/ange.202017102
-
[57]
M.B.Z. Hegazy, F. Hassan, M. Hu, Small 20 (2023) 2306709.
-
[58]
T. Wang, Y. Wu, Y. Han, et al., ACS Appl. Nano Mater. 4 (2021) 14161–14168.
doi: 10.1021/acsanm.1c03619
-
[59]
R. Zhang, Z. Wu, Z. Huang, et al., Chin. Chem. Lett. 34 (2023) 107600.
-
[60]
J.Y. Li, Z.P. Ni, Z. Yan, et al., CrystEngComm 16 (2014) 6444–6449.
-
[61]
V. García-López, F. Marques-Moros, J. Troya, et al., J. Mater. Chem. C 12 (2023) 161–169.
-
[62]
M.B. Zakaria, E.Z.M. Ebeid, M.M. Abdel-Galeil, et al., New J. Chem. 41 (2017) 14890–14897.
-
[63]
H.F. Liu, S.Z. Cong, X.R. Yan, et al., J. Membr. Sci. 669 (2023) 121282.
-
[64]
V. Rubio-Giménez, G. Escorcia-Ariza, C. Bartual-Murgui, et al., Chem. Mat. 31 (2019) 7277–7287.
doi: 10.1021/acs.chemmater.9b01634
-
[65]
W. Wang, L. Li, J. Ouyang, et al., Chin. Chem. Lett. 34 (2023) 107597.
-
[66]
J. Chen, G. Qian, B. Chu, et al., Small 18 (2022) 2106773.
-
[67]
H. Li, C. Zhang, W. Xiang, et al., Chem. Eng. J 452 (2023) 139104.
-
[68]
B. Zhang, L. Wang, Z. Cao, et al., Nat. Catal. 3 (2020) 985–992.
doi: 10.1038/s41929-020-00525-6
-
[69]
J. Zhou, Y. Dou, T. He, et al., Nano Res. 14 (2021) 4548–4555.
doi: 10.1007/s12274-021-3370-7

 Login In
Login In






 DownLoad:
DownLoad: