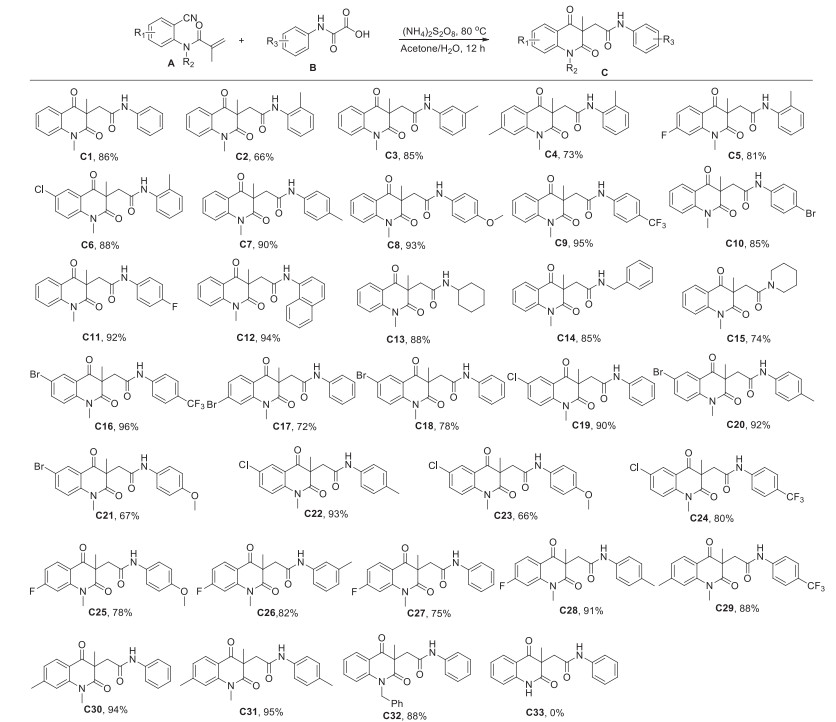
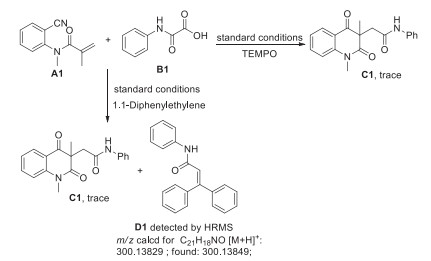
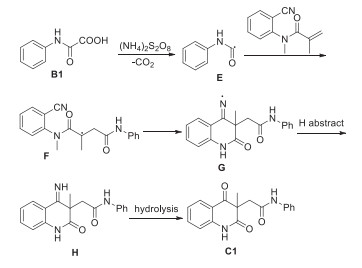
-
[1]
(a) S. Han, F.F. Zhang, H.Y. Qian, et al., J. Med. Chem. 58 (2015) 5751-5769;
(b) Y.X. Liu, H.P. Zhao, Z.W. Wang, et al., Mol. Divers. 17 (2013) 701-710;
(c) J.L. McCormick, T.C. McKee, J.H. Cardellina, M.R. Boyd, J. Nat. Prod. 59 (1996) 469-471;
(d) Z. Liu, X. Zhang, H. Zhang, et al., Chin. J. Org. Chem. 40 (2020) 2755;
(e) Q.W. Gui, F. Teng, S.N. Ying, et al., Chin. Chem. Lett. 31 (2020) 3241-3244;
(f) Y.F. Si, K. Sun, X.L. Chen, et al., Org. Lett. 22 (2020) 6960-6965.
-
[2]
(a) T. Yang, W.J. Xia, J.Q. Shang, et al., Org. Lett. 21 (2019) 444-447;
(b) L.J. Wu, Y. Yang, R.J. Song, et al., Chem. Commun. 54 (2018) 1367-1370;
(c) Y. Zhang, T. Zhang, H. Zhan, X. Li, Chin. J. Catal. 35 (2014) 1840-1845;
(d) L. Tao, C. Li, Y. Ren, et al., Chin. J. Catal. 40 (2019) 1548-1556;
(e) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30 (2019) 2287-2290;
(f) H.Y. Guo, Y. Yu, Chin. Chem. Lett. 21 (2010) 1435-1438;
(g) Z. Wang, L. Chen, G. Mao, C. Wang, Chin. Chem. Lett. 31 (2020) 1890-1894.
-
[3]
(a) L. Xiong, H. Hu, C.W. Wei, B. Yu, Eur. J. Org. Chem. 2020 (2020) 1588-1597;
(b) J. Xuan, A. Studer, Chem. Soc. Rev. 46 (2017) 4329-4346;
(c) F. Zhao, X. Jia, D. Wang, et al., Chin. J. Org. Chem. (2017) 37;
(d) X.X. Meng, Q.Q. Kang, J.Y. Zhang, et al., Green Chem 22 (2020) 1388-1392;
(e) Z. Wang, W.M. He, Chin. J. Org. Chem. 39 (2019) 3594-3595;
(f) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258;
(g) D. Dong, Y. Sun, G. Li, et al., Chin. J. Org. Chem. 40 (2020) 4071-4086;
(h) Z.Y. Gan, G.Q. Li, X.B. Yang, et al., Sci. China Chem. 63 (2020) 1652-1658;
(i) K. Sun, G. Li, Y. Li, et al., Adv. Synth. Catal. 362 (2020) 1947-1954;
(j) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30 (2019) 2259-2262;
(k) L. Wang, Y. Zhang, M. Zhang, et al., Tetrahedron Lett 60 (2019) 1845-1848;
(l) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30 (2019) 1361-1368;
(m) X. Ren, Z. Lu, Chin. J. Catal. 40 (2019) 1003-1019;
(n) R. Yi, L. Qian, B. Wan, Chin. J. Catal. 40 (2019) 177-183;
(o) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258;
(p) Q.W. Gui, F. Teng, Z.C. Li, et al., Chin. Chem. Lett. 32 (2021) 1907-1910;
(q) W.H. Bao, Z. Wang, Z. Cao, et al., Adv. Synth. Catal. 363 (2021) 757-761;
(r) D.Q. Dong, H. Yang, J.L. Shi, et al., Org. Chem. Front. 7 (2020) 2538-2575;
(s) D.Q. Dong, Q.Q. Han, S.H. Yang, et al., ChemistrySelect 5 (2020) 13103-13134.
-
[4]
T. Yang, W.J. Xia, B. Zhou, et al., Eur. J. Org. Chem. 2019 (2019) 5749–5755.
doi: 10.1002/ejoc.201900918
-
[5]
Y.M. Li, T. Yang, J.L. Zhou, et al., Synthesis(Mass)50 (2018) 3460–3466.
doi: 10.1055/s-0037-1610070
-
[6]
F. Ding, Y. Fang, Y. Jiang, K. Lin, L. Shi, Chem. Asian J. 13 (2018) 636–640.
doi: 10.1002/asia.201701780
-
[7]
H. Sun, Y. Jiang, Y.S. Yang, et al., Org. Biomol. Chem. 17 (2019) 6629–6638.
doi: 10.1039/c9ob01213c
-
[8]
(a) S.S. Wang, H. Fu, G. Wang, M. Sun, Y.M. Li, RSC Adv. 6 (2016) 52391-52394;
(b) S.S. Wang, H. Fu, Y. Shen, M. Sun, Y.M. Li, J. Org. Chem. 81 (2016) 2920-2929.
-
[9]
S. Wang, X. Huang, Q. Wang, et al., RSC Adv. 6 (2016) 11754–11757.
doi: 10.1039/C5RA27878C
-
[10]
H. Fu, S.S. Wang, Y.M. Li, Adv. Synth. Catal. 358 (2016) 3616–3626.
doi: 10.1002/adsc.201600693
-
[11]
Y.M. Li, S.S. Wang, F. Yu, Y. Shen, K.J. Chang, Org. Biomol. Chem. 13 (2015) 5376–5380.
doi: 10.1039/C5OB00617A
-
[12]
(a) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30 (2019) 2304-2308;
(b) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173;
(c) Y. Wu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 31 (2020) 2999-3000;
(d) D.Q. Dong, W.J. Chen, Y. Yang, X. Gao, Z.L. Wang, Chemistryselect 4 (2019) 2480-2483;
(e) J.Y. Chen, H.Y. Wu, Q.W. Gui, et al., Chin. J. Catal. 42 (2021) 1445-1450;
(f) D.Q. Dong, W.J. Chen, D.M. Chen, et al., Chin. J. Org. Chem. 39 (2019) 3190-3198;
(g) X.Y. Li, Y. Liu, X.L. Chen, et al., Green Chem. 22 (2020) 4445-4449;
(h) K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500;
(i) J. Jiang, F. Xiao, W.M. He, L. Wang, Chin. Chem. Lett. 32 (2021) 1637-1644.
-
[13]
(a) J.F. Zhao, X.H. Duan, L.N. Guo, Chin. J. Org. Chem. 37 (2017) 2498-2511;
(b) S. Mandal, T. Bera, G. Dubey, J. Saha, J.K. Laha, ACS Catal. 8 (2018) 5085-5144;
(c) number>L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. Chin. Chem. 62 (2019) 460-464;
(d) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30 (2019) 2132-2138;
(e) W. Wu, S. Yi, W. Huang, D. Luo, H. Jiang, Org. Lett. 19 (2017) 2825-2828;
(f) J. Liu, D. Yu, Y. Yang, et al., Org. Lett. 22 (2020) 4844-4847;
(g) Q.Q. Han, G.H. Li, Y.Y. Sun, et al., Tetrahedron Lett. 61 (2020) 151704;
(h) H.B. Qiu, P.C. Guo, L. Yuan, G.P. Sheng, Chin. Chem. Lett. 31 (2020) 2614-2618;
(i) Y. Li, W. Xiang, T. Zhou, et al., Chin. Chem. Lett. 31 (2020) 2757-2761;
(j) Z. Chen, Q. Huang, B. Huang, F. Zhang, C. Li, Chin. J. Catal. 40 (2019) 38-42.
-
[14]
(a) D. Dong, G. Li, D. Chen, et al., Chin. J. Org. Chem. 40 (2020) 1766-1771;
(b) D.M. Chen, Y.Y. Sun, Q.Q. Han, Z.L. Wang, Tetrahedron Lett. 61 (2020) 152482;
(c) S.Q. Yan, D.Q. Dong, C.W. Xie, W.S. Wang, Z.L. Wang, Chin. J. Org. Chem. 39 (2019) 2560-2566;
(d) G.H. Li, D.Q. Dong, Y. Yang, X.Y. Yu, Z.L. Wang, Adv. Synth. Catal. 361 (2019) 832-835.
-
[15]
(a) J.W. Yuan, J.L. Zhu, H.L. Zhu, et al., Org. Chem. Front. 7 (2020) 273-285;
(b) J.W. Yuan, Q. Chen, C. Li, et al., Org. Biomol. Chem. 18 (2020) 2747-2757;
(c) M.T. Westwood, C.J.C. Lamb, D.R. Sutherland, A.L. Lee, Org. Lett. 21 (2019) 7119-7123;
(d) Q.Q. Han, D.M. Chen, Z.L. Wang, et al., Chin. Chem. Lett. 32 (2021) 2559-2561;
(e) D. Chen, Y. Sun, D. Dong, Q. Han, Z. Wang, Chin. J. Org. Chem. 40 (2020) 4267-4273;
(f) D.Q. Dong, L.X. Li, G.H. Li, et al., Chin. J. Catal. 40 (2019) 1494-1498;
(g) Z. Gan, X. Zhu, Q. Yan, X. Song, D. Yang, Chin. Chem. Lett. 32 (2020) 1705-1708;
(h) J.Y. Chen, Y.W. Lin, W.M. He, Chin. Chem. Lett. 31 (2020) 2989-2990;
(i) K. Sun, S.J. Li, X.L. Chen, et al., Chem. Commun. 55 (2019) 2861-2864;
(j) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378;
(k) M.M. Xu, L.Y. Kou, X.G. Bao, X.P. Xu, S.J. Ji, Chin. Chem. Lett. 32 (2021) 1915-1919.

 Login In
Login In






 DownLoad:
DownLoad: