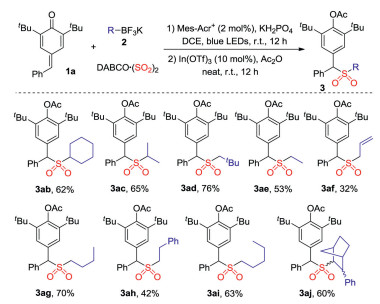
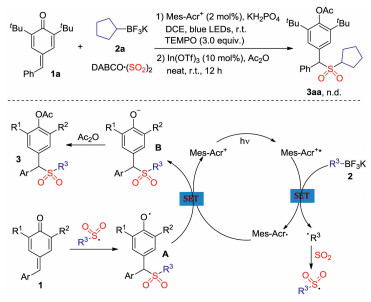
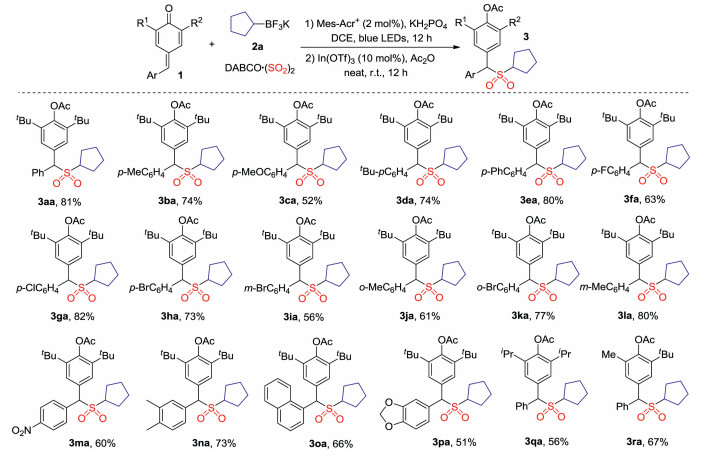
-
[1]
(a) M.R. Prinsep, J.W. Blunt, M.H.G. Munro, J. Nat. Prod. 54 (1991) 1068-1076;
(b) M. Teall, P. Oakley, T. Harrison, et al., Bioorg. Med. Chem. Lett. 15 (2005) 2685-2688;
(c) L. Legros, J.R. Dehli, C. Bolm, Adv. Synth. Catal. 347 (2005) 19-31;
(d) I. Churcher, D. Beher, J.D. Best, et al., Bioorg. Med. Chem. Lett. 16 (2006) 280-284;
(e) Y. Harrak, G. Casula, J. Basset, et al., J. Med. Chem. 53 (2010) 6560-6571.
-
[2]
N.S. Simpkins, Sulfones in Organic Synthesis, Pergamon Press, Oxford, 1993.
-
[3]
(a) J. Zhou, M.L. Wang, X. Gao, G.F. Jiang, Y.G. Zhou, Chem. Commun. 53 (2017) 3531-3534;
(b) M. Nambo, Z.T. Ariki, D. Canseco-Gonzalez, D.D. Beattie, C.M. Crudden, Org. Lett. 18 (2016) 2339-2342;
(c) M. Nambo, C.M. Crudden, Angew. Chem. Int. Ed. 53 (2014) 742-746.
-
[4]
(a) D.F. Duxbury, Chem. Rev. 93 (1993) 381-433;
(b) M.S. Shchepinov, V.A. Korshun, Chem. Soc. Rev. 32 (2003) 170-180;
(c) V. Nair, S. Thomas, S.C. Mathew, K.G. Abhilash, Tetrahedron 62 (2006) 6731-6747.
-
[5]
(a) P. Thirupathi, S.S. Kim, Eur. J. Org. Chem. 2010 (2010) 1798-1808;
(b) J.L. Zhao, S.H. Guo, J. Qiu, et al., Tetrahedron Lett 57 (2016) 2375-2378;
(c) R.R. Kuchukulla, F. Li, Z. He, L. Zhou, Q. Zeng, Green Chem 21 (2019) 5808-5812.
-
[6]
(a) M.A. Reddy, P.S. Reddy, B. Sreedhar, Adv. Synth. Catal. 352 (2010) 1861-1869;
(b) K. Xu, L. Li, W. Yan, et al., Green Chem 19 (2017) 4494-4497;
(c) H. Yamamoto, K. Nakata, Eur. J. Org. Chem. 2019 (2019) 4906-4910.
-
[7]
(a) B. Zheng, T. Jia, P.J. Walsh, Org. Lett. 15 (2013) 1690-1693;
(b) M. Nambo, C.M. Crudden, Angew. Chem. Int. Ed. 53 (2014) 742-746.
-
[8]
(a) T. Liu, J. Liu, S. Xia, et al., ACS Omega 3 (2018) 1409-1415;
(b) X.Y. Guan, L.D. Zhang, P.S. You, S.S. Liu, Z.Q. Liu, Tetrahedron Lett 60 (2019) 244-247;
(c) Z.Q. Liu, P.S. You, L.D. Zhang, et al., Molecules 25 (2020) 539-553.
-
[9]
(a) M.G. Peter, 1989 Angew. Chem. Int. Ed., 28555-570;
(b) H.U. Wagner, R. Gompper, Quinone Methides, in S. Patai, Z. Rappport (Eds. ), The Chemistry of Quinonoid Compounds, Wiley, New York, 1974, pp. 1145-1178;
(c) A.B.Q. Turner, 1964 Q. Rev. Chem. Soc., 18347-360;
(d) D.J. Hart, P.A. Cain, D.A. Evans, 1978 J. Am. Chem. Soc., 1001548-1557.
-
[10]
(a) V. Reddy, R. Vijaya Anand, Org. Lett. 17 (2015) 3390-3393;
(b) F.S. He, J.H. Jin, Z.T. Yang, et al., ACS Catal 6 (2016) 652-656;
(c) K. Zhao, Y. Zhi, A. Wang, D. Enders, ACS Catal 6 (2016) 657-660;
(d) N. Dong, Z.P. Zhang, X.S. Xue, X. Li, J.P. Cheng, Angew. Chem. Int. Ed. 55 (2016) 1460-1464;
(e) Y.H. Deng, X.Z. Zhang, K.Y. Yu, et al., Chem. Commun. 52 (2016) 4183-4186;
(f) Z.P. Zhang, N. Dong, X. Li, Chem. Commun. 53 (2017) 1301-1304;
(g) B.M. Sharma, D.R. Shinde, R. Jain, et al., Org. Lett. 20 (2018) 2787-2791;
(h) Z. Liu, H. Xu, T. Yao, J. Zhang, L. Liu, Org. Lett. 21 (2019) 7539-7543;
(i) G.M. Nan, X. Li, T.Y. Yao, et al., Org. Biomol. Chem. 18 (2020) 1780-1784;
(j) J.Y. Wang, W.J. Hao, S.J. Tu, B. Jiang, Org. Chem. Front. 7 (2020) 1743-1778.
-
[11]
(a) M. Ke, Q. Song, Adv. Synth. Catal. 359 (2017) 384-389;
(b) Q.Y. Wu, Q.Q. Min, G.Z. Ao, F. Liu, Org. Biomol. Chem. 16 (2018) 6391-6394;
(c) Q.Y. Wu, G.Z. Ao, F. Liu, Org. Chem. Front. 5 (2018) 2061-2064;
(d) W. Zhang, C. Yang, Z.P. Zhang, X. Li, J.P. Cheng, Org. Lett. 21 (2019) 4137-4142;
(e) H.D. Zuo, W.J. Hao, C.F. Zhu, et al., Org. Lett. 22 (2020) 4471-4477.
-
[12]
(a) P. Bisseret, N. Blanchard, 2013 Org. Biomol. Chem., 115393-5398;
(b) A.S. Deeming, E.J. Emmett, C.S. Richards-Taylor, M.C. Willis, 2014 Synthesis (Mass), 462701-2710;
(c) G. Liu, C. Fan, J. Wu, 2015 Org. Biomol. Chem., 131592-1599;
(d) E.J. Emmett, M.C. Willis, 2015 Asian J. Org. Chem., 4602-611;
(e) D. Zheng, J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017;
(f) G. Qiu, K. Zhou, L. Gao, J. Wu, 2018 Org. Chem. Front., 5691-705;
(g) K. Hofman, N.W. Liu, G. Manolikakes, 2018 Chem. Eur. J., 2411852-11863;
(h) G. Qiu, L. Lai, J. Cheng, J. Wu, 2018 Chem. Commun., 5410405-10414;
(i) G. Qiu, K. Zhou, J. Wu, 2018 Chem. Commun., 5412561-12569;
(j) S. Ye, G. Qiu, J. Wu, 2019 Chem. Commun., 551013-1019;
(k) S. Ye, M. Yang, J. Wu, 2020 Chem. Commun., 564145-4155;
(l) S. Ye, X. Li, W. Xie, J. Wu, 2020 Eur. J. Org. Chem. 1274-1287.
-
[13]
(a) X. Wang, L. Xue, Z. Wang, Org. Lett. 16 (2014) 4056-4058;
(b) N.W. Liu, S. Liang, G. Manolikakes, Adv. Synth. Catal. 359 (2017) 1308-1319;
(c) H. Wang, S. Sun, J. Cheng, Org. Lett. 19 (2017) 5844-5847;
(d) N. von Wolff, J. Char, X. Frogneux, T. Cantat, Angew. Chem. Int. Ed. 56 (2017) 5616-5619;
(e) J. Sheng, Y. Li, G. Qiu, Org. Chem. Front. 4 (2017) 95-100;
(f) Y. Wang, L. Deng, J. Zhou, et al., Adv. Synth. Catal. 360 (2018) 1060-1065;
(g) A.L. Tribby, I. Rodríguez, S. Shariffudin, N.D. Ball, J. Org. Chem. 82 (2017) 2294-2299.
-
[14]
(a) D. Zheng, J. Yu, J. Wu, Angew. Chem. Int. Ed. 55 (2016) 11925-11929;
(b) J. Zhang, Y. An, J. Wu, Chem. Eur. J. 23 (2017) 9477-9480;
(c) T. Liu, D. Zheng, Z. Li, J. Wu, Adv. Synth. Catal. 359 (2017) 2653-2659;
(d) Y. An, J. Wu, Org. Lett. 19 (2017) 6028-6031;
(e) T. Liu, D. Zheng, Y. Ding, X. Fan, J. Wu, Chem. Asian J. 12 (2017) 465-469;
(f) Y. An, J. Zhang, H. Xia, J. Wu, Org. Chem. Front. 4 (2017) 1318-1321;
(g) X. Wang, T. Liu, Q. Zhong, J. Wu, Org. Chem. Front. 4 (2017) 2455-2458;
(h) T. Liu, D. Zheng, J. Wu, Org. Chem. Front. 4 (2017) 1079-1083;
(i) J. Zhang, K. Zhou, J. Wu, Org. Chem. Front. 5 (2018) 813-816;
(j) T. Liu, D. Zheng, Z. Li, J. Wu, Adv. Synth. Catal. 360 (2018) 865-869;
(k) J. Zhang, F. Zhang, L. Lai, et al., Chem. Commun. 54 (2018) 3891-3894;
(l) Zhou K., J. Zhang, G. Qiu, J. Wu, Org. Lett. 21 (2019) 275-278;
(m) X. Gong, X. Li, W. Xie, J. Wu, S. Ye, Org. Chem. Front. 6 (2019) 1863-1867;
(n) X. Wang, Y. Lin, J.B. Liu, et al., Chin. J. Chem. 38 (2020) 1098-1102;
(o) F.S. He, Y. Yao, W. Xie, J. Wu, Chem. Commun. 56 (2020) 9469-9472;
(p) T. Zhu, J. Wu, Org. Lett. 22(18) (2020) 7094-7097;
(q) J. Huang, F. Ding, Z. Chen, G. Yang, J. Wu, Org. Chem. Front. 8 (2021) 1461-1465;
(r) Y. Yao, Z. Yin, F.S. He, et al., Chem. Commun. 57 (2021) 2883-2886.
-
[15]
(a) K. Zhou, J. Huang, J. Wu, G. Qiu, Chin. Chem. Lett. 32 (2021) 37-39;
(b) T. Liu, Y. Ding, X. Fan, J. Wu, Org. Chem. Front. 5 (2018) 3153-3157;
(c) S. Ye, X. Li, W. Xie, J. Wu, Asian J. Org. Chem. 8 (2019) 893-898;
(d) X. Gong, M. Yang, J.B. Liu, F.S. He, J. Wu, Org. Chem. Front. 7 (2020) 938-943.
-
[16]
(a) X. Wang, Y. Kuang, S. Ye, J. Wu, Chem. Commun. 55 (2019) 14962-14964;
(b) T. Zhu, J. Shen, Y. Sun, J. Wu, Chem. Commun. 57 (2021) 915-918.
-
[17]
(a) X. Wang, M. Yang, W. Xie, X. Fan, J. Wu, Chem. Commun. 55 (2019) 6010-6013;
(b) X. Wang, H. Li, G. Qiu, J. Wu, Chem. Commun. 55 (2019) 2062-2065;
(c) X. Wang, M. Yang, W. Xie, X. Fan, J. Wu, Chem. Commun. 55 (2019) 6010-6013;
(d) X. Gong, M. Yang, J.B. Liu, et al., Green Chem 22 (2020) 1906-1910.
-
[18]
(a) Y. Zong, Y. Lang, M. Yang, et al., Org. Lett. 21 (2019) 1935-1938;
(b) F.S. He, M. Yang, S. Ye, J. Wu, Chin. Chem. Lett. 32 (2021) 461-464;
(c) K. Zhou, J. Chen, J. Wu, Chin. Chem. Lett. 31 (2020) 2996-2998;
(d) J. Chen, L. Wu, J. Wu, Chin. Chem. Lett. 31 (2020) 2993-2995.
-
[19]
(a) D. Richter, N. Hampel, T. Singer, A.R. Ofial, H. Mayr, Eur. J. Org. Chem. (2009) 3203-3211;
(b) Y.J. Fan, L. Zhou, S. Li, Org. Chem. Front. 5 (2018) 1820-1824.
-
[20]
K.K. Chauhan, C.G. Frost, I. Love, D. Waite, Synlett 10 (1999) 1743-1744.
-
[21]
P. Goswami, S. Sharma, G. Singh, R.V. Anand, J. Org. Chem. 83 (2018) 4213-4220.

 Login In
Login In






 DownLoad:
DownLoad: