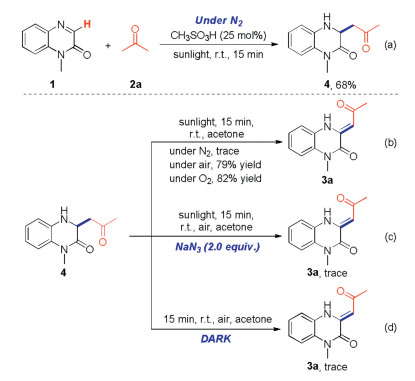
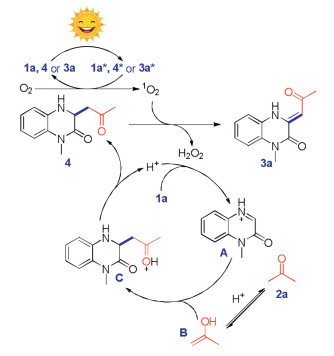
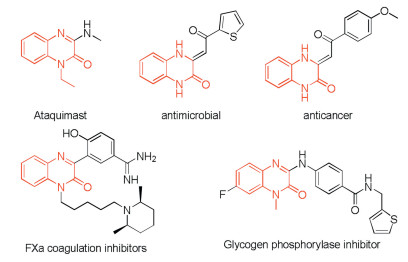
-
[1]
(a) R.E. TenBrink, W.B. Im, V.H. Sethy, et al., J. Med. Chem. 37 (1994) 758-768;
(b) A. Monge, F.J. Martinez-Crespo, A.L. Cerai, et al., J. Med. Chem. 38 (1995) 4488-4494;
(c) M.M. Badran, K.A.M. Abouzid, M.H.M. Hussein, Arch. Pharmacal Res. 26 (2003) 107-113;
(d) H.M. Refaat, A.A. Moneer, O.M. Khalil, Arch. Pharmacal Res. 27 (2004) 1093-1098;
(e) A. Carta, S. Piras, G. Loriga, G. Paglietti, Mini-Rev. Med. Chem. 6 (2006) 1179-1200;
(f) J.H. Fu, J.W. Yuan, Y. Zhang, et al., Org. Chem. Front. 5 (2018) 3382-3390;
(g) W. Wei, L.L. Wang, H.L. Yue, et al., ACS Sustain. Chem. Eng. 6 (2018) 17252-17257;
(h) J.W. Yuan, J.H. Fu, S.N. Liu, et al., Org. Biomol. Chem. 16 (2018) 3203-3212.
-
[2]
X.B. Zeng, C.L. Liu, X.Y. Wang, et al., Org. Biomol. Chem. 15 (2017) 8929-8935.
doi: 10.1039/C7OB02187A
-
[3]
(a) J.A. Willardsen, D.A. Dudley, W.L. Cody, et al., J. Med. Chem. 47 (2004) 4089-4099;
(b) S.Y. Zhang, F.M. Zhang, Y.Q. Tu, Chem. Soc. Rev. 40 (2011) 1937-1949;
(c) J.R. Zbieg, E. Yamaguchi, E.L. Mclnturff, M.J. Krische, Science 336 (2012) 324-327;
(d) T.Y. Chen, M.J. Krische, Org. Lett. 15 (2013) 2994-2997;
(e) D. Liu, C. Liu, H. Li, A. Lei, Angew. Chem. Int. Ed. 52 (2013) 4453-4456;
(f) X.Q. Chu, H. Meng, Y. Zi, X.P. Xu, S.J. Ji, Chem. Commun. 50 (2014) 9718-9721;
(g) X. Qin, X. Hao, H. Han, et al., J. Med. Chem. 58 (2015) 1254-1267;
(h) J.K. Cheng, T.P. Loh, J. Am. Chem. Soc. 137 (2015) 42-45.
-
[4]
(a) K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533;
(b) A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792;
(c) Q.M. Yang, Z.B. Yang, Y.S. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667.
-
[5]
X. Li, K.H. Yang, W.L. Li, W.F. Xu, Drugs Fut. 31 (2006) 979.
doi: 10.1358/dof.2006.031.11.1037128
-
[6]
J. Lu, X.K. He, X. Cheng, et al., Adv. Synth. Catal. 362 (2020) 2178-2182.
doi: 10.1002/adsc.202000116
-
[7]
Q. Ke, G. Yan, J. Yu, X. Wu, Org. Biomol. Chem. 17 (2019) 5863-5881.
doi: 10.1039/C9OB00782B
-
[8]
(a) J.Z. Jin, J.Y. Tong, W.B. Yu, J. Qiao, C. Shen, Catal. Commun. 141 (2020) 106008;
(b) J. Xu, H. Yang, H. Cai, et al., Org. Lett. 21 (2019) 4698-4702;
(c) J. Zhou, P. Zhou, T. Zhao, Q. Ren, J. Li, Adv. Synth. Catal. 361 (2019) 5371-5382;
(d) S. Peng, D. Hu, J.L. Hu, et al., Adv. Synth. Catal. 361 (2019) 5721-5726;
(e) L. Zhao, L. Wang, Y. Gao, Z. Wang, P. Li, Adv. Synth. Catal. 361 (2019) 5363-5370;
(f) Q. Yang, X. Han, J. Zhao, H.Y. Zhang, Y. Zhang, J. Org. Chem. 84 (2019) 11417-11424.
-
[9]
(a) K.J. Li, Y.Y. Jiang, K. Xu, C.C. Zeng, B.G. Sun, Green Chem. 21 (2019) 4412-4421;
(b) W.P. Mai, J.W. Yuan, J.L. Zhu, et al., ChemistrySelect 4 (2019) 11066-11070;
(c) J. Wang, J. Li, Y. Wei, J. Yang, C. Huo, Org. Chem. Front. 5 (2018) 3534-3537;
(d) Y. Kim, D.Y. Kim, Tetrahedron Lett. 59 (2018) 2443-2446;
(e) M. Gao, Y. Li, L. Xie, R. Chauvin, X. Cui, Chem. Commun. 52 (2016) 2846-2849.
-
[10]
(a) L.Y. Xie, Y.L. Chen, L. Qin, et al., Org. Chem. Front. 6 (2019) 3950-3955;
(b) Q.H. Teng, Y. Yao, W.X. Wei, et al., Green Chem. 21 (2019) 6241-6245.
-
[11]
(a) J. Yuan, J. Zhu, J. Fu, et al., Org. Chem. Front. 6 (2019) 925-935;
(b) L.Y. Xie, J.L. Hu, Y.X. Song, et al., ACS Sustain. Chem. Eng. 7 (2019) 19993-19999;
(c) J.W. Yuan, J.L. Zhu, B. Li, et al., Org. Biomol. Chem. 17 (2019) 10178-10187;
(d) Q. Yang, Z. Yang, Y. Tan, et al., Adv. Synth. Catal. 361 (2019) 1662-1667;
(e) Q. Yang, Y. Zhang, Q. Sun, et al., Adv. Synth. Catal. 360 (2018) 4509-4514;
(f) W. Wei, L. Wang, P. Bao, et al., Org. Lett. 20 (2018) 7125-7130;
(g) T.T. Hoang, T.A. To, V.T.T. Cao, et al., Catal. Commun. 101 (2017) 20-25;
(h) A. Gupta, M.S. Deshmukh, N. Jain, J. Org. Chem. 82 (2017) 4784-4792.
-
[12]
(a) L.Y. Xie, Y.S. Bai, X.Q. Xu, et al., Green Chem. 22 (2020) 1720-1725;
(b) P. Bao, F. Liu, Y. Lv, et al., Org. Chem. Front. 7 (2020) 492-498;
(c) J. Xu, H. Yang, L. He, et al., Org. Lett. 23 (2021) 195-201;
(d) J. Wang, B. Sun, L. Zhang, et al., Org. Chem. Front. 7 (2020) 113-118;
(e) J. Xu, H. Zhang, J. Zhao, et al., Org. Chem. Front. 7 (2020) 4031-4042;
(f) J. Shen, J. Xu, L. Huang, Q. Zhu, P. Zhang, Adv. Synth. Catal. 362 (2020)230-241;
(g) H. Zhang, J. Xu, M. Zhou, et al., Org. Biomol. Chem. 17 (2019) 10201-10208;
(h) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. Chin. Chem. 62 (2019) 460-464;
(i) L.X. Liu, N. Pan, W. Sheng, et al., Adv. Synth. Catal. 361 (2019) 4126-4132;
(j) W. Zhang, Y.L. Pan, C. Yang, et al., J. Org. Chem. 84 (2019) 7786-7795;
(k) L.Y. Xie, L.L. Jiang, J.X. Tan, etal., ACSSustain. Chem. Eng. 7 (2019)14153-14160;
(l) G. Hong, J. Yuan, J. Fu, et al., Org. Chem. Front. 6 (2019) 1173-1182;
(m) L. Wang, H. Liu, F. Li, et al., Adv. Synth. Catal. 361 (2019) 2354-2359;
(n) C. Jin, X. Zhuang, B. Sun, D. Li, R. Zhu, AsianJ. Org. Chem. 8 (2019) 1490-1494;
(o) W. Xue, Y. Su, K.H. Wang, et al., Asian J. Org. Chem. 8 (2019) 887-892;
(p) J. Wang, B. Sun, L. Zhang, et al., Asian J. Org. Chem. 8 (2019) 1942-1946;
(q) W. Wei, L. Wang, H. Yue, etal., ACSSustain. Chem. Eng. 6 (2018)17252-17257;
(r) S. Liu, Y. Huang, F.L. Qing, X.H. Xu, Org. Lett. 20 (2018) 5497-5501;
(s) L. Hu, J. Yuan, J. Fu, et al., Eur. J. Org. Chem. 2018 (2018) 4113-4120;
(t) J. Fu, J. Yuan, Y. Zhang, Org. Chem. Front. 5 (2018) 3382-3390;
(u) J. Yuan, J. Fu, J. Yin, et al., Org. Chem. Front. 5 (2018) 2820-2828;
(v) K. Yin, R. Zhang, Synlett 29 (2018) 597-602;
(w) B. Ramesh, C.R. Reddy, G.R. Kumar, B.V.S. Reddy, Tetrahedron Lett. 59 (2018) 628-631;
(x) L. Wang, Y. Zhang, F. Li, Adv. Synth. Catal. 360 (2018) 3969-3977;
(y) K. Yin, R. Zhang, Org. Lett. 19 (2017) 1530-1533;
(z) J. Yuan, S. Liu, L. Qu, Adv. Synth. Catal. 359 (2017) 4197-4207.
-
[13]
(a) M.H. Shaw, V.W. Shurtleff, J.A. Terrett, J.D. Cuthbertson, D.W.C. MacMillan, Science 352 (2016) 1304-1308;
(b) Q.W. Gui, F. Teng, Z.C. Li, et al., Chin. Chem. Lett. 32 (2021)1907-1910;
(c) B. Sun, P. Huang, Z. Yan, et al., Org. Lett. 23 (2021) 1026-1031;
(d) L.Y. Xie, S. Peng, L.H. Yang, et al., Green Chem. 23 (2021) 374-378;
(e) K.J. Liu, Z. Wang, L.H. Lu, et al., Green Chem. 23 (2021) 496-500;
(f) J. Yang, B. Sun, H. Ding, et al., Green Chem. 23 (2021) 575-581;
(g) G.H. Li, Q.Q. Han, Y.Y. Sun, et al., Chin. Chem. Lett. 31 (2020) 3255-3258;
(h) W. Ou, R. Zou, M. Han, L. Yu, C. Su, Chin. Chem. Lett. 31 (2020) 1899-1902;
(i) S. He, X. Chen, F. Zeng, et al., Chin. Chem. Lett. 31 (2020) 1863-1867;
(j) L. Wang, M. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 31 (2020) 67-70;
(k) L. Zou, P. Li, B. Wang, L. Wang, Green Chem. 21 (2019) 3362-3369;
(l) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30 (2019) 2295-2298;
(m) J. Shen, J. Xu, L. He, Y. Ouyang, et al., Org. Lett. 23 (2021) 1204-1208;
(n) J.M.R. Narayanam, C.R.J. Stephenson, Chem. Soc. Rev. 40 (2011) 102-113.
-
[14]
(a) P. Esser, B. Pohlmann, H.D. Scharf, Angew. Chem. Int. Ed. 33 (1994) 2009-2023;
(b) M. Okada, T. Fukuyama, K. Yamada, et al., Chem. Sci. 5 (2014) 2893-2898;
(c) S. Park, W.H. Jeon, W.S. Yong, P.H. Yong, Org. Lett. 17 (2015) 5060-5063;
(d) S.Y. Ni, J. Cao, H.B. Mei, et al., Green Chem. 18 (2016) 3935-3939.
-
[15]
(a) J. Xu, L. Huang, L. He, et al., Green Chem. 23 (2021) 2123-2129;
(b) C. Shen, A. Wang, J. Xu, et al., Chem 5 (2019) 1059-1107;
(c) J. Xu, K. Du, J. Shen, et al., ChemCatChem 10 (2018) 3675-3679;
(d) J. Xu, K. Cheng, C. Shen, et al., ChemCatChem 10 (2018) 965-970;
(e) C. Shen, M. Yang, J. Xu, et al., RSC Adv. 7 (2017) 49436-49439;
(f) J. Xu, C. Shen, X. Zhu, et al., Chem. Asian J. 11 (2016) 882-892;
(g) J. Xu, X. Zhu, G. Zhou, et al., Org. Biomol. Chem. 14 (2016) 3016-3021.
-
[16]
E.E. Stepanova, D.N. Lukmanova, S.O. Kasatkina, M.V. Dmitriev, A.N. Maslivets, ChemistrySelect 4 (2019) 12774-12778.
doi: 10.1002/slct.201902900
-
[17]
(a) L.Y. Xie, Y.S. Liu, H.R. Ding, et al., Chin. J. Catal. 41 (2020) 1168-1173;
(b) D. Rawat, R. Kumar, A. Subbarayappa, Green Chem. 22 (2020) 6170-6175.

 Login In
Login In






 DownLoad:
DownLoad: