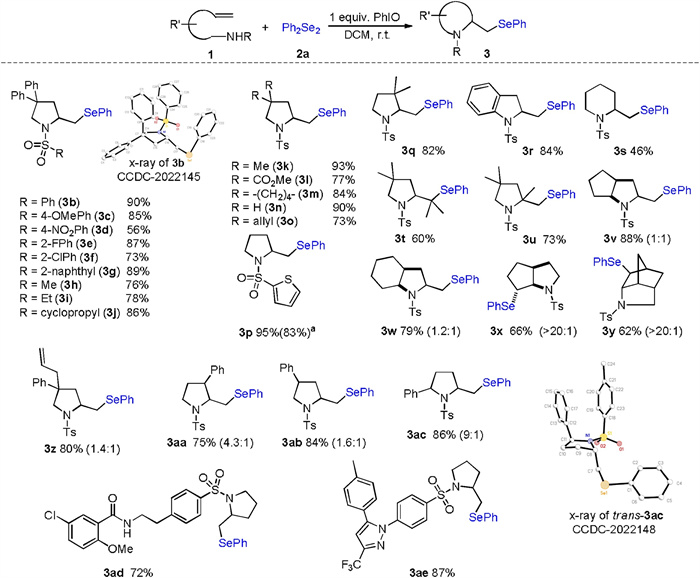
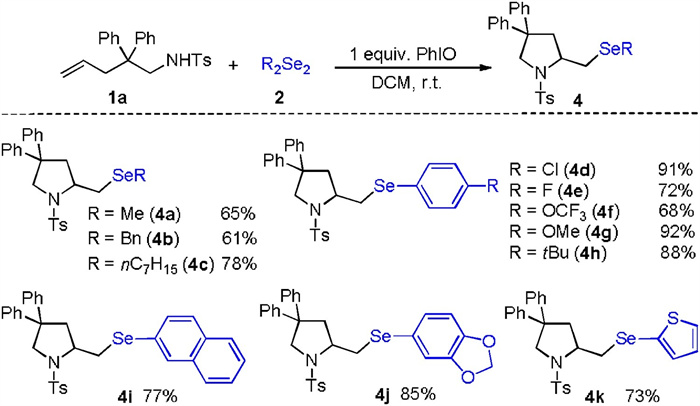
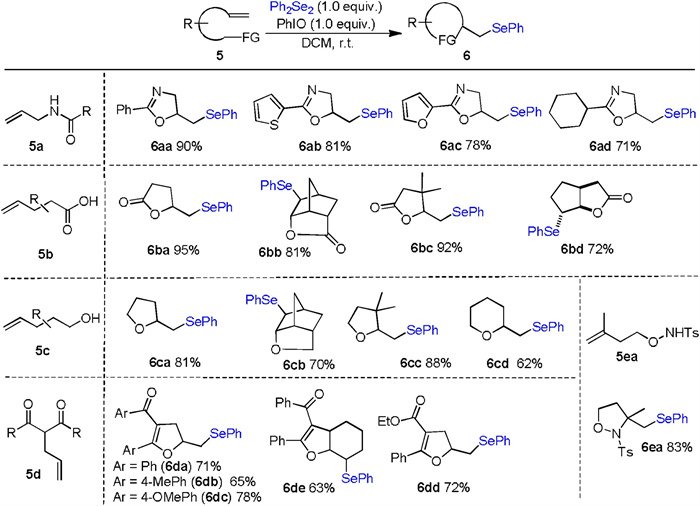
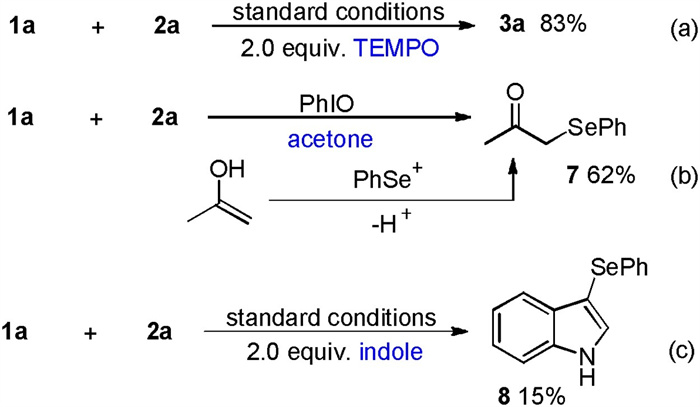
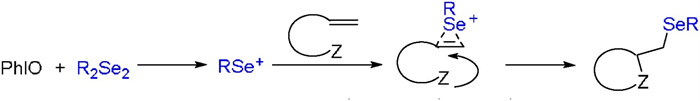
-
[1]
(a) Z. Chen, H. Lai, L. Hou, T. Chen, Chem. Commun. 56 (2020) 179–196;
(b) C.W. Nogueira, G. Zeni, J.B.T. Rocha, Chem. Rev. 104 (2004) 6255–6285;
(c) G. Mugesh, W.W. du Mont, H. Sies, Chem. Rev. 101 (2001) 2125–2179.
-
[2]
(a) S. Panda, A. Panda, S.S. Zade, Coord. Chem. Rev. 300 (2015) 86–100;
(b) S.T. Manjare, Y. Kim, D.G. Churchill, Acc. Chem. Res. 47 (2014) 2985–2998.
-
[3]
(a) A. Breder, C. Depken, Angew. Chem. Int. Ed. 58 (2019) 17130–17147;
(b) J. Luo, X. Liu, X. Zhao, Synlett 28 (2017) 397–401;
(c) V. Rathore, C. Jose, S. Kumar, New J. Chem. 43 (2019) 8852–8864;
(d) P.N. Makhal, A. Nandi, V.R. Kaki, ChemistrySelect 6 (2021) 663–679.
-
[4]
(a) A.D. Sonawane, M. Koketsu, Curr. Org. Chem. 23 (2019) 3206–3225;
(b) A.J. Mukherjee, S.S. Zade, H.B. Singh, R.B. Sunoj, Chem. Rev. 110 (2010) 4357–4416;
(c) K. Sun, X. Wang, C. Li, H. Wang, L. Li, Org. Chem. Front. 7 (2020) 3100–3119.
-
[5]
(a) X.W. Lan, N.X. Wang, Y. Xing, Eur. J. Org. Chem. 2017 (2017) 5821–5851;
(b) T. Courant, G. Masson, J. Org. Chem. 81 (2016) 6945–6952;
(c) Z.L. Li, G.C. Fang, Q.S. Gu, X.Y. Liu, Chem. Soc. Rev. 49 (2020) 32–48;
(d) J.S. Zhang, L. Liu, T. Chen, L.B. Han, Chem. Asian J. 13 (2018) 2277–2291.
-
[6]
(a) X. Franck, S. Leleu, F. Outurquin, Tetrahedron Lett. 51 (2010) 4437–4440;
(b) B. Berthe, F. Outurquin, C. Paulmier, Tetrahedron Lett. 38 (1997) 1393–1396.
-
[7]
(a) T. Izumi, M. Sugano, T. Konno, J. Heterocycl. Chem. 29 (1992) 899–904;
(b) A.D. Jones, D.W. Knight, A.L. Redfern, J. Gilmore, Tetrahedron Lett. 40 (1999) 3267–3270;
(c) A.D. Jones, A.L. Redfern, D.W. Knight, I.R. Morgan, A.C. Williams, Tetrahedron 62 (2006) 9247–9257;
(d) A. Toshimitsu, K. Terao, S. Uemura, Tetrahedron Lett. 25 (1984) 5917–5920;
(e) A. Toshimitsu, K. Terao, S. Uemura, J. Org. Chem. 51 (1986) 1724–1729.
-
[8]
(a) R. Grigg, M. Hadjisoteriou, P. Kennewell, J. Markandu, J. Chem. Soc. Chem. Commun. (1992) 1537–1538;
(b) M. Tiecco, L. Testaferri, M. Tingoli, L. Bagnoli, J. Chem. Soc. Chem. Commun. (1995) 235–236.
-
[9]
K. Terao, M. Kunishima, S. Tani, Synlett 6 (1999) 733–734.
doi: 10.1055/s-1999-2728
-
[10]
(a) R.R. Webb II, S. Danishefsky, Tetrahedron Lett. 24 (1983) 1357–1360;
(b) M.A. Cooper, A.D. Ward, Tetrahedron Lett. 33 (1992) 5999–6002;
(c) A.M. Morella, A.D. Ward, Aust. J. Chem. 48 (1995) 445–467;
(d) D.L.J. Clive, C.K. Wong, W.A. Kiel, S.M. Menchen, J. Chem. Soc. Chem. Commun. (1978) 379–380;
(e) D.L.J. Clive, V. Farina, A. Singh, et al., J. Org. Chem. 45 (1980) 2120–2126.
-
[11]
(a) N. De Kimpe, M. Boelens, J. Chem. Soc. Chem. Commun. (1993) 916–918;
(b) D. Schley, J. Liebscher, Eur. J. Org. Chem. 2007 (2007) 2945–2957.
-
[12]
(a) K. Terao, A. Toshimitsu, S. Uemura, J. Chem. Soc. Perkin Trans. 1 (1986) 1837–18431709c(b) A. Toshimitsu, K. Terao, S. Uemura, J. Chem. Soc. Chem. Commun. (1986) 530–531.
-
[13]
M. Tiecco, L. Testaferri, F. Marini, et al., Tetrahedron 53 (1997) 10591–10602.
doi: 10.1016/S0040-4020(97)00670-4
-
[14]
M. Tiecco, L. Testaferri, F. Marini, et al., Tetrahedron 53 (1997) 7311–7318.
doi: 10.1016/S0040-4020(97)00414-6
-
[15]
(a) X. Pannecoucke, F. Outurquin, C. Paulmier, Eur. J. Org. Chem. 2002 (2002) 995–1006;
(b) F. Outurquin, X. Pannecoucke, B. Berthe, C. Paulmier, Eur. J. Org. Chem. 2002 (2002) 1007–1014.
-
[16]
(a) M. Tiecco, L. Testaferri, M. Tingoli, D. Chianelli, D. Bartoli, Tetrahedron 44 (1988) 2261–2272:
(b) M. Tiecco, L. Testaferri, M. Tingoli, D. Chianelli, D. Bartoli, Tetrahedron 44 (1988) 2273–2282.
-
[17]
(a) M. Tiecco, L. Testaferri, F. Marini, et al., Tetrahedron 53 (1997) 4441–4446;
(b) M. Tiecco, L. Testaferri, M. Tingoli, L. Bagnoli, F. Marini, J. Chem. Soc. Perkin Trans. 1 (1993) 1989–1993;
(c) M. Tiecco, L. Testaferri, F. Marini, Tetrahedron 52 (1996) 11841–11848;
(d) M. Tiecco, L. Testaferri, M. Tingoli, C. Santi, Tetrahedron Lett. 36 (1995) 163–166.
-
[18]
(a) W. Yi, P.F. Wang, M. Lu, et al., ACS Sustain. Chem. Eng. 7 (2019) 16777–16785;
(b) W. Yi, Q.Y. Liu, X.X. Fang, S.C. Lou, G.Q. Liu, Org. Biomol. Chem. 16 (2018) 7012–7018;
(c) J. Liu, Q.Y. Liu, X.X. Fang, G.Q. Liu, Y. Ling, Org. Biomol. Chem. 16 (2018) 7454–7460;
(d) G.Q. Liu, Y.M. Li, J. Org. Chem. 79 (2014) 10094–10109.
-
[19]
(a) G. Illuminati, L. Mandolini, Acc. Chem. Res. 14 (1981) 95–102;
(b) A.J. Kirby, Effective Molarities for Intramolecular Reactions, Vol. 17, Academic Press, 1980, pp. 183–278;
(c) C. Galli, L. Mandolini, Eur. J. Org. Chem. 2000 (2000) 3117–3125.
-
[20]
M. Tiecco, L. Testaferri, L. Bagnoli, C. Scarponi, Tetrahedron: Asymmetry 19 (2008) 2411–2416.
doi: 10.1016/j.tetasy.2008.10.006
-
[21]
C.J. Carmalt, J.G. Crossley, J.G. Knight, et al., J. Chem. Soc. Chem. Commun. (1994) 2367–2368.
-
[22]
N. Miyoshi, T. Yamamoto, N. Kambe, S. Murai, N. Sonoda, Tetrahedron Lett. 23 (1982) 4813–4816.
doi: 10.1016/S0040-4039(00)85720-2
-
[23]
(a) Q.B. Zhang, Y.L. Ban, P.F. Yuan, et al., Green Chem. 19 (2017) 5559–5563;
(b) V. Rathore, S. Kumar, Green Chem. 21 (2019) 2670–2676.
-
[24]
(a) M. Shi, B.Y. Wang, J. Li, Eur. J. Org. Chem. 2005 (2005) 759–765;
(b) M. Tiecco, M. Tingoli, L. Testaferri, Pure Appl. Chem. 65 (1993) 715–722;
(c) L. Yu, B. Chen, X. Huang, Tetrahedron Lett. 48 (2007) 925–927.

 Login In
Login In

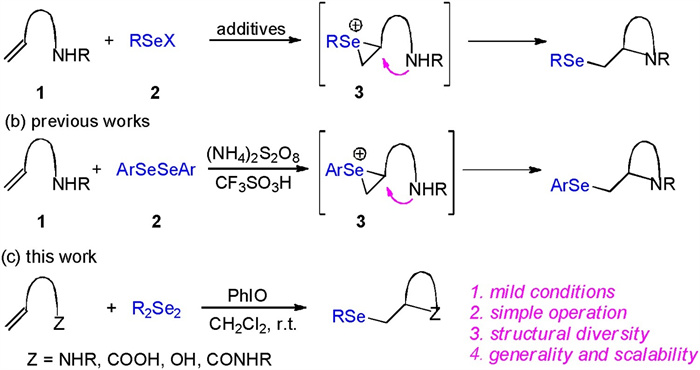





 DownLoad:
DownLoad: